Total Intravenous Anesthesia with Ketofol versus Combination of Ketofol and Lidocaine for Short-Term Anesthesia in Pediatric Patients; Double Blind, Randomized Clinical Trial of Effects on Recovery
Abstract
1. Introduction
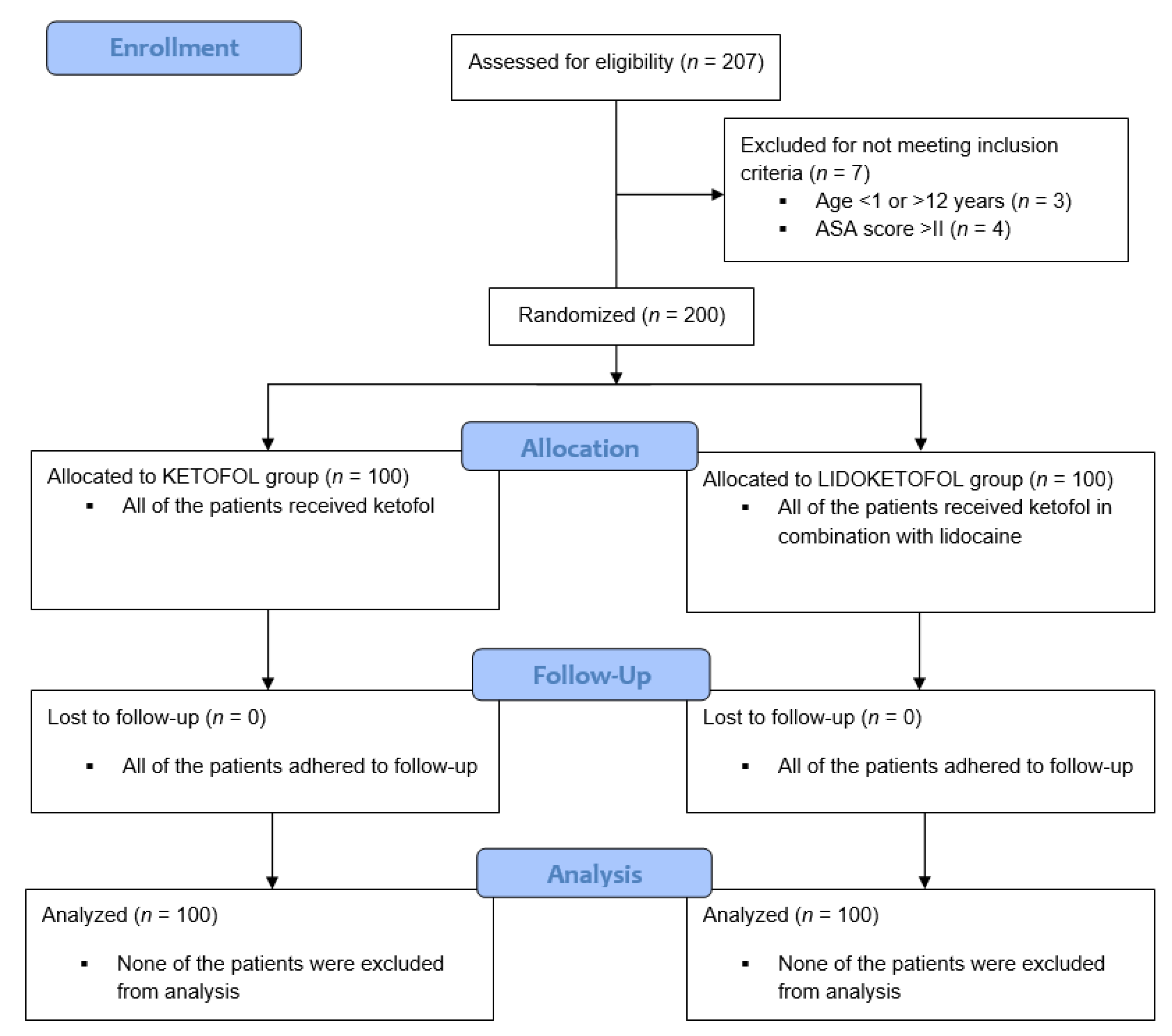
2. Materials and Methods
2.1. Patients
2.2. Intraoperative Monitoring
2.3. Study Design
2.4. Outcomes of the Study
2.5. Sample Size Calculation
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Motoyoma, E.K.; Davis, P.J. Smith’s Anesthesia for Infants and Children, 7th ed.; Mosby: St. Louis, MI, USA, 2006; pp. 3–7. [Google Scholar]
- Nevešćanin, B.A.; Ivančev, B.; Pogorelić, Z. Effects on recovery of pediatric patients undergoing total intravenous anesthesia with propofol versus ketofol for short-lasting laparoscopic procedures. Children 2021, 8, 610. [Google Scholar]
- Lerman, J.; Jöhr, M. Inhalational anesthesia vs total intravenous anesthesia (TIVA) for pediatric anesthesia. Peditr. Anesth. 2009, 19, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Kurdi, M.S.; Theerth, K.A.; Deva, R.S. Ketamine: Current applications in anesthesia, pain, and critical care. Anesth. Essays Res. 2014, 8, 283–290. [Google Scholar] [CrossRef]
- Sahinovic, M.M.; Struys, M.M.R.F.; Absalom, A.R. Clinical pharmacokinetics and pharmacodynamics of propofol. Clin. Pharmacokinet. 2018, 57, 1539–1558. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Zemedu, A.; Jemal, B. Evidence based guideline on use of ketofol (Ketamine and Propofol admixture) for procedural sedation and analgesia (PSA) in pediatrics surgery: Review article. Int. J. Sur. Open 2020, 25, 52–58. [Google Scholar]
- Amornyotin, S. Ketofol: A Combination of Ketamine and Propofol. J. Anesth. Crit. Care Open Access 2014, 1, 00031. [Google Scholar] [CrossRef][Green Version]
- Golzari, S.E.J.; Soleimanpour, H.; Mahmoodpoor, A.; Safari, S.; Ala, A. Lidocaine and pain management in the emergency department: A review article. Anesth. Pain Med. 2014, 4, e1544. [Google Scholar] [CrossRef]
- Nakhli, M.S.; Kahloul, M.; Guizani, T.; Zedini, C.; Chaouch, A.; Naija, W. Intravenous lidocaine as adjuvant to general anesthesia in renal surgery. Libyan J. Med. 2018, 13, 1433418. [Google Scholar] [CrossRef]
- Ghimire, A.; Subedi, A.; Bhattarai, B. The effect of intraoperative lidocaine infusion on opioid consumption and pain after totally extraperitoneal laparoscopic inguinal hernioplasty: A randomized controlled trial. BMC Anesthesiol. 2020, 20, 137. [Google Scholar] [CrossRef]
- McFarlan, C.S.; Anderson, B.J.; Short, T.G. The use of propofol infusions in pediatric anesthesia: A practical guide. Pediatr. Anesth. 1999, 9, 209–216. [Google Scholar]
- Vereecke, H.E.M.; Struys, M.M.R.F.; Mortier, E.P. A comparison of bispectral index and ARX-derived auditory evoked potential index in measuring the clinical interaction between ketamine and propofol anaesthesia. Anaesthesia 2003, 58, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Hans, P.; Dewandre, P.Y.; Brichant, J.F.; Bonhomme, V. Comparative effects of ketamine on bispectral index and spectral entropy of the electroencephalogram under sevoflurane anesthesia. Br. J. Anaesth. 2005, 94, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Nevešćanin, A.; Vickov, J.; Elezović Baloević, S.; Pogorelić, Z. Laryngeal mask airway versus tracheal intubation for laparoscopic hernia repair in children: Analysis of respiratory complications. J. Laparoendosc. Adv. Surg. Tech. A 2020, 30, 76–80. [Google Scholar] [CrossRef]
- Koo, B.-W.; Oh, A.-Y.; Hwang, J.-W.; Na, H.-S.; Min, S.-W. Comparison of standard versus 90° rotation technique for LMA Flexible™ insertion: A randomized controlled trial. BMC Anesthesiol. 2019, 19, 95. [Google Scholar] [CrossRef]
- Aldrete, J. The post-anesthesia recovery score revisited. J. Clin. Anesth. 1995, 7, 89–91. [Google Scholar] [CrossRef]
- Lauder, G.R. Total intravenous anesthesia will supercede inhalational anesthesia in pediatric anesthetic practice. Pediatr. Anesth. 2015, 25, 52–64. [Google Scholar] [CrossRef]
- Dallimore, D.; Anderson, B.J.; Short, T.G.; Herd, D. Ketamine anesthesia in children exploring infusion regimens. Pediatr. Anesth. 2008, 18, 708–714. [Google Scholar] [CrossRef]
- Herd, D.W.; Anderson, B.J.; Keene, N.A.; Holford, N.H. Investigating the pharmacodynamics of ketamine in children. Pediatr. Anesth. 2007, 18, 36–42. [Google Scholar] [CrossRef]
- Biricik, E.; Karacaer, F. Comparasion of TIVA with different combinations of ketamine-propofol mixtures in pediatric patients. J. Anesth. 2018, 32, 104–111. [Google Scholar] [CrossRef]
- Coulter, F.L.S.; Hannam, J.A.; Anderson, B.J. Ketofol simulations for dosing in pediatric anesthesia. Pediatr. Anesth. 2014, 24, 806–812. [Google Scholar] [CrossRef]
- Koppert, W.; Weigand, M.; Neumann, F.; Sittl, R.; Schüttler, J.; Schmelz, M.; Hering, W. Perioperative intravenous lidocaine has preventive effects on postoperative pain and morphine consumption after major abdominal surgery. Anesth. Analg. 2004, 98, 1050–1055. [Google Scholar] [CrossRef]
- Pogorelić, Z.; Gaberc, T.; Jukić, M.; Tintor, G.; Biliškov, A.N.; Mrklić, I.; Jerončić, A. The effect of subcutaneous and intraperitoneal instillation of local anesthetics on postoperative pain after laparoscopic varicocelectomy: A randomized controlled trial. Children 2021, 8, 1051. [Google Scholar] [CrossRef]
- Lin, R.; Zhu, K.; Poznikoff, A.K.; Görges, M.; Brown, Z.E. The effect of intraoperative lidocaine infusion on postoperative opioid consumption in adolescents undergoing posterior spinal instrumentation and fusion for idiopathic scoliosis. Spine J. 2021, 21, 1047–1048. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, T.; Wang, N.; Yun, Y.; Gan, T.J. Perioperative systemic lidocaine for postoperative analgesia and recovery after abdominal surgery. Dis. Colon. Rectum. 2012, 55, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Both, C.P.; Thomas, J.; Bühler, P.K.; Schmitz, A.; Weiss, M.; Piegeler, T. Factors associated with intravenous lidocaine in pediatric patients undergoing laparoscopic appendectomy—A retrospective, single-centre experience. BMC Anesthesiol. 2018, 18, 88. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Li, H.; Yang, M.; Zhang, F.; Liao, R.; Wang, R.; Wang, Q.; Zheng, P.; Zhang, J. Effect of ketamine combined with lidocaine in pediatric anesthesia. J. Clin. Lab. Anal. 2020, 34, e23115. [Google Scholar] [CrossRef]
| Group I | Group II | p | |
|---|---|---|---|
| KETOFOL | KETOFOL + LIDOCAINE | ||
| Age (years) (median, IQR) | 5 | 5 | 0.681 * |
| (4, 7) | (3, 7) | ||
| Sex (M) (n, %) (F) (n, %) | 78 (78%) | 81 (81%) | 0.599 ** |
| 22 (22%) | 19 (19%) | ||
| Weight (kg) (median, IQR) | 21 | 10.5 | 0.841 * |
| (16, 28) | (15, 32.5) |
| Variable | Group I | Group II | p |
|---|---|---|---|
| KETOFOL | KETOFOL + LIDOCAINE | ||
| Sevoran (iv cannula) (n, %) | 64 (64%) | 54 (54%) | 0.150 ** |
| Length of iv infusion (min) (median, IQR) | 23 (16, 30.5) | 23 (16, 30) | 0.944 * |
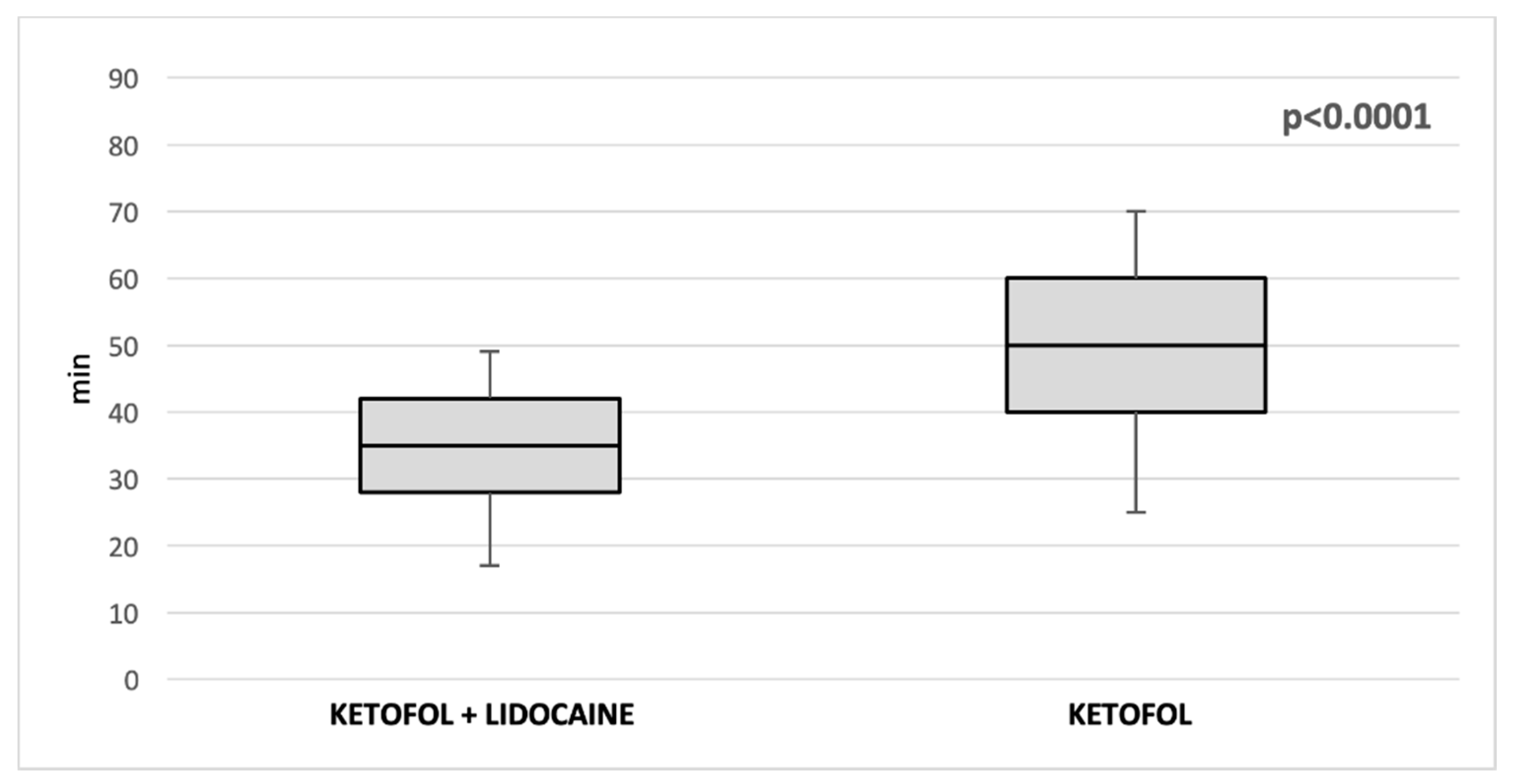
| Duration of anesthesia (min) (median, IQR) | 50 (40, 60) | 35 (28, 42) | <0.00001 * |
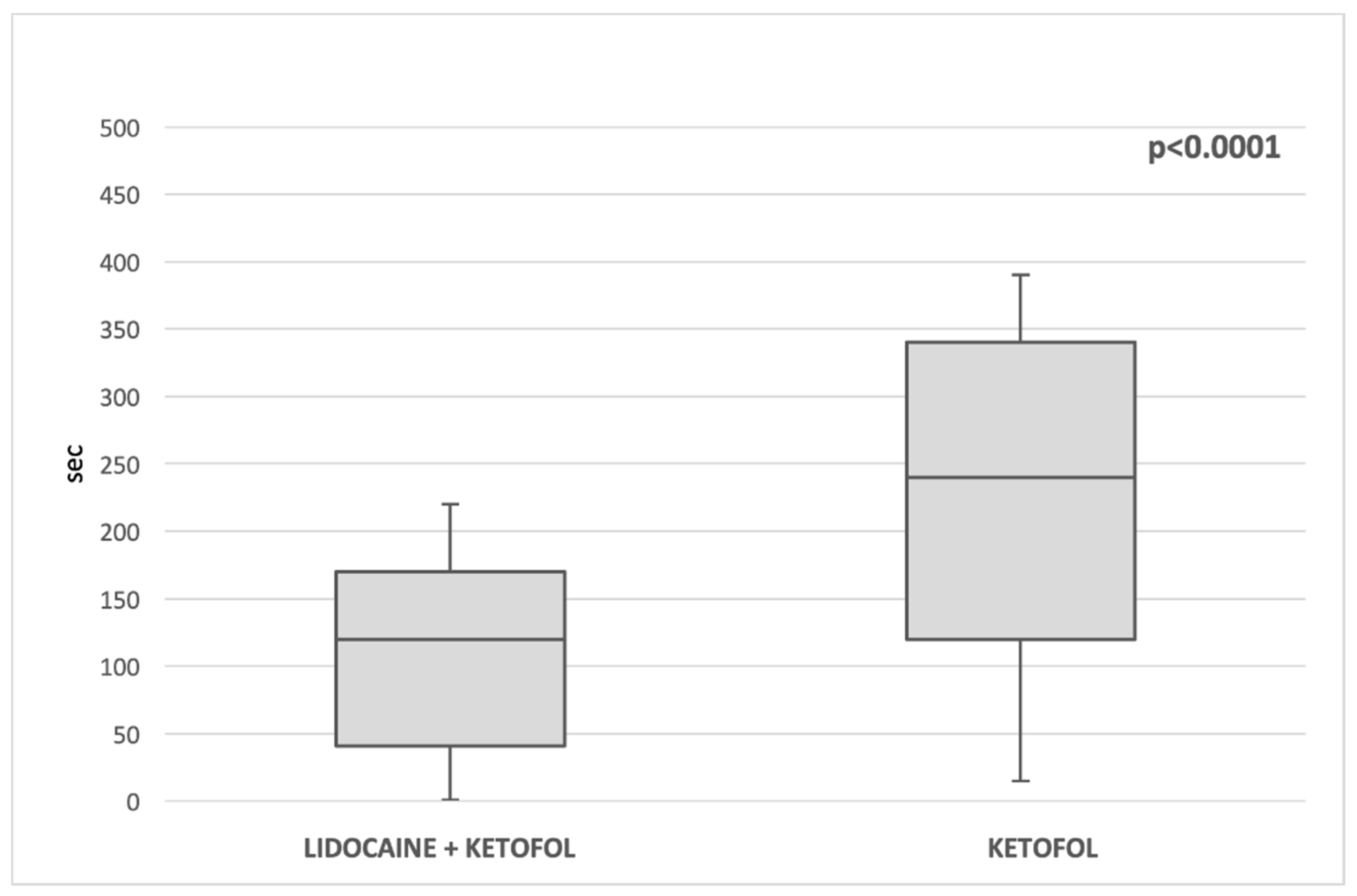
| Extubation time (s) (median, IQR) | 240 (120, 340) | 120 (41.5, 170) | <0.00001 * |
| Fentanyl (μg/kg) (median, IQR) | 2.3 (2, 3) | 2.1 (2, 2.8) | <0.0056 * |
| Fentanyl—TOTAL (μg) (median, IQR) | 50 (40, 60) | 45 (30, 65) | 0.250 * |
| Propofol (mg/kg) (median, IQR) | 7.55 (5, 10.5) | 6.6 (4, 7.5) | 0.032 * |
| Propofol—TOTAL (mg) (median, IQR) | 162.5 (122.5, 217.5) | 135 (106.5, 204) | 0.014 * |
| Ketamin (mg/kg) (median, IQR) | 1.6 (1.2, 2.1) | 1.3 (1, 1.5) | <0.0001 * |
| Ketamin—TOTAL (mg) (median, IQR) | 32 (24, 36) | 29 (22, 32) | 0.378 |
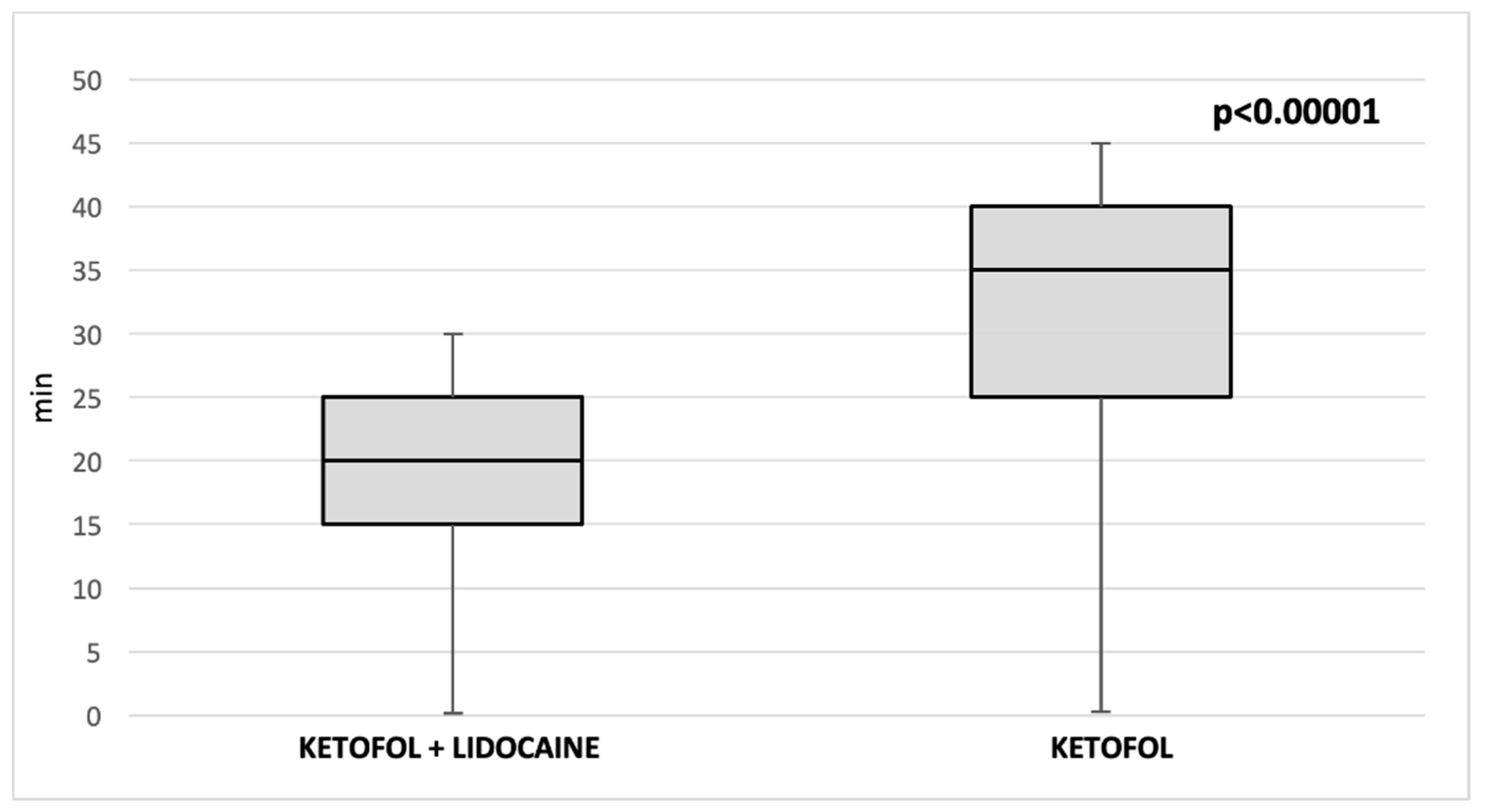
| PACU (min) (median, IRQ) | 35 (30, 40) | 20 (20, 25) | <0.00001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nevešćanin Biliškov, A.; Gulam, D.; Žaja, M.; Pogorelić, Z. Total Intravenous Anesthesia with Ketofol versus Combination of Ketofol and Lidocaine for Short-Term Anesthesia in Pediatric Patients; Double Blind, Randomized Clinical Trial of Effects on Recovery. Children 2022, 9, 282. https://doi.org/10.3390/children9020282
Nevešćanin Biliškov A, Gulam D, Žaja M, Pogorelić Z. Total Intravenous Anesthesia with Ketofol versus Combination of Ketofol and Lidocaine for Short-Term Anesthesia in Pediatric Patients; Double Blind, Randomized Clinical Trial of Effects on Recovery. Children. 2022; 9(2):282. https://doi.org/10.3390/children9020282
Chicago/Turabian StyleNevešćanin Biliškov, Ana, Danijela Gulam, Marija Žaja, and Zenon Pogorelić. 2022. "Total Intravenous Anesthesia with Ketofol versus Combination of Ketofol and Lidocaine for Short-Term Anesthesia in Pediatric Patients; Double Blind, Randomized Clinical Trial of Effects on Recovery" Children 9, no. 2: 282. https://doi.org/10.3390/children9020282
APA StyleNevešćanin Biliškov, A., Gulam, D., Žaja, M., & Pogorelić, Z. (2022). Total Intravenous Anesthesia with Ketofol versus Combination of Ketofol and Lidocaine for Short-Term Anesthesia in Pediatric Patients; Double Blind, Randomized Clinical Trial of Effects on Recovery. Children, 9(2), 282. https://doi.org/10.3390/children9020282







