Understanding Foot Loading and Balance Behavior of Children with Motor Sensory Processing Disorder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
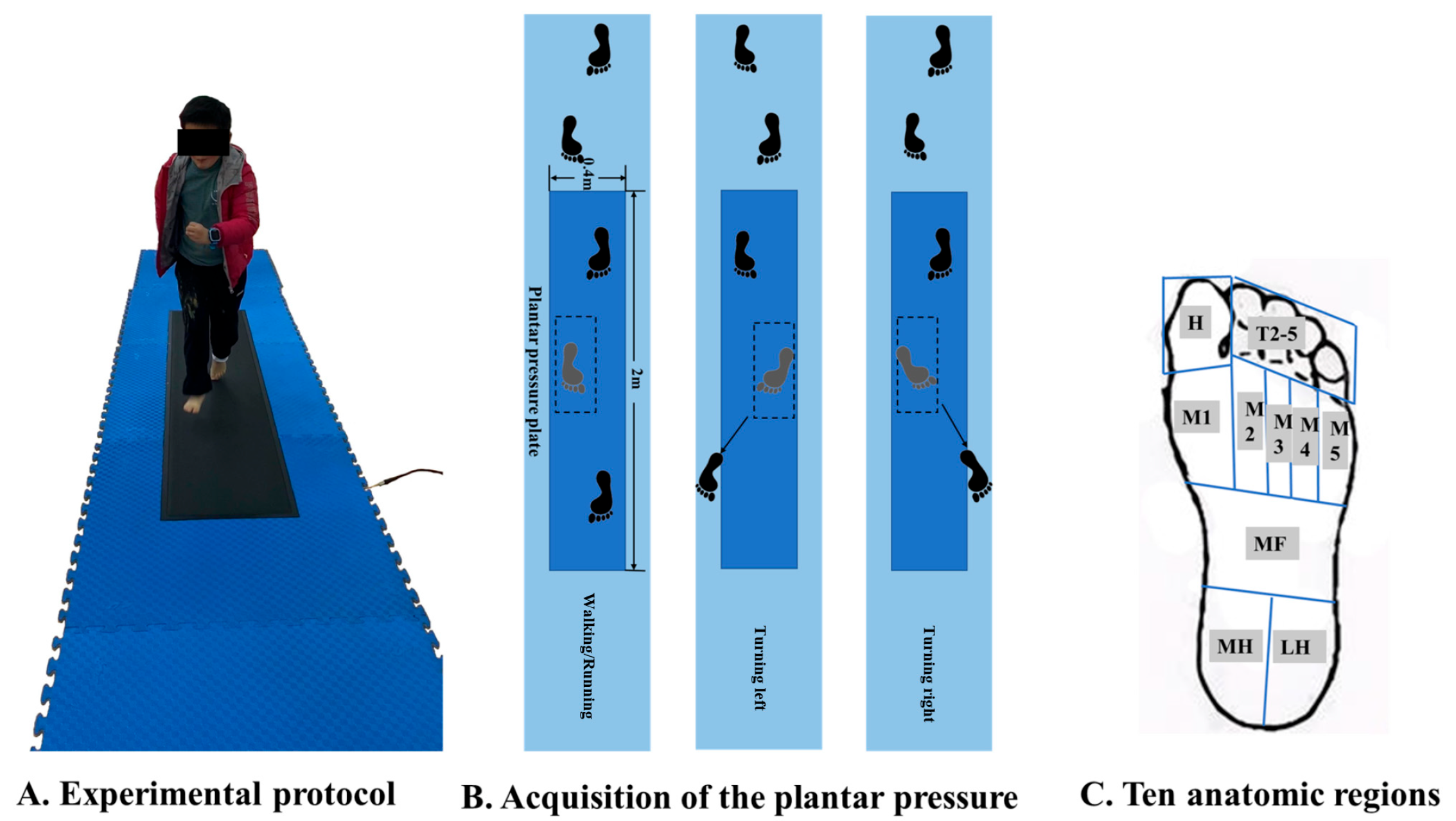
2.2. Test Protocol
2.3. Data Processing
2.4. Statistical Analysis
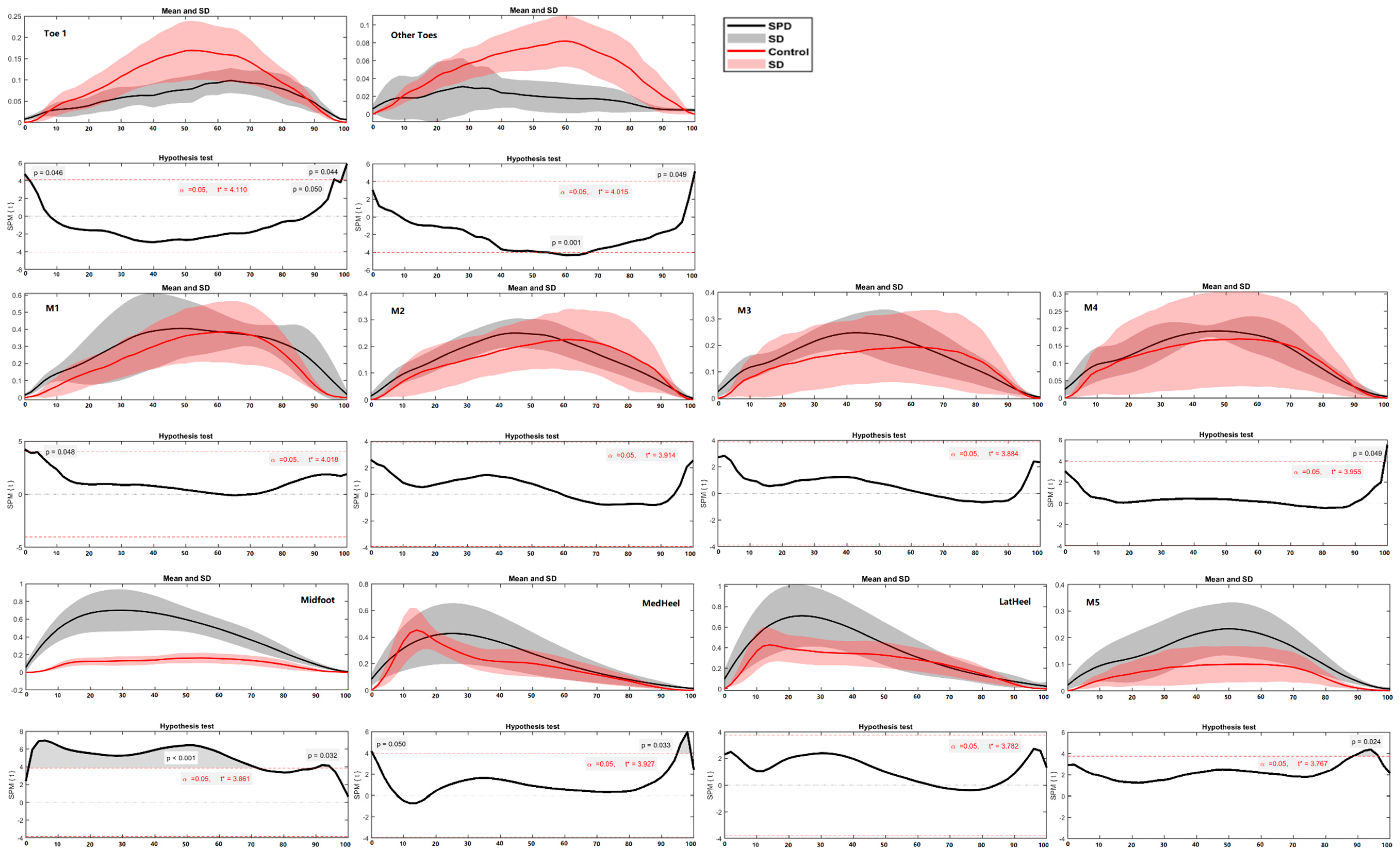
3. Results
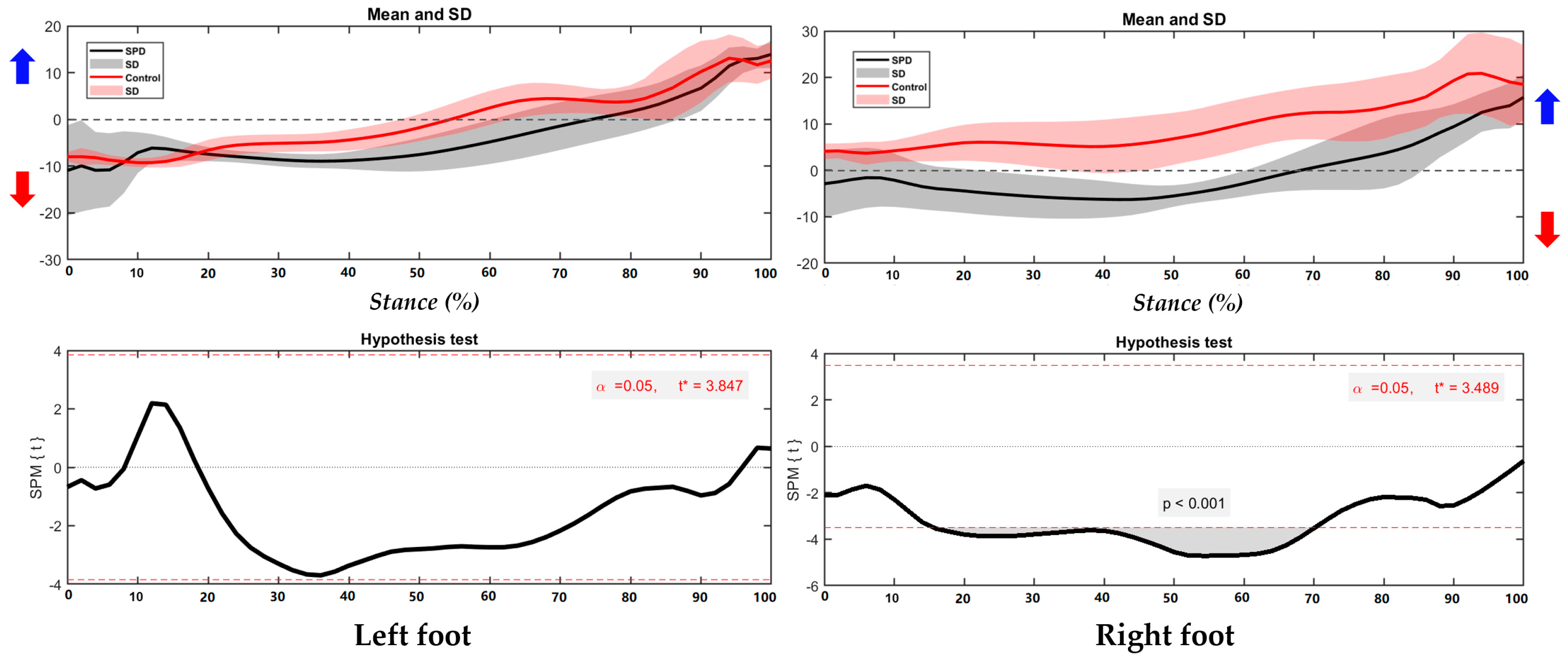
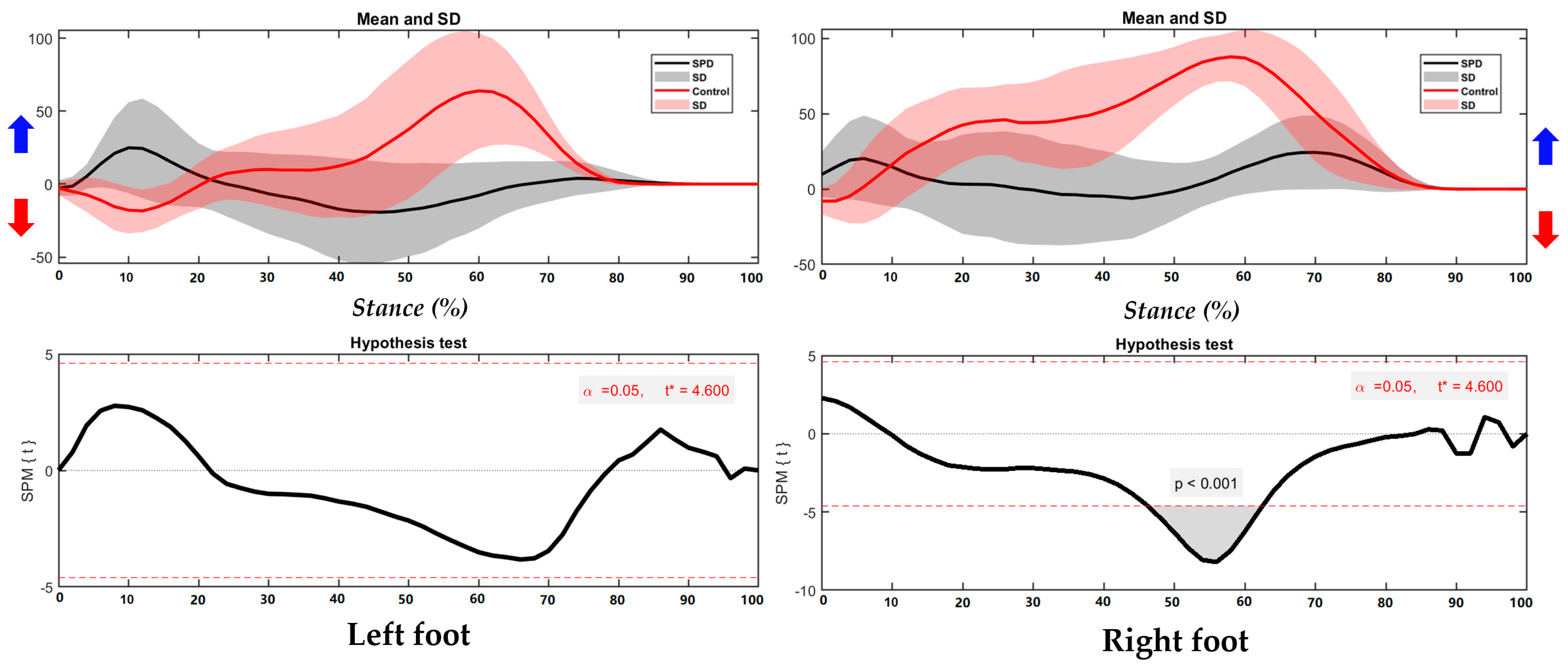
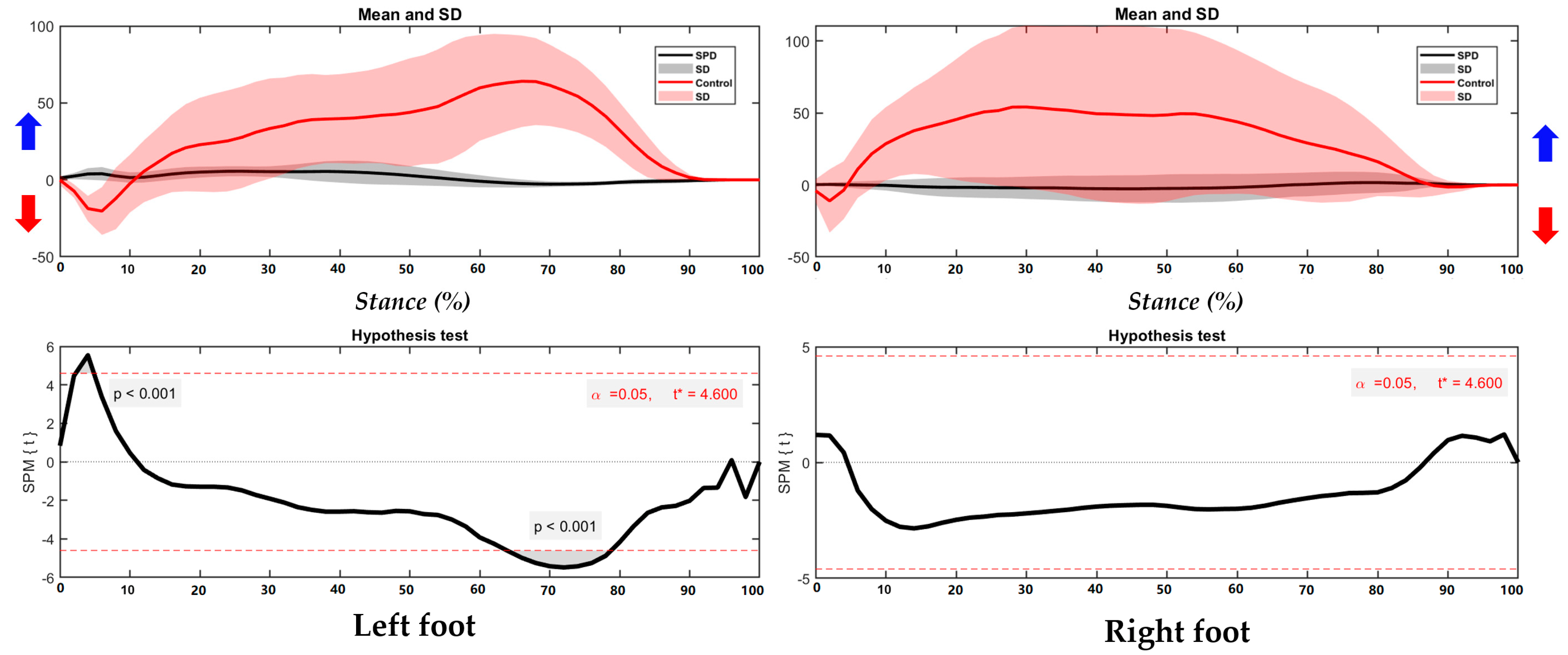
3.1. Center of Pressure Trajectory
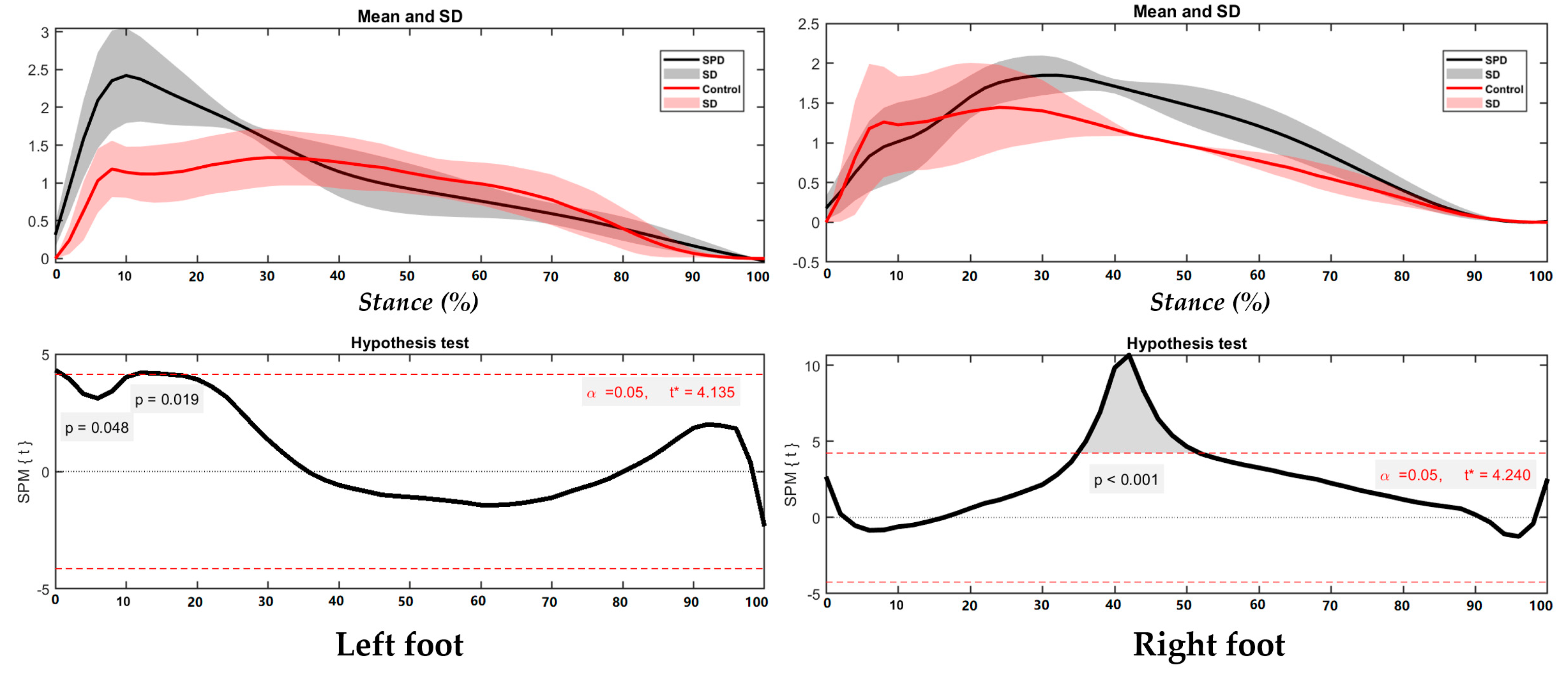
3.2. Foot Balance Index
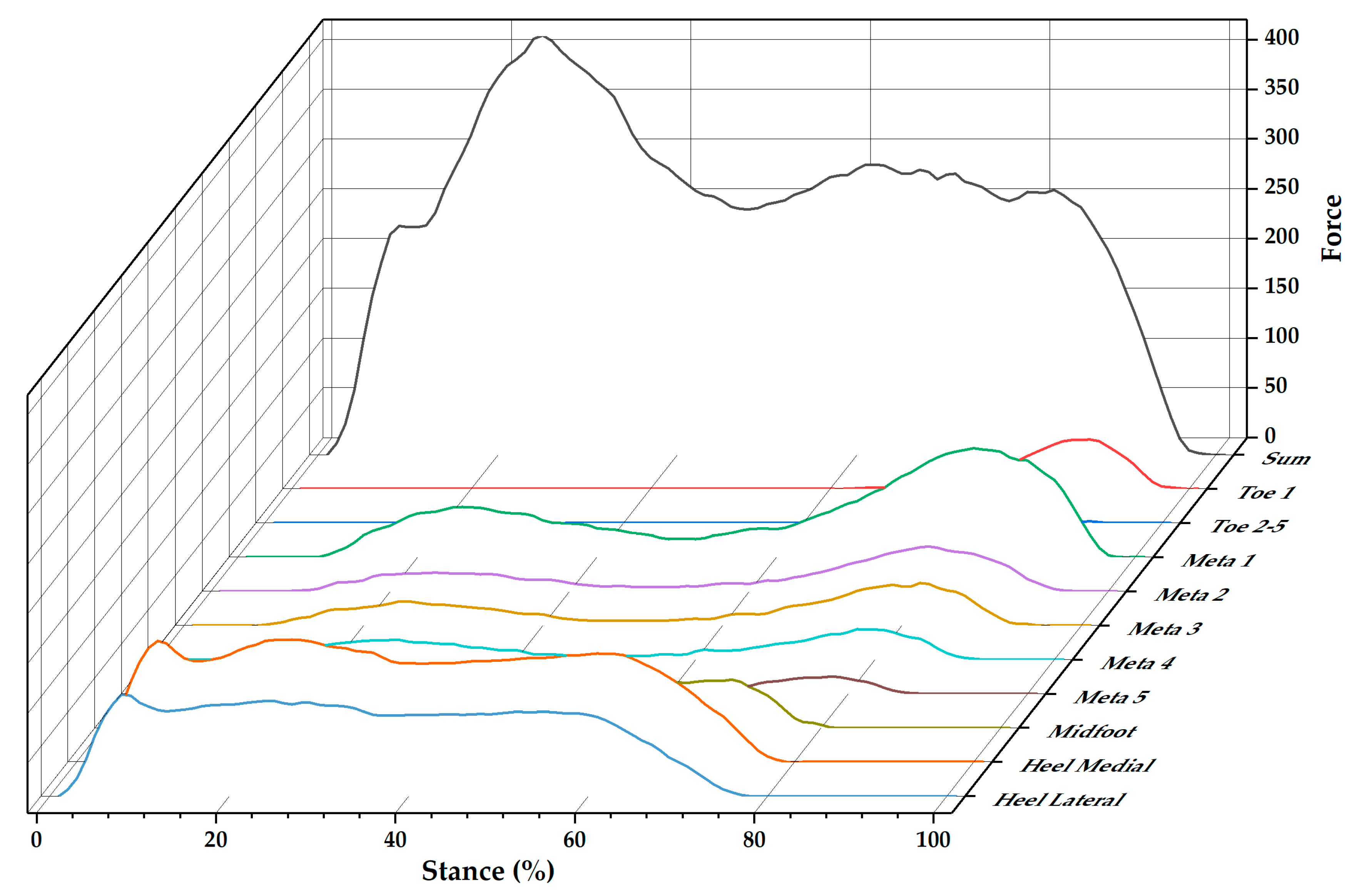
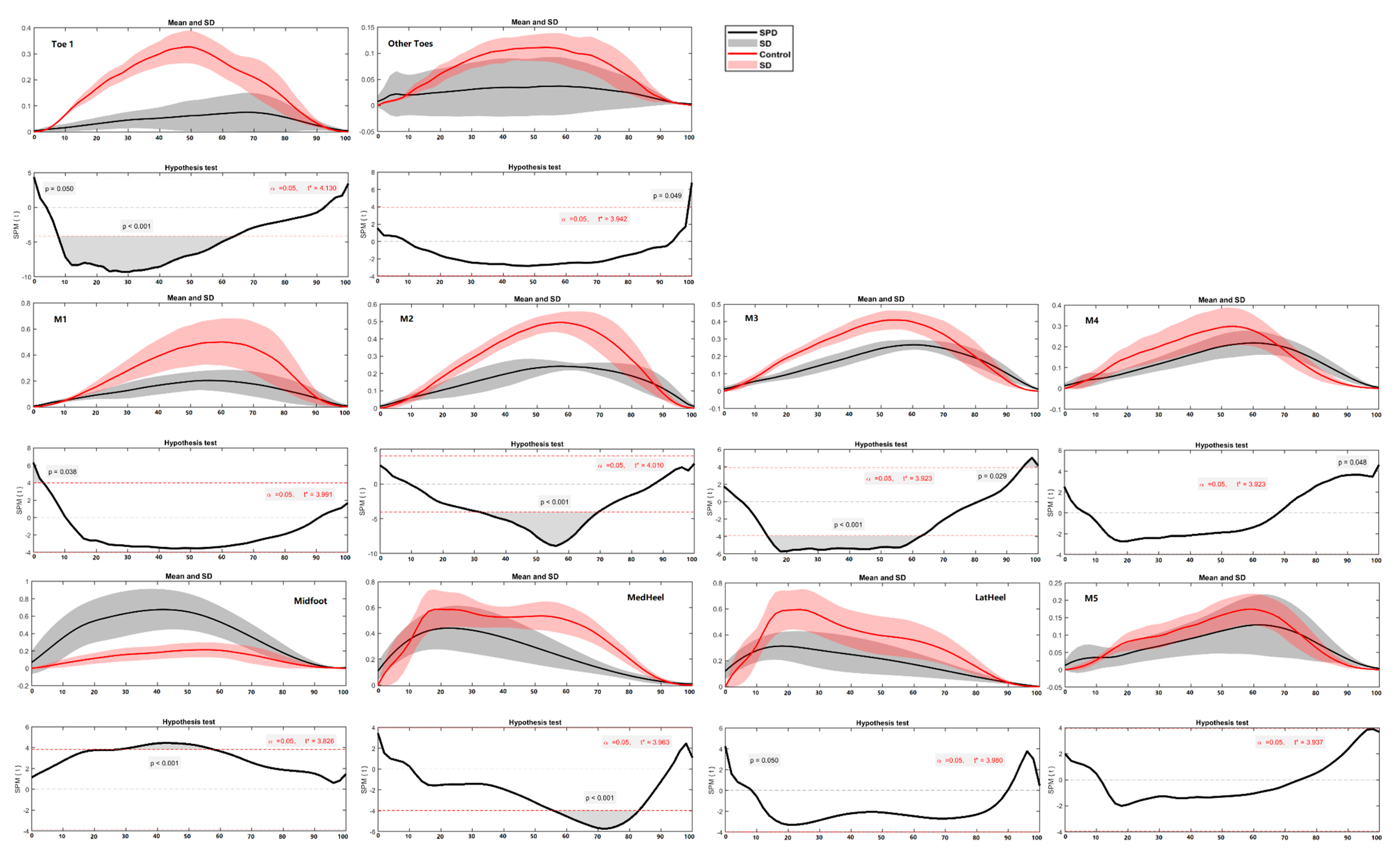
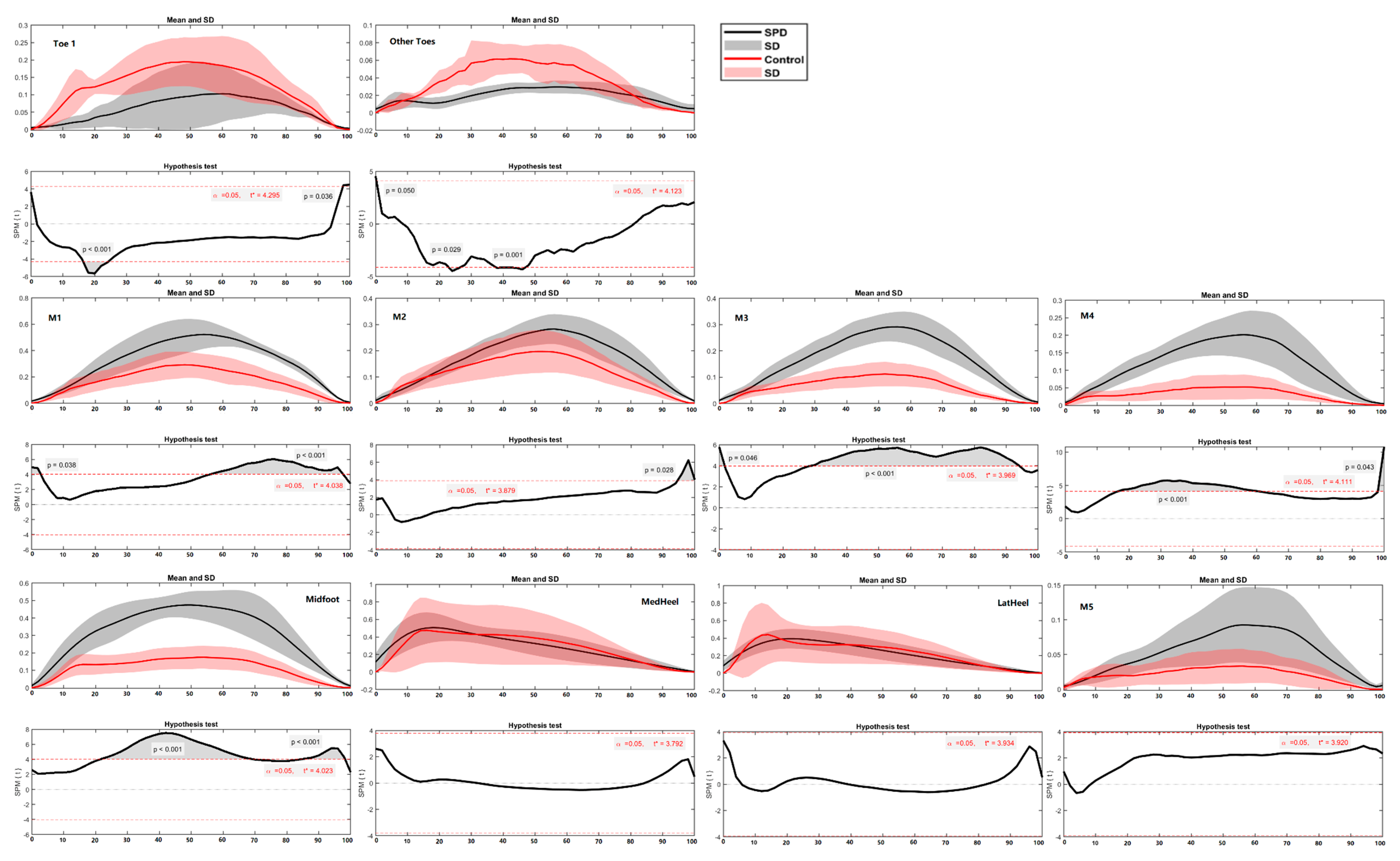
3.3. Ground Reaction Force and Regional Plantar Forces
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jussila, K.; Junttila, M.; Kielinen, M.; Ebeling, H.; Joskitt, L.; Moilanen, I.; Mattila, M.L. Sensory Abnormality and Quantitative Autism Traits in Children with and without Autism Spectrum Disorder in an Epidemiological Population. J. Autism. Dev. Disord. 2020, 50, 180–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gentil-Gutiérrez, A.; Cuesta-Gómez, J.L.; Rodríguez-Fernández, P.; González-Bernal, J.J. Implication of the sensory environment in children with autism spectrum disorder: Perspectives from school. Int. J. Environ. Res. Public Health 2021, 18, 7670. [Google Scholar] [CrossRef] [PubMed]
- Molholm, S.; Murphy, J.W.; Bates, J.; Ridgway, E.M.; Foxe, J.J. Multisensory Audiovisual Processing in Children With a Sensory Processing Disorder (I): Behavioral and Electrophysiological Indices Under Speeded Response Conditions. Front. Integr. Neurosci. 2020, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Foxe, J.J.; Bene, D.; Ross, V.A.; Ridgway, L.A.; Francisco, E.M.; Molholm, A.A. Multisensory Audiovisual Processing in Children With a Sensory Processing Disorder (II): Speech Integration Under Noisy Environmental Conditions. Front. Integr. Neurosci. 2020, 14, 39. [Google Scholar] [CrossRef] [PubMed]
- Crasta, J.E.; Salzinger, E.; Lin, M.H.; Gavin, W.J.; Davies, P.L. Sensory Processing and Attention Profiles Among Children With Sensory Processing Disorders and Autism Spectrum Disorders. Front. Integr. Neurosci. 2020, 14, 22. [Google Scholar] [CrossRef]
- Ghanizadeh, A. Sensory processing problems in children with ADHD, a systematic review. Psychiatry Investig. 2011, 8, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Ahn, R.R.; Miller, L.J.; Milberger, S.; McIntosh, D.N. Prevalence of parents’ perceptions of sensory processing disorders among kindergarten children. Am. J. Occup. Ther. 2004, 58, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Ben-Sasson, A.; Carter, A.S.; Briggs-Gowan, M.J. Sensory over-responsivity in elementary school: Prevalence and social-emotional correlates. J. Abnorm. Child. Psychol. 2009, 37, 705–716. [Google Scholar] [CrossRef]
- Niutanen, U.; Harra, T.; Lano, A.; Metsäranta, M. Systematic review of sensory processing in preterm children reveals abnormal sensory modulation, somatosensory processing and sensory-based motor processing. Acta Paediatr. Int. J. Paediatr. 2020, 109, 45–55. [Google Scholar] [CrossRef]
- Miller, L.J.; Nielsen, D.M.; Schoen, S.A.; Brett-Green, B.A. Perspectives on sensory processing disorder: A call for translational research. Front. Integr. Neurosci. 2009, 3, 22. [Google Scholar] [CrossRef] [Green Version]
- Suarez, H.; Alonso, R.; Arocena, S.; Ferreira, E.; Roman, C.S.; Suarez, A.; Lapilover, V. Sensorimotor interaction in deaf children. Relationship between gait performance and hearing input during childhood assessed in pre-lingual cochlear implant users. Acta Otolaryngol. 2017, 137, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, J.; Young, W. Agility literature review: Classifications, training and testing. J. Sports Sci. 2006, 24, 919–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dellal, A.; Keller, D.; Carling, C.; Chaouachi, A.; Wong, D.P.; Chamari, K. Physiologic effects of directional changes in intermittent exercise in soccer players. J. Strength Cond. Res. 2010, 24, 3219–3226. [Google Scholar] [CrossRef] [PubMed]
- Alexander, R.M. Energy-saving mechanisms in walking and running. J. Exp. Biol. 1991, 160, 55–69. [Google Scholar] [CrossRef]
- Cong, Y.; Lam, W.K.; Cheung, J.T.M.; Zhang, M. In-shoe plantar tri-axial stress profiles during maximum-effort cutting maneuvers. J. Biomech. 2014, 47, 3799–3806. [Google Scholar] [CrossRef]
- Glaister, B.C.; Orendurff, M.S.; Schoen, J.A.; Bernatz, G.C.; Klute, G.K. Ground reaction forces and impulses during a transient turning maneuver. J. Biomech. 2008, 41, 3090–3093. [Google Scholar] [CrossRef]
- Xu, D.; Carlton, L.G.; Rosengren, K.S. Anticipatory postural adjustments for altering direction during walking. J. Mot. Behav. 2004, 36, 316–326. [Google Scholar] [CrossRef]
- Xiang, L.; Mei, Q.; Fernandez, J.; Gu, Y. A biomechanical assessment of the acute hallux abduction manipulation intervention. Gait Posture 2020, 76, 210–217. [Google Scholar] [CrossRef]
- Gao, Z.; Mei, Q.; Xiang, L.; Gu, Y. Difference of walking plantar loadings in experienced and novice long-distance runners. Acta Bioeng. Biomech. 2020, 22, 127–147. [Google Scholar] [CrossRef]
- Mei, Q.; Gu, Y.; Fernandez, J. Alterations of Pregnant Gait during Pregnancy and Post-Partum. Sci. Rep. 2018, 8, 2217. [Google Scholar] [CrossRef] [Green Version]
- Mei, Q.; Feng, N.; Ren, X.J.X.; Lake, M.; Gu, Y.D.Y.Y.D. Foot Loading Patterns With Different Unstable Soles Structure. J. Mech. Med. Biol. 2015, 15, 1550014. [Google Scholar] [CrossRef]
- Hessert, M.J.; Vyas, M.; Leach, J.; Hu, K.; Lipsitz, L.A.; Novak, V. Foot pressure distribution during walking in young and old adults. BMC Geriatr. 2005, 5, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mei, Q.; Gu, Y.; Xiang, L.; Yu, P.; Gao, Z.; Shim, V.; Fernandez, J. Foot shape and plantar pressure relationships in shod and barefoot populations. Biomech. Model. Mechanobiol. 2020, 19, 1211–1224. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Mei, Q.C.; Gu, Y.D. Plantar Loading Reflects Ulceration Risks of Diabetic Foot with Toe Deformation. Biomed. Res. Int. 2015, 2015, 326493. [Google Scholar] [CrossRef] [PubMed]
- Hyun, G.J.; Jung, T.W.; Park, J.H.; Kang, K.D.; Kim, S.M.; Son, Y.D.; Cheong, J.H.; Kim, B.N.; Han, D.H. Changes in Gait Balance and Brain Connectivity in Response to Equine-Assisted Activity and Training in Children with Attention Deficit Hyperactivity Disorder. J. Altern. Complement. Med. 2016, 22, 286–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, B.O.; O’Sull Ivan, D.; Choi, B.G.; Kim, M.Y. Comparative gait analysis between children with autism and age-matched controls: Analysis with temporal-spatial and foot pressure variables. J. Phys. Ther. Sci. 2016, 28, 286–292. [Google Scholar] [CrossRef] [Green Version]
- Nsenga Leunkeu, A.; Lelard, T.; Shephard, R.J.; Doutrellot, P.L.; Ahmaidi, S. Gait cycle and plantar pressure distribution in children with cerebral palsy: Clinically useful outcome measures for a management and rehabilitation. NeuroRehabilitation 2014, 35, 657–663. [Google Scholar] [CrossRef]
- Chang, W.D.; Chang, N.J.; Lin, H.Y.; Lai, P.T. Changes of plantar pressure and gait parameters in children with mild cerebral palsy who used a customized external strap orthosis: A crossover study. Biomed Res. Int. 2015, 2015, 813942. [Google Scholar] [CrossRef] [Green Version]
- Provost, B.; Oetter, P. The Sensory Rating Scale for Infants and Young Children: Development and Reliabity. Phys. Occup. Ther. Pediatr. 1994, 13, 15–35. [Google Scholar] [CrossRef]
- Hilton, C.L. Sensory Processing and Motor Issues in Autism Spectrum Disorders. In International Handbook of Autism and Pervasive Developmental Disorders; Springer: New York, NY, USA, 2011; pp. 175–193. [Google Scholar] [CrossRef]
- Jorquera-Cabrera, S.; Romero-Ayuso, D.; Rodriguez-Gil, G.; Triviño-Juárez, J.M. Assessment of sensory processing characteristics in children between 3 and 11 years old: A systematic review. Front. Pediatr. 2017, 5, 57. [Google Scholar] [CrossRef] [Green Version]
- Chapman, J.P.; Chapman, L.J.; Allen, J.J. The measurement of foot preference. Neuropsychologia 1987, 25, 579–584. [Google Scholar] [CrossRef]
- Gao, Z.; Mei, Q.; Xiang, L.; Baker, J.S.; Fernandez, J.; Gu, Y. Effects of limb dominance on the symmetrical distribution of plantar loading during walking and running. Proc. Inst. Mech. Eng. Part P J. Sport. Eng. Technol. 2020, 236, 1754337120960962. [Google Scholar] [CrossRef]
- Mei, Q.; Xiang, L.; Li, J.; Fernandez, J.; Gu, Y. Analysis of Running Ground Reaction Forces Using the One-Dimensional Statistical Parametric Mapping (SPM1d). J. Med. Biomech. 2021, 36, 684–691. [Google Scholar] [CrossRef]
- Carpinella, I.; Crenna, P.; Calabrese, E.; Rabuffetti, M.; Mazzoleni, P.; Nemni, R.; Ferrarin, M. Locomotor function in the early stage of Parkinson’s disease. IEEE Trans. Neural Syst. Rehabil. Eng. 2007, 15, 543–551. [Google Scholar] [CrossRef]
- Willems, T.; Witvrouw, E.; Delbaere, K.; De Cock, A.; De Clercq, D. Relationship between gait biomechanics and inversion sprains: A prospective study of risk factors. Gait Posture 2005, 21, 379–387. [Google Scholar] [CrossRef]
- Gao, Z.; Mei, Q.; Fekete, G.; Baker, J.S.; Gu, Y. The Effect of Prolonged Running on the Symmetry of Biomechanical Variables of the Lower Limb Joints. Symmetry 2020, 12, 720. [Google Scholar] [CrossRef]
- Wen, J.; Ding, Q.; Yu, Z.; Sun, W.; Wang, Q.; Wei, K. Adaptive changes of foot pressure in hallux valgus patients. Gait Posture 2012, 36, 344–349. [Google Scholar] [CrossRef]
- Pataky, T.C. One-dimensional statistical parametric mapping in Python. Comput. Methods Biomech. Biomed. Eng. 2012, 15, 295–301. [Google Scholar] [CrossRef]
- Saxby, D.J.; Modenese, L.; Bryant, A.L.; Gerus, P.; Killen, B.; Fortin, K.; Wrigley, T.V.; Bennell, K.L.; Cicuttini, F.M.; Lloyd, D.G. Tibiofemoral contact forces during walking, running and sidestepping. Gait Posture 2016, 49, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, H.; Allard, P.; Prince, F.; Labelle, H. Symmetry and limb dominance in able-bodied gait: A review. Gait Posture 2000, 12, 34–45. [Google Scholar] [CrossRef]
- Mesquita, P.R.; Neri, S.G.R.; Lima, R.M.; Manfio, E.F.; De David, A.C. Running and walking foot loading in children aged 4–10 years. J. Appl. Biomech. 2019, 35, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Bosch, K.; Gerß, J.; Rosenbaum, D. Development of healthy children’s feet-Nine-year results of a longitudinal investigation of plantar loading patterns. Gait Posture 2010, 32, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, C.; De Vera, M.; Chhina, H.; Black, A. Normative data for the dynamic pedobarographic profiles of children. Gait Posture 2008, 28, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Mei, Q.; Fernandez, J.; Li, Z.; Feng, N.; Gu, Y. Foot Morphological Difference between Habitually Shod and Unshod Runners. PLoS ONE 2015, 10, e0131385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mei, Q.; Fernandez, J.; Fu, W.; Feng, N.; Gu, Y. A comparative biomechanical analysis of habitually unshod and shod runners based on a foot morphological difference. Hum. Mov. Sci. 2015, 42, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Mei, Q.; Gu, Y.; Fernandez, J. A biomechanical assessment of running with hallux unstable shoes of different material stiffness. Acta Bioeng. Biomech. 2019, 21, 121–128. [Google Scholar] [CrossRef]
- Lambrinudi, C. Use and Abuse of Toes. Postgrad. Med. J. 1932, 8, 459–464. [Google Scholar] [CrossRef] [Green Version]
- Nawata, K.; Nishihara, S.; Hayashi, I.; Teshima, R. Plantar pressure distribution during gait in athletes with functional instability of the ankle joint: Preliminary report. J. Orthop. Sci. 2005, 10, 298–301. [Google Scholar] [CrossRef]
- Chuckpaiwong, B.; Nunley, J.A.; Mall, N.A.; Queen, R.M. The effect of foot type on in-shoe plantar pressure during walking and running. Gait Posture 2008, 28, 405–411. [Google Scholar] [CrossRef]
- Koziol, L.F.; Budding, D.E.; Chidekel, D. Sensory integration, sensory processing, and sensory modulation disorders: Putative functional neuroanatomic underpinnings. Cerebellum 2011, 10, 770–792. [Google Scholar] [CrossRef]
- Nigg, B.; Behling, A.V.; Hamill, J. Foot pronation. Footwear Sci. 2019, 11, 131–134. [Google Scholar] [CrossRef]
- Orendurff, M.S.; Rohr, E.S.; Segal, A.D.; Medley, J.W.; Green, J.R.; Kadel, N.J. Regional foot pressure during running, cutting, jumping, and landing. Am. J. Sports Med. 2008, 36, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Best, A.N.; Wu, A.R. Upper body and ankle strategies compensate for reduced lateral stability at very slow walking speeds. Proc. R. Soc. B Biol. Sci. 2020, 287, 20201685. [Google Scholar] [CrossRef]
- Segal, A.; Rohr, E.; Orendurff, M.; Shofer, J.; O’Brien, M.; Sangeorzan, B. The effect of walking speed on peak plantar pressure. Foot Ankle Int. 2004, 25, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Havens, K.L.; Sigward, S.M. Cutting mechanics: Relation to performance and anterior cruciate ligament injury risk. Med. Sci. Sports Exerc. 2015, 47, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Mei, Q.; Fernandez, J.; Hume, P.; Gu, Y. Investigating biomechanical function of toes through external manipulation integrating analysis. Acta Bioeng. Biomech. 2016, 18, 87–92. [Google Scholar] [CrossRef]
- Lane, S.J.; Mailloux, Z.; Schoen, S.; Bundy, A.; May-Benson, T.A.; Parham, L.D.; Roley, S.S.; Schaaf, R.C. Neural foundations of ayres sensory integration. Brain Sci. 2019, 9, 153. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Yu, P.; Liu, W.; Gao, Z.; Sun, D.; Mei, Q.; Fernandez, J.; Gu, Y. Understanding Foot Loading and Balance Behavior of Children with Motor Sensory Processing Disorder. Children 2022, 9, 379. https://doi.org/10.3390/children9030379
Yu L, Yu P, Liu W, Gao Z, Sun D, Mei Q, Fernandez J, Gu Y. Understanding Foot Loading and Balance Behavior of Children with Motor Sensory Processing Disorder. Children. 2022; 9(3):379. https://doi.org/10.3390/children9030379
Chicago/Turabian StyleYu, Lin, Peimin Yu, Wei Liu, Zixiang Gao, Dong Sun, Qichang Mei, Justin Fernandez, and Yaodong Gu. 2022. "Understanding Foot Loading and Balance Behavior of Children with Motor Sensory Processing Disorder" Children 9, no. 3: 379. https://doi.org/10.3390/children9030379
APA StyleYu, L., Yu, P., Liu, W., Gao, Z., Sun, D., Mei, Q., Fernandez, J., & Gu, Y. (2022). Understanding Foot Loading and Balance Behavior of Children with Motor Sensory Processing Disorder. Children, 9(3), 379. https://doi.org/10.3390/children9030379








