Differential Diagnosis of Histiocytic Necrotizing Lymphadenitis and Malignant Lymphoma with Simple Clinical Findings
Abstract
:1. Introduction
2. Materials and Methods
3. Results
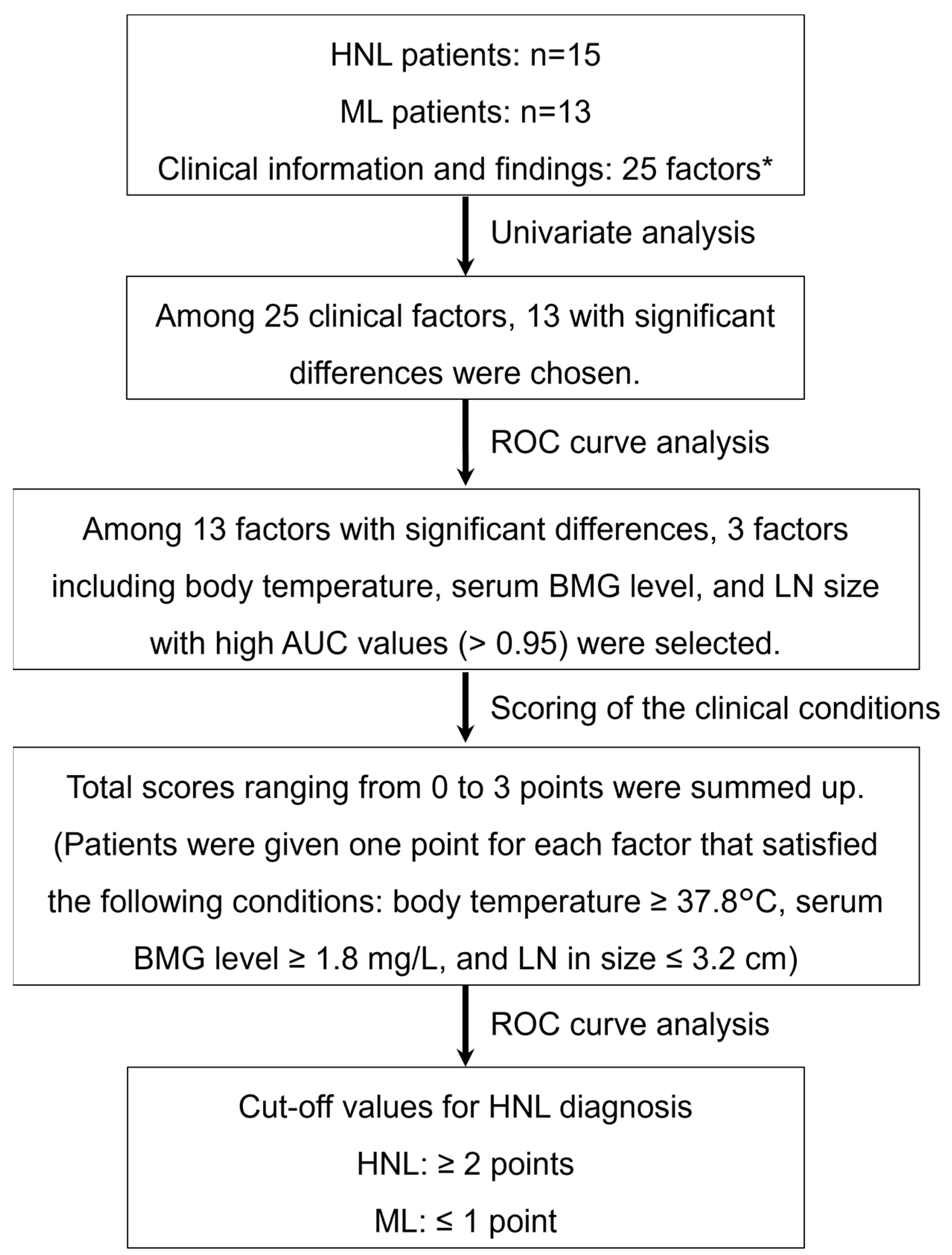
3.1. Patients
3.2. Establishment and Evaluation of the Scoring Model
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kikuchi, M. Lymphadenitis showing focal reticulum cells hyperplasia with nuclear debris and phagocytosis: A clinicopathological study. Acta Haematol. Jpn. 1972, 35, 379–380. [Google Scholar]
- Bosch, X.; Guilabert, A.; Miquel, R.; Campo, E. Enigmatic Kikuchi-Fujimoto disease: A comprehensive review. Am. J. Clin. Pathol. 2004, 122, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.M.; Choi, S.M. Kikuchi-Fujimoto Disease: A Review. Arch. Pathol. Lab. Med. 2018, 142, 1341–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Tani, L.Y.; Burns, J.C.; Shulman, S.T.; Bolger, A.F.; et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation 2017, 135, e927–e999. [Google Scholar] [CrossRef]
- Xu, S.; Sun, W.; Liu, J. Kikuchi-Fujimoto disease: A case report and the evaluation of diagnostic procedures. BMC Oral Health 2019, 19, 223. [Google Scholar] [CrossRef] [PubMed]
- Bosch, X.; Guilabert, A. Kikuchi-Fujimoto disease. Orphanet J. Rare Dis. 2006, 1, 18. [Google Scholar] [CrossRef] [Green Version]
- Team, R.C. R: A Language and Environment for Statistical Computing. 2021. Available online: https://www.R-project.org/ (accessed on 3 August 2021).
- Allaire, J. RStudio: Integrated Development Environment for R (Version 0.99. 892); R-Studio: Boston, MA, USA, 2012. [Google Scholar]
- Lo, W.-C.; Chang, W.-C.; Lin, Y.-C.; Hsu, Y.-P.; Liao, L.-J. Ultrasonographic differentiation between Kikuchi’s disease and lymphoma in patients with cervical lymphadenopathy. Eur. J. Radiol. 2012, 81, 1817–1820. [Google Scholar] [CrossRef]
- Ishimura, M.; Yamamoto, H.; Mizuno, Y.; Takada, H.; Goto, M.; Doi, T.; Hoshina, T.; Ohga, S.; Ohshima, K.; Hara, T. A Non-invasive Diagnosis of Histiocytic Necrotizing Lymphadenitis by Means of Gene Expression Profile Analysis of Peripheral Blood Mononuclear Cells. J. Clin. Immunol. 2013, 33, 1018–1026. [Google Scholar] [CrossRef]
- Zou, Q.; Jiao, J.; Yang, T.; Zhang, Y. Role of 18F-FDG PET/CT in the Assessment of Kikuchi-Fujimoto Disease and Its Differential Diagnosis from non-Hodgkin Lymphoma. J. Nucl. Med. 2019, 60 (Suppl. 1), 1073. [Google Scholar]
- Jung, H.J.; Lee, I.J.; Yoon, S.-H. Risk Assessment of Recurrence and Autoimmune Disorders in Kikuchi Disease. Risk Manag. Heal. Policy 2020, 13, 1687–1693. [Google Scholar] [CrossRef]
- Smith, A.; Crouch, S.; Lax, S.; Li, J.; Painter, D.; Howell, D.; Patmore, R.; Jack, A.; Roman, E. Lymphoma incidence, survival and prevalence 2004–2014: Sub-type analyses from the UK’s Haematological Malignancy Research Network. Br. J. Cancer 2015, 112, 1575–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.M.; Kim, J.Y.; Choi, E.H.; Lee, H.J.; Yun, K.W.; Lee, H. Clinical Characteristics of Severe Histiocytic Necrotizing Lymphadenitis (Kikuchi-Fujimoto Disease) in Children. J. Pediatr. 2016, 171, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, M.; Fan, H.; Hu, F.; Liu, T. Comparison of pediatric and adult lymphomas involving the mediastinum characterized by distinctive clinicopathological and radiological features. Sci. Rep. 2017, 7, 2577. [Google Scholar] [CrossRef] [PubMed]
- Storck, K.; Brandstetter, M.; Keller, U.; Knopf, A. Clinical presentation and characteristics of lymphoma in the head and neck region. Head Face Med. 2019, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Yeon, Y.H.; Lee, B.C. Kikuchi-Fujimoto disease with prolonged fever in children. Pediatrics 2004, 114, e752–e756. [Google Scholar] [CrossRef] [Green Version]
- Cooper, E.H.; Forbes, M.A.; Hambling, M.H. Serum beta 2-microglobulin and C reactive protein concentrations in viral infections. J. Clin. Pathol. 1984, 37, 1140–1143. [Google Scholar] [CrossRef] [Green Version]
- Yoo, C.; Yoon, D.H.; Suh, C. Serum beta-2 microglobulin in malignant lymphomas: An old but powerful prognostic factor. Blood Res. 2014, 49, 148–153. [Google Scholar] [CrossRef] [Green Version]
- Kwon, S.-Y.; Kim, T.-K.; Kim, Y.-S.; Lee, K.Y.; Lee, N.J.; Seol, H.Y. CT findings in Kikuchi disease: Analysis of 96 cases. Am. J. Neuroradiol. 2004, 25, 1099–1102. [Google Scholar]
- Nixon, S.; Bezverbnaya, K.; Maganti, M.; Gullane, P.; Reedijk, M.; Kuruvilla, J.; Prica, A.; Kridel, R.; Kukreti, V.; Bennett, S.; et al. Evaluation of Lymphadenopathy and Suspected Lymphoma in a Lymphoma Rapid Diagnosis Clinic. JCO Oncol. Pract. 2020, 16, e29–e36. [Google Scholar] [CrossRef]
- Cooper, G. The Development and Causes of Cancer, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Gaddey, H.L.; Riegel, A.M. Unexplained Lymphadenopathy: Evaluation and Differential Diagnosis. Am. Fam. Physician 2016, 94, 896–903. [Google Scholar]
- Lu, T.-X.; Wu, S.; Cai, D.-Y.; Hong, T.-T.; Zhang, Y.; Gao, H.-Q.; Hua, H.-Y.; Wu, X.-H. Prognostic significance of serum aspartic transaminase in diffuse large B-cell lymphoma. BMC Cancer 2019, 19, 553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, F.K.; Moriwaki, K.; De Rosa, M.J. Detection of necrosis by release of lactate dehydrogenase activity. Methods Mol. Biol. 2013, 979, 65–70. [Google Scholar] [PubMed] [Green Version]
- Yoshioka, K.; Miyashita, T.; Nakamura, T.; Inoue, T.; Yamagami, K. Treatment of histiocytic necrotizing lymphadenitis (Kikuchi’s disease) with prolonged fever by a single course of methylprednisolone pulse therapy without maintenance therapy: Experience with 13 cases. Intern. Med. 2010, 49, 2267–2270. [Google Scholar] [CrossRef] [Green Version]
- Canna, S.W.; Marsh, R.A. Pediatric hemophagocytic lymphohistiocytosis. Blood 2020, 135, 1332–1343. [Google Scholar] [CrossRef] [PubMed]
- Tsujikawa, T.; Tsuchida, T.; Imamura, Y.; Kobayashi, M.; Asahi, S.; Shimizu, K.; Tsuji, K.; Okazawa, H.; Kimura, H. Kikuchi-Fujimoto disease: PET/CT assessment of a rare cause of cervical lymphadenopathy. Clin. Nucl. Med. 2011, 36, 661–664. [Google Scholar] [CrossRef] [Green Version]
- Cieckiewicz, S.A.; Wojciechowska, U.; Didkowska, J.; Rymkiewicz, G.; Paszkiewicz-Kozik, E.; Sokół, K.; Prochorec-Sobieszek, M.; Walewski, J. Population-based epidemiological data of follicular lymphoma in Poland: 15 years of observation. Sci. Rep. 2020, 10, 14610. [Google Scholar] [CrossRef]
- Tai, E.; Pollack, L.A.; Townsend, J.; Li, J.; Steele, C.B.; Richardson, L.C. Differences in non-Hodgkin lymphoma survival between young adults and children. Arch. Pediatr. Adolesc. Med. 2010, 164, 218–224. [Google Scholar] [CrossRef] [Green Version]
- Cairo, M.S.; Sposto, R.; Gerrard, M.; Auperin, A.; Goldman, S.C.; Harrison, L.; Pinkerton, R.; Raphael, M.; McCarthy, K.; Perkins, S.L.; et al. Advanced stage, increased lactate dehydrogenase, and primary site, but not adolescent age (≥ 15 years), are associated with an increased risk of treatment failure in children and adolescents with mature B-cell non-Hodgkin’s lymphoma: Results of the FAB LMB 96 study. J Clin Oncol. 2012, 30, 387–393. [Google Scholar]



| ML (n = 13) | HNL (n = 15) | p-Value | |
|---|---|---|---|
| Body temperature (°C), median (IQR) | 36.90 (36.60, 37.20) | 39.00 (38.05, 39.15) | <0.001 |
| Duration of fever (days), median (IQR) | 0.00 (0.00, 0.00) | 14.00 (9.00, 19.00) | <0.001 |
| LN in size (cm), median (IQR) | 4.00 (3.45, 5.95) | 1.90 (1.55, 2.00) | <0.001 |
| Presence of LN tenderness, No (%) | 2 (15.4) | 15 (100.0) | <0.001 |
| Skin rash | 0 (0) | 2 (13.3) | 0.4841 |
| ML (n = 13) | HNL (n = 15) | p-Value | |
|---|---|---|---|
| WBC (/μL) | 7400.00 (5800.00, 9700.00) | 2600.00 (2200.00, 3750.00) | <0.001 |
| Neutrophil (/μL) | 4030.00 (2020.00, 4320.00) | 1711.00 (1182.50, 2087.50) | <0.005 |
| Monocyte (/μL) | 248.00 (140.00, 481.00) | 152.00 (94.75, 395.00) | 0.4327 |
| Lymphocyte (/μL) | 3040.00 (1450.00, 3626.00) | 1189.00 (603.50, 1507.50) | <0.01 |
| Atypical lymphocyte (/μL) | 0.00 (0.00, 0.00) | 0.00 (0.00, 17.25) | 0.5963 |
| RBC (M/μL) | 5.02 (4.76, 5.15) | 4.64 (4.38, 4.80) | 0.0295 |
| Hb (g/dL) | 13.70 (12.60, 14.00) | 12.60 (11.65, 13.50) | 0.1658 |
| HCT (%) | 39.40 (36.80, 41.50) | 35.70 (34.50, 39.05) | 0.0779 |
| MCV (fL) | 78.50 (75.50, 83.60) | 80.00 (77.45, 82.85) | 0.8301 |
| RDW (fL) | 38.10 (36.20, 38.20) | 36.70 (35.30, 37.50) | 0.1656 |
| Plt (109/L) | 2840.00 (2480.00, 3480.00) | 1710.00 (1525.00, 1940.00) | <0.001 |
| AST (U/L) | 22.00 (18.00, 27.00) | 43.00 (29.00, 55.50) | <0.01 |
| ALT (U/L) | 17.00 (12.00, 18.00) | 22.00 (14.50, 30.50) | 0.1801 |
| TG (mg/dL) | 73.00 (56.00, 91.00) | 90.00 (61.00, 104.50) | 0.2786 |
| LDH (U/L) | 260.00 (210.00, 319.00) | 449.00 (307.00, 617.50) | 0.01116 |
| Na (mEq/L) | 140.00 (140.00, 140.00) | 138.00 (135.50, 140.00) | <0.01 |
| CRP (mg/dL) | 0.07 (0.03, 0.28) | 0.34 (0.16, 0.87) | 0.06475 |
| BMG (mg/L) | 1.60 (1.30, 1.70) | 2.50 (2.25, 2.80) | <0.001 |
| Ferritin (ng/mL) | 35.00 (28.00, 192.00) | 282.00 (102.00, 382.00) | <0.001 |
| sIL-2R (U/mL) | 684.00 (550.00, 1358.00) | 798.00 (654.50, 1033.50) | 0.751 |
| HNL > ML | Cut-Off | AUC |
| Body temperature (°C) | 37.8 | 0.9641 |
| Tenderness of LN (%) | 50 | 0.9231 |
| AST (U/L) | 23.3 | 0.7897 |
| LDH (U/L) | 292 | 0.7795 |
| Ferritin (ng/mL) | 53 | 0.8538 |
| BMG (mg/L) | 1.8 | 0.9538 |
| HNL < ML | Cut-Off | AUC |
| LN in size (cm) | 3.2 | 0.9641 |
| WBC (/μL) | 5600 | 0.9436 |
| Neutrophil (/μL) | 3554 | 0.8051 |
| Lymphocyte (/μL) | 2632.5 | 0.7897 |
| RBC | 4.965 | 0.741 |
| Plt (109/L) | 2445 | 0.8564 |
| Na (mEq/L) | 138.5 | 0.7923 |
| <2 | 2≤ | Total | |
|---|---|---|---|
| HNL | 0 | 15 | 15 |
| ML | 13 | 0 | 13 |
| Total | 13 | 15 | 28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omachi, T.; Atsumi, N.; Yamazoe, T.; Yamanouchi, S.; Matsuno, R.; Kitawaki, T.; Kaneko, K. Differential Diagnosis of Histiocytic Necrotizing Lymphadenitis and Malignant Lymphoma with Simple Clinical Findings. Children 2022, 9, 290. https://doi.org/10.3390/children9020290
Omachi T, Atsumi N, Yamazoe T, Yamanouchi S, Matsuno R, Kitawaki T, Kaneko K. Differential Diagnosis of Histiocytic Necrotizing Lymphadenitis and Malignant Lymphoma with Simple Clinical Findings. Children. 2022; 9(2):290. https://doi.org/10.3390/children9020290
Chicago/Turabian StyleOmachi, Taichi, Naho Atsumi, Takashi Yamazoe, Sohsaku Yamanouchi, Ryosuke Matsuno, Tomoki Kitawaki, and Kazunari Kaneko. 2022. "Differential Diagnosis of Histiocytic Necrotizing Lymphadenitis and Malignant Lymphoma with Simple Clinical Findings" Children 9, no. 2: 290. https://doi.org/10.3390/children9020290
APA StyleOmachi, T., Atsumi, N., Yamazoe, T., Yamanouchi, S., Matsuno, R., Kitawaki, T., & Kaneko, K. (2022). Differential Diagnosis of Histiocytic Necrotizing Lymphadenitis and Malignant Lymphoma with Simple Clinical Findings. Children, 9(2), 290. https://doi.org/10.3390/children9020290







