Early Use of Sotrovimab in Children: A Case Report of an 11-Year-Old Kidney Transplant Recipient Infected with SARS-CoV-2
Abstract
:1. Introduction
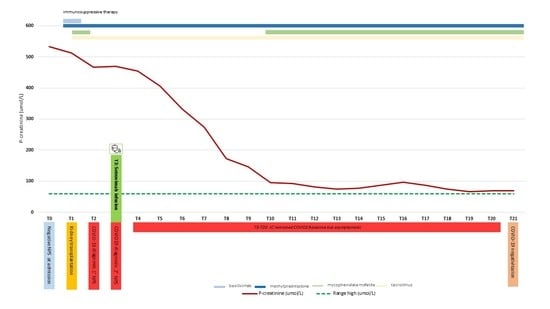
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khairallah, P.; Aggarwal, N.; Awan, A.A.; Vangala, C.; Airy, M.; Pan, J.S.; Murthy, B.V.; Winkelmayer, W.C.; Ramanathan, V. The impact of COVID-19 on kidney transplantation and the kidney transplant recipient—One year into the pandemic. Transpl. Int. 2021, 34, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Thaunat, O.; Legeai, C.; Anglicheau, D.; Couzi, L.; Blancho, G.; Hazzan, M.; Pastural, M.; Savoye, E.; Bayer, F.; Morelon, E.; et al. IMPact of the COVID-19 epidemic on the moRTAlity of kidney transplant recipients and candidates in a French Nationwide registry sTudy (IMPORTANT). Kidney Int. 2020, 98, 1568–1577. [Google Scholar] [CrossRef] [PubMed]
- Kremer, D.; Pieters, T.T.; Verhaar, M.C.; Berger, S.P.; Bakker, S.J.; van Zuilen, A.D.; Joles, J.A.; Vernooij, R.W.; van Balkom, B.W. A systematic review and meta-analysis of COVID-19 in kidney transplant recipients: Lessons to be learned. Am. J. Transpl. 2021, 21, 3936–3945. [Google Scholar] [CrossRef]
- Weinreich, D.M.; Sivapalasingam, S.; Norton, T.; Ali, S.; Gao, H.; Bhore, R.; Musser, B.J.; Soo, Y.; Rofail, D.; Im, J.; et al. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with COVID-19. N. Engl. J. Med. 2021, 384, 238–251. [Google Scholar] [CrossRef]
- Catalano, C.; Servais, S.; Bonvoisin, C.; Couturier, B.; Hildebrand, M.; Etienne, I.; Meuris, C.; Goffard, J.C.; Wissing, M.; Goldman, M.; et al. Preemptive Antibody Therapy for Vaccine Breakthrough SARS-CoV-2 Infection in Immunocompromised Patients. Transplantation 2021, 105, e282. [Google Scholar] [CrossRef]
- Del Bello, A.; Marion, O.; Vellas, C.; Faguer, S.; Izopet, J.; Kamar, N. Anti-SARS-CoV-2 Monoclonal Antibodies in Solid-organ Transplant Patients. Transplantation 2021, 105, e146–e147. [Google Scholar] [CrossRef]
- Cravedi, P.; Mothi, S.S.; Azzi, Y.; Haverly, M.; Farouk, S.S.; Pérez-Sáez, M.J.; Redondo-Pachón, M.D.; Murphy, B.; Florman, S.; Cyrino, L.G.; et al. COVID-19 and kidney transplantation: Results from the TANGO International Transplant Consortium. Am. J. Transpl. 2020, 20, 3140–3148. [Google Scholar] [CrossRef]
- Aleem, A.; Slenker, A.K. Monoclonal Antibody Therapy for High-Risk Coronavirus (COVID 19) Patients with Mild to Moderate Disease Presentations. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Malik, B.; Kalantary, A.; Rikabi, K.; Abdelazeem, B.; Kunadi, A. Outpatient Management of COVID-19 With Monoclonal Antibody Therapy in a Young Renal Transplant Patient. Cureus 2021, 13, e17672. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Fact Sheet for Health Care Providers; Emergency Use Authorization (EUA) of Casirivimab and Imdevimab. Available online: https://www.fda.gov/media/145611/download (accessed on 30 July 2021).
- Food and Drug Administration. Fact Sheet for Healthcare Providers—Emergency Use Authorization of Sotrovimab. Available online: https://www.fda.gov/media/149534/download (accessed on 12 July 2021).
- Food and Drug Administration. Fact Sheet for Health Care Providers; Emergency Use Authorization of Bamlanivimab and Etesevimab. Available online: https://www.fda.gov/media/145802/download (accessed on 3 December 2021).
- COVID-19: EMA Recommends Authorisation of Two Monoclonal Antibody Medicines. Available online: https://www.ema.europa.eu/en/news/covid-19-ema-recommends-authorisation-two-monoclonal-antibody-medicines (accessed on 11 November 2021).
- COVID-19: EMA Recommends Authorisation of Antibody Medicine Xevudy. Available online: www.ema.europa.eu/en/news/covid-19-ema-recommends-authorisation-antibody-medicine-xevudy (accessed on 16 December 2021).
- Takashita, E.; Kinoshita, N.; Yamayoshi, S.; Sakai-Tagawa, Y.; Fujisaki, S.; Ito, M.; Iwatsuki-Horimoto, K.; Chiba, S.; Halfmann, P.; Nagai, H.; et al. Efficacy of Antibodies and Antiviral Drugs against COVID-19 Omicron Variant. N. Engl. J. Med. 2022, 386, 995–998. [Google Scholar] [CrossRef]
- Ding, C.; He, J.; Zhang, X.; Jiang, C.; Sun, Y.; Zhang, Y.; Chen, Q.; He, H.; Li, W.; Xie, J.; et al. Crucial Mutations of Spike Protein on SARS-CoV-2 Evolved to Variant Strains Escaping Neutralization of Convalescent Plasmas and RBD-Specific Monoclonal Antibodies. Front. Immunol. 2021, 12, 693775. [Google Scholar] [CrossRef]
- Gupta, A.; Gonzalez-Rojas, Y.; Juarez, E.; Crespo Casal, M.; Moya, J.; Falci, D.R.; Sarkis, E.; Solis, J.; Zheng, H.; Scott, N.; et al. Early Treatment for COVID-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. N. Engl. J. Med. 2021, 385, 1941–1950. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Mo, X.; Hu, Y.; Qi, X.; Jiang, F.; Jiang, Z.; Tong, S. Epidemiology of COVID-19 Among Children in China. Pediatrics 2020, 145, e20200702. [Google Scholar] [CrossRef] [Green Version]
- Graff, K.; Smith, C.; Silveira, L.; Jung, S.; Curran-Hays, S.; Jarjour, J.; Carpenter, L.; Pickard, K.; Mattiucci, M.; Fresia, J.; et al. Risk Factors for Severe COVID-19 in Children. Pediatr. Infect. Dis. J. 2021, 40, e137–e145. [Google Scholar] [CrossRef] [PubMed]
- Tsankov, B.K.; Allaire, J.M.; Irvine, M.A.; Lopez, A.A.; Sauvé, L.J.; Vallance, B.A.; Jacobson, K. Severe COVID-19 Infection and Pediatric Comorbidities: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2021, 103, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Lanari, M.; Venturini, E.; Pierantoni, L.; Stera, G.; Gattinara, G.C.; Esposito, S.M.R.; Favilli, S.; Franzoni, E.; Fusco, E.; Lionetti, P.; et al. Eligibility criteria for pediatric patients who may benefit from anti SARS-CoV-2 monoclonal antibody therapy administration: An Italian inter-society consensus statement. Ital. J. Pediatr. 2022, 48, 7. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.J.; Mark, P.B.; Patel, R.K.; Stevens, K.K.; Palmer, N. Renal association clinical practice guideline in post-operative care in the kidney transplant recipient. BMC Nephrol. 2017, 18, 174. [Google Scholar] [CrossRef] [Green Version]
- Akalin, E.; Azzi, Y.; Bartash, R.; Seethamraju, H.; Parides, M.; Hemmige, V.; Ross, M.; Forest, S.; Goldstein, Y.D.; Ajaimy, M.; et al. COVID-19 and Kidney Transplantation. N. Engl. J. Med. 2020, 382, 2475–2477. [Google Scholar] [CrossRef]
- Bansal, N.; Ovchinsky, N.; Foca, M.; Lamour, J.M.; Kogan-Liberman, D.; Hsu, D.T.; Beddows, K.; Abraham, L.; Coburn, M.; Cunningham, R.; et al. COVID-19 infection in pediatric solid organ transplant patients. Pediatr. Transpl. 2022, 26, e14156. [Google Scholar] [CrossRef]
- Dhand, A.; Lobo, S.A.; Wolfe, K.; Feola, N.; Lee, L.; Nog, R.; Chen, D.; Glicklich, D.; Diflo, T.; Nabors, C. Casirivimab-imdevimab for Treatment of COVID-19 in Solid Organ Transplant Recipients: An Early Experience. Transplantation 2021, 105, e68–e69. [Google Scholar] [CrossRef]
- Verderese, J.P.; Stepanova, M.; Lam, B.; Racila, A.; Kolacevski, A.; Allen, D.; Hodson, E.; Aslani-Amoli, B.; Homeyer, M.; Stanmyre, S.; et al. Neutralizing Monoclonal Antibody Treatment Reduces Hospitalization for Mild and Moderate COVID-19: A Real-World Experience. Clin. Infect. Dis. 2021, ciab579. [Google Scholar] [CrossRef] [PubMed]
- Venturini, E.; Montagnani, C.; Garazzino, S.; Donà, D.; Pierantoni, L.; Vecchio, A.L.; Krzysztofiak, A.; Nicolini, G.; Bianchini, S.; Galli, L.; et al. Treatment of children with COVID-19: Update of the Italian Society of Pediatric Infectious Diseases position paper. Ital. J. Pediatr. 2021, 47, 199. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Veklury: EPAR—Medicine Overview. Available online: https://www.ema.europa.eu/en/documents/overview/veklury-epar-medicine-overview_en.pdf (accessed on 18 March 2022).
- Elias, G.P.; Antoniali, C.; Mariano, R.C. Comparative study of rules employed for calculation of pediatric drug dosage. J. Appl. Oral Sci. 2005, 13, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Temrikar, Z.H.; Suryawanshi, S.; Meibohm, B. Pharmacokinetics and Clinical Pharmacology of Monoclonal Antibodies in Pediatric Patients. Paediatr. Drugs. 2020, 22, 199–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.I.; Dallmann, A.; Wang, Y.M.; Green, D.J.; Burnham, J.M.; Chiang, B.; Wu, P.; Sheng, M.; Lu, K.; van den Anker, J.N.; et al. Monoclonal Antibodies and Fc-Fusion Proteins for Pediatric Use: Dosing, Immunogenicity, and Modeling and Simulation in Data Submitted to the US Food and Drug Administration. J. Clin. Pharmacol. 2019, 59, 1130–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munzenberger, P.J.; McKercher, P. Pediatric dosing—The pharmacist’s dilemma. Contemp. Pharm. Pract. 1980, 3, 11–14. [Google Scholar] [PubMed]
- Delgado, B.J.; Safadi, A.O.; Bajaj, T. Clark’s Rule. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541104/ (accessed on 8 February 2022).
- Garegnani, L.; Styrmisdóttir, L.; Roson Rodriguez, P.; Escobar Liquitay, C.M.; Esteban, I.; Franco, J.V. Palivizumab for preventing severe respiratory syncytial virus (RSV) infection in children. Cochrane Database Syst. Rev. 2021, 11, CD013757. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Chiara, C.; Mengato, D.; De Pieri, M.; Longo, G.; Benetti, E.; Venturini, F.; Giaquinto, C.; Donà, D. Early Use of Sotrovimab in Children: A Case Report of an 11-Year-Old Kidney Transplant Recipient Infected with SARS-CoV-2. Children 2022, 9, 451. https://doi.org/10.3390/children9040451
Di Chiara C, Mengato D, De Pieri M, Longo G, Benetti E, Venturini F, Giaquinto C, Donà D. Early Use of Sotrovimab in Children: A Case Report of an 11-Year-Old Kidney Transplant Recipient Infected with SARS-CoV-2. Children. 2022; 9(4):451. https://doi.org/10.3390/children9040451
Chicago/Turabian StyleDi Chiara, Costanza, Daniele Mengato, Marica De Pieri, Germana Longo, Elisa Benetti, Francesca Venturini, Carlo Giaquinto, and Daniele Donà. 2022. "Early Use of Sotrovimab in Children: A Case Report of an 11-Year-Old Kidney Transplant Recipient Infected with SARS-CoV-2" Children 9, no. 4: 451. https://doi.org/10.3390/children9040451
APA StyleDi Chiara, C., Mengato, D., De Pieri, M., Longo, G., Benetti, E., Venturini, F., Giaquinto, C., & Donà, D. (2022). Early Use of Sotrovimab in Children: A Case Report of an 11-Year-Old Kidney Transplant Recipient Infected with SARS-CoV-2. Children, 9(4), 451. https://doi.org/10.3390/children9040451