House Dust Mite Subcutaneous Immunotherapy and Lung Function Trajectory in Children and Adolescents with Asthma
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Study Population
2.2. Lung Function
2.3. Statistical Analysis
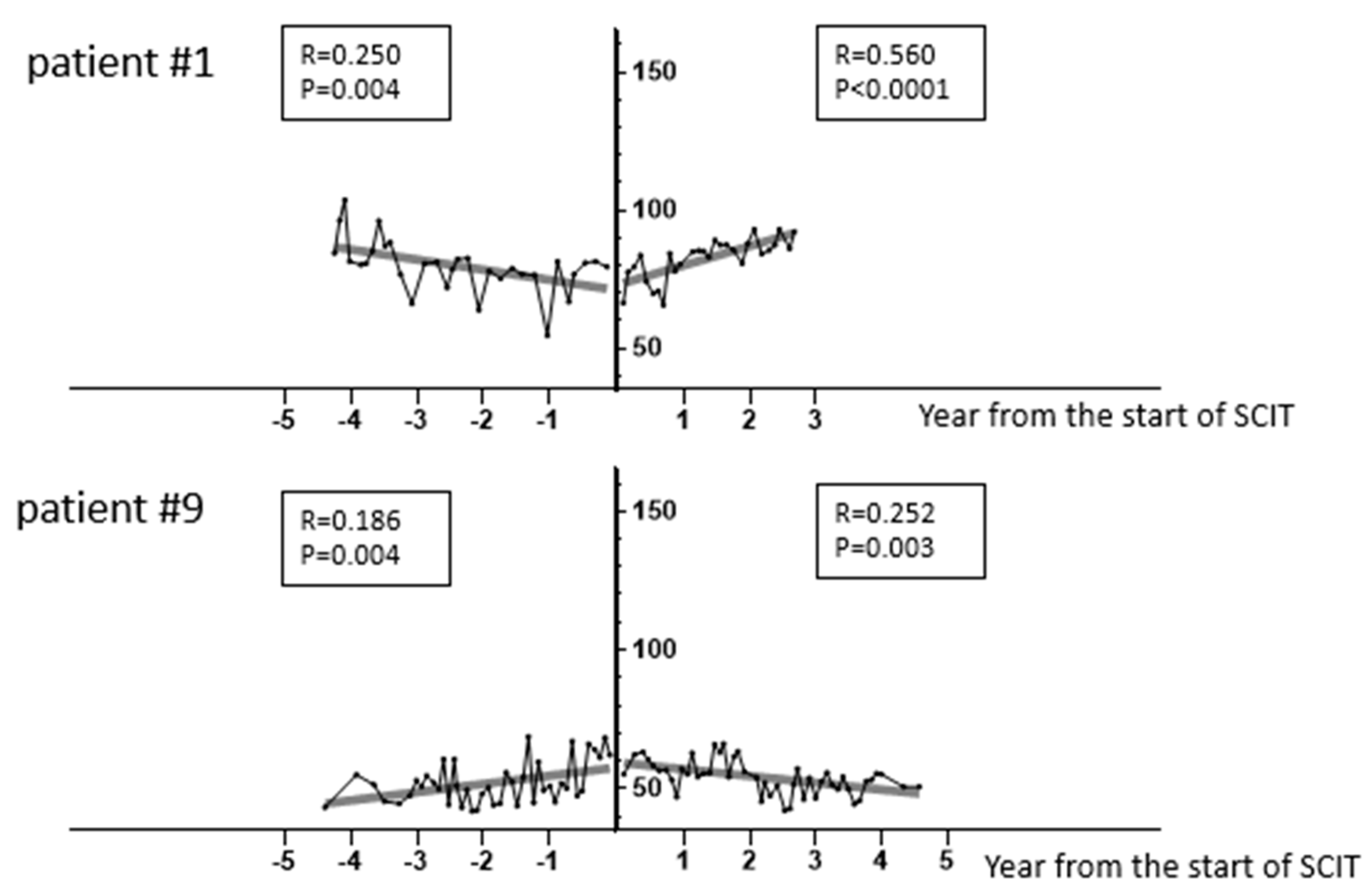
3. Results
3.1. Subjects
3.2. Clinical Characteristics
3.3. Factors Associated with a Favorable Lung Function Trajectory with HDM-SCIT
3.4. Clinical Outcome of the Subjects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Platts-Mills, T.A.; Ward, G.W., Jr.; Sporik, R.; Gelber, L.E.; Chapman, M.D.; Heymann, P.W. Epidemiology of the relationship between exposure to indoor allergens and asthma. Int. Arch. Allergy Appl. Immunol. 1991, 94, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Holt, P.G.; Rowe, J.; Kusel, M.; Parsons, F.; Hollams, E.M.; Bosco, A.; McKenna, K.; Subrata, L.; de Klerk, N.; Serralha, M.; et al. Toward improved prediction of risk for atopy and asthma among preschoolers: A prospective cohort study. J. Allergy Clin. Immunol. 2010, 125, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Kucuksezer, U.C.; Ozdemir, C.; Cevhertas, L.; Ogulur, I.; Akdis, M.; Akdis, C.A. Mechanisms of allergen-specific immunotherapy and allergen tolerance. Allergol. Int. 2020, 69, 549–560. [Google Scholar] [CrossRef]
- Agache, I.; Lau, S.; Akdis, C.A.; Smolinska, S.; Bonini, M.; Cavkaytar, O.; Flood, B.; Gajdanowicz, P.; Izuhara, K.; Kalayci, O.; et al. EAACI guidelines on allergen immunotherapy: House dust mite-driven allergic asthma. Allergy 2019, 74, 855–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rice, J.L.; Diette, G.B.; Suarez-Cuervo, C.; Brigham, E.P.; Lin, S.Y.; Ramanathan, M., Jr.; Robinson, K.A.; Azar, A. Allergen-specific immunotherapy in the treatment of pediatric asthma: A systematic review. Pediatrics 2018, 141, e20173833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virchow, J.C. Allergen immunotherapy (AIT) in asthma. Semin. Immunol. 2019, 46, 101334. [Google Scholar] [CrossRef] [PubMed]
- Coordinating, C.; Cloutier, M.M.; Baptist, A.P.; Blake, K.V.; Brooks, E.G.; Bryant-Stephens, T.; DiMango, E.; Dixon, A.E.; Elward, K.S.; Hartert, T.; et al. 2020 Focused Updates to the Asthma Management Guidelines: A Report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J. Allergy Clin. Immunol. 2020, 146, 1217–1270. [Google Scholar]
- Dhami, S.; Kakourou, A.; Asamoah, F.; Agache, I.; Lau, S.; Jutel, M.; Muraro, A.; Roberts, G.; Akdis, C.A.; Bonini, M.; et al. Allergen immunotherapy for allergic asthma: A systematic review and meta-analysis. Allergy 2017, 72, 1825–1848. [Google Scholar] [CrossRef]
- Unal, D. Effects of perennial allergen immunotherapy in allergic rhinitis in patients with/without asthma: A-randomized controlled real-life study. Int. Arch. Allergy Immunol. 2020, 181, 141–148. [Google Scholar] [CrossRef]
- McGeachie, M.J.; Yates, K.P.; Zhou, X.; Guo, F.; Sternberg, A.L.; Van Natta, M.L.; Wise, R.A.; Szefler, S.J.; Sharma, S.; Kho, A.T.; et al. Patterns of growth and decline in lung function in persistent childhood asthma. N. Engl. J. Med. 2016, 374, 1842–1852. [Google Scholar] [CrossRef]
- Belgrave, D.C.M.; Granell, R.; Turner, S.W.; Curtin, J.A.; Buchan, I.E.; Le Souef, P.N.; Simpson, A.; Henderson, A.J.; Custovic, A. Lung function trajectories from pre-school age to adulthood and their associations with early life factors: A retrospective analysis of three population-based birth cohort studies. Lancet Respir. Med. 2018, 6, 526–534. [Google Scholar] [CrossRef] [Green Version]
- Bui, D.S.; Lodge, C.J.; Burgess, J.A.; Lowe, A.J.; Perret, J.; Bui, M.Q.; Bowatte, G.; Gurrin, L.; Johns, D.P.; Thompson, B.R.; et al. Childhood predictors of lung function trajectories and future COPD risk: A prospective cohort study from the first to the sixth decade of life. Lancet Respir. Med. 2018, 6, 535–544. [Google Scholar] [CrossRef]
- Bui, D.S.; Lodge, C.J.; Perret, J.L.; Lowe, A.; Hamilton, G.S.; Thompson, B.; Giles, G.; Tan, D.; Erbas, B.; Pirkis, J.; et al. Trajectories of asthma and allergies from 7 years to 53 years and associations with lung function and extrapulmonary comorbidity profiles: A prospective cohort study. Lancet Respir. Med. 2020, 32, 465–478. [Google Scholar] [CrossRef]
- O’Byrne, P.M.; Pedersen, S.; Busse, W.W.; Tan, W.C.; Chen, Y.Z.; Ohlsson, S.V.; Ullman, A.; Lamm, C.J.; Pauwels, R.A. Effects of early intervention with inhaled budesonide on lung function in newly diagnosed asthma. Chest 2006, 129, 1478–1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busse, W.W.; Pedersen, S.; Pauwels, R.A.; Tan, W.C.; Chen, Y.Z.; Lamm, C.J.; O’Byrne, P.M. The inhaled steroid treatment as regular therapy in early asthma (START) study 5-year follow-up: Effectiveness of early intervention with budesonide in mild persistent asthma. J. Allergy Clin. Immunol. 2008, 121, 1167–1174. [Google Scholar] [CrossRef] [Green Version]
- O’Byrne, P.M.; Pedersen, S.; Lamm, C.J.; Tan, W.C.; Busse, W.W. Severe exacerbations and decline in lung function in asthma. Am. J. Respir. Crit. Care Med. 2009, 179, 19–24. [Google Scholar] [CrossRef]
- Childhood Asthma Management Program Research Group; Szefler, S.; Weiss, S.; Tonascia, J.; Adkinson, N.F.; Bender, B.; Cherniack, R.; Donithan, M.; Kelly, H.W.; Reisman, J.; et al. Long-term effects of budesonide or nedocromil in children with asthma. N. Engl. J. Med. 2000, 343, 1054–1063. [Google Scholar]
- Busse, W.W.; Szefler, S.J.; Haselkorn, T.; Iqbal, A.; Ortiz, B.; Lanier, B.Q.; Chipps, B.E. Possible protective effect of omalizumab on lung function decline in patients experiencing asthma exacerbations. J. Allergy Clin. Immunol. Pract. 2021, 9, 1201–1211. [Google Scholar] [CrossRef]
- Hoshi, M.; Matsunaga, M.; Nogami, K.; Hamada, K.; Kobori, T.; Kainuma, K.; Nagao, M.; Fujisawa, T. Three cases of severe adolescent asthma treated with mepolizumab: Lung function trajectories. Asia Pac. Allergy 2020, 10, e13. [Google Scholar] [CrossRef]
- Fujisawa, T.; Shimoda, T.; Masuyama, K.; Okubo, K.; Honda, K.; Okano, M.; Katsunuma, T.; Urisu, A.; Kondo, Y.; Odajima, H.; et al. Long-term safety of subcutaneous immunotherapy with TO-204 in Japanese patients with house dust mite-induced allergic rhinitis and allergic bronchial asthma: Multicenter, open label clinical trial. Allergol. Int. 2018, 67, 347–356. [Google Scholar] [CrossRef]
- Takase, M.; Sakata, H.; Shikada, M.; Tatara, K.; Fukushima, T.; Miyakawa, T. Development of reference equations for spirometry in Japanese children aged 6-18 years. Pediatr. Pulmonol. 2013, 48, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motulsky, H.J.; Brown, R.E. Detecting outliers when fitting data with nonlinear regression—A new method based on robust nonlinear regression and the false discovery rate. BMC Bioinform. 2006, 7, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, D.; Agusti, A.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Criner, G.J.; Frith, P.; Halpin, D.M.G.; Han, M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: The GOLD science committee report 2019. Eur. Respir. J. 2019, 53, 1900164. [Google Scholar] [CrossRef]
- Arakawa, H.; Adachi, Y.; Ebisawa, M.; Fujisawa, T. Committee for Japanese Pediatric Guideline for Childhood A, Japanese Society of Pediatric A, Clinical I, Japanese Society of A. Japanese guidelines for childhood asthma 2020. Allergol. Int. 2020, 69, 314–330. [Google Scholar] [CrossRef]
- Nagata, M.; Nakagome, K.; Soma, T. Mechanisms of eosinophilic inflammation. Asia Pac. Allergy 2020, 10, e14. [Google Scholar] [CrossRef]
- Hartl, S.; Breyer, M.K.; Burghuber, O.C.; Ofenheimer, A.; Schrott, A.; Urban, M.H.; Agusti, A.; Studnicka, M.; Wouters, E.F.M.; Breyer-Kohansal, R. Blood eosinophil count in the general population: Typical values and potential confounders. Eur. Respir. J. 2020, 55, 1901874. [Google Scholar] [CrossRef]
- Price, D.B.; Rigazio, A.; Campbell, J.D.; Bleecker, E.R.; Corrigan, C.J.; Thomas, M.; Wenzel, S.E.; Wilson, A.M.; Small, M.B.; Gopalan, G.; et al. Blood eosinophil count and prospective annual asthma disease burden: A UK cohort study. Lancet Respir. Med. 2015, 3, 849–858. [Google Scholar] [CrossRef]
- Mallah, N.; Rodriguez-Segade, S.; Gonzalez-Barcala, F.J.; Takkouche, B. Blood eosinophil count as predictor of asthma exacerbation. A meta-analysis. Pediatr. Allergy Immunol. 2020, 32, 465–478. [Google Scholar] [CrossRef]
- Motomura, C.; Odajima, H.; Yamada, A.; Taba, N.; Murakami, Y.; Nishima, S. Pale nasal mucosa affects airflow limitations in upper and lower airways in asthmatic children. Asia Pac. Allergy 2016, 6, 220–225. [Google Scholar] [CrossRef] [Green Version]
- Mogensen, I.; Vonk, J.M.; Wijnant, S.R.A.; Zhou, X.; Boezen, H.M.; Brusselle, G.; Lahousse, L.; Janson, C.; Malinovschi, A. Blood eosinophil level and lung function trajectories: Cross-sectional and longitudinal studies in European cohorts. ERJ Open Res. 2020, 6. [Google Scholar] [CrossRef]
- Backman, H.; Lindberg, A.; Hedman, L.; Stridsman, C.; Jansson, S.A.; Sandstrom, T.; Lundback, B.; Ronmark, E. FEV1 decline in relation to blood eosinophils and neutrophils in a population-based asthma cohort. World Allergy Organ. J. 2020, 13, 100110. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Qin, R.; Hu, Q.; Zhu, Z.; Liu, Y.; Luo, T.; Li, J. Effect of Dermatophagoides pteronyssinus Immunotherapy on Upper and Lower Airway Eosinophilic Inflammatory Response to Nasal Allergen Challenge. Allergy Asthma Immunol. Res. 2020, 12, 844–858. [Google Scholar] [CrossRef] [PubMed]
- Lovinsky-Desir, S. The use of biologic therapies for the management of pediatric asthma. Pediatr. Pulmonol. 2020, 55, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Vähätalo, I.; Ilmarinen, P.; Tuomisto, L.E.; Tommola, M.; Niemelä, O.; Lehtimäki, L.; Nieminen, P.; Kankaanranta, H. 12-year adherence to inhaled corticosteroids in adult-onset asthma. ERJ Open Res. 2020, 6, 00324–02019. [Google Scholar] [CrossRef]
- Durham, S.R. The allergen-specificity of allergen immunotherapy—Doubt no more. Allergy 2019, 74, 2054–2056. [Google Scholar] [CrossRef] [Green Version]
- Di Lorenzo, G.; Mansueto, P.; Pacor, M.L.; Rizzo, M.; Castello, F.; Martinelli, N.; Ditta, V.; Lo Bianco, C.; Leto-Barone, M.S.; D’Alcamo, A.; et al. Evaluation of serum s-IgE/total IgE ratio in predicting clinical response to allergen-specific immunotherapy. J. Allergy Clin. Immunol. 2009, 123, 1103–1110.e4. [Google Scholar] [CrossRef] [Green Version]
- Dhami, S.; Nurmatov, U.; Arasi, S.; Khan, T.; Asaria, M.; Zaman, H.; Agarwal, A.; Netuveli, G.; Roberts, G.; Pfaar, O.; et al. Allergen immunotherapy for allergic rhinoconjunctivitis: A systematic review and meta-analysis. Allergy 2017, 72, 1597–1631. [Google Scholar] [CrossRef] [Green Version]


| ID | Group I | ID | Group D | ||
|---|---|---|---|---|---|
| Before SCIT | During SCIT | Before SCIT | During SCIT | ||
| 1 | −3.8 | 2.4 | 9 | 3.0 | −1.8 |
| 2 | 1.3 | 1.4 | 10 | 6.7 | −4.6 |
| 3 | −9.0 | 1.1 | 11 | 4.4 | −0.3 |
| 4 | −0.8 | −0.6 | 12 | 8.0 | −15.2 |
| 5 | −3.7 | 7.0 | 13 | −3.7 | −7.0 |
| 6 | −11.3 | −1.3 | 14 | 13.0 | 4.8 |
| 7 | −10.9 | −8.3 | 15 | 7.6 | −0.4 |
| 8 | −5.0 | −2.5 | 16 | 10.6 | 3.1 |
| Mean | −5.4 | −0.1 | Mean | 6.2 | −2.7 |
| ID | Group I | ID | Group D | ||
|---|---|---|---|---|---|
| Before SCIT | During SCIT | Before SCIT | During SCIT | ||
| 1 | −4.3 | 1.9 | 9 | 0.7 | −2.8 |
| 2 | −0.4 | 5.6 | 10 | 3.2 | −2.6 |
| 3 | −9.1 | −0.8 | 11 | −0.9 | −12.2 |
| 4 | 0.4 | 0.5 | 12 | −0.8 | −6.1 |
| 5 | −3.2 | 4.4 | 13 | −2.6 | −2.9 |
| 6 | −15.3 | 0.7 | 14 | 3.8 | −0.5 |
| 7 | −16.2 | −6.2 | 15 | 3.9 | −5.7 |
| 8 | −8.1 | −2.5 | 16 | 10.6 | 1.8 |
| Mean | −7.0 | 0.5 | Mean | 2.2 | −3.9 |
| Characteristic | Group I (n = 8) | Group D (n = 8) | p Value |
|---|---|---|---|
| Age (years); mean ± SD | |||
| at the start of SCIT | 10.5 ± 2.0 | 11.8 ± 1.9 | 0.252 |
| at diagnosis of asthma | 6.4 ± 1.8 | 8.6 ± 1.8 | 0.033 |
| Gender; M/F | 8/0 | 4/4 | 0.077 |
| Observation period (years); mean (range) | |||
| Pre-SCIT | 3.8 (0.6–7.5) | 3.0 (1.6–7.2) | 0.65 |
| SCIT | 4.1 (1.3–4.7) | 3.9 (1.5–7.9) | 0.74 |
| Co-morbid allergic disease; n (%) | |||
| Allergic rhinitis | 8 (100%) | 6 (75%) | 0.467 |
| Atopic dermatitis | 2 (25%) | 2 (25%) | >0.999 |
| Food allergy | 4 (50%) | 2 (25%) | 0.608 |
| Pharmacological treatment at the start of SCIT | |||
| Median dose of ICS (range) | 200 (0–1000) | 200 (0–400) | 0.563 |
| No use of ICS | 1 (13%) | 2 (25%) | >0.999 |
| Use of omalizumab | 2 (25%) | 1 (13%) | >0.999 |
| Pharmacological treatment at the last visit of SCIT | |||
| Median dose (range) | 100 (0–200) | 150 (0–250) | 0.563 |
| No use of ICS | 4 (50%) | 3 (38%) | >0.999 |
| Use of omalizumab | 2 (25%) | 1 (13%) | >0.999 |
| Lung function (A) at the start and (B) at the last visit of SCIT | |||
| FEV1% predicted (A) | 85.8 ± 5.9 | 85.2 ± 8.1 | 0.958 |
| (B) | 88.6 ± 7.6 | 83.5 ± 8.6 | 0.156 |
| FEV1/FVC ratio (%); mean ± SD (A) | 90.8 ± 8.2 | 92.1 ± 8.9 | 0.713 |
| (B) | 92.8 ± 15.4 | 87.7 ± 10.5 | 0.637 |
| MEF50% predicted; mean ± SD (A) | 78.3 ± 13.7 | 94.7 ± 31.1 | 0.318 |
| (B) | 85.2 ± 23.6 | 85.5 ± 27.6 | 0.958 |
| FeNO (ppb); median (range) (A) | 33 (8–80) | 25 (13–82) | >0.999 |
| (B) | 29 (16–105) | 47 (6–107) | 0.793 |
| Blood eosinophil count at the start of SCIT (/µL); median (range) | 200 (60–400) | 500 (260–1100) | 0.006 |
| Total serum IgE at the start of SCIT (IU/mL); median (range) | 652 (105–2976) | 559 (135–2572) | 0.959 |
| Specific IgE (kUA/L) at the start of SCIT; median (range) | |||
| HDM (Dermatophagoides pteronyssinus) | 160 (60–273) | 70 (5.5–144) | 0.038 |
| Japanese cedar pollen | 13.1 (4.7–221) | 6.7 (0.1–144) | 0.328 |
| Dog dander | 0.3 (0.1–34.6) | 0.4 (0.1–47.4) | 0.485 |
| Cat dander | 0.1 (0.1–1.7) | 0.3 (0.1–10.9) | 0.114 |
| Ragweed | 0.2 (0.1–3.9) | 0.3 (0.1–1.7) | 0.657 |
| Orchard grass | 0.2 (0.1–29.3) | 0.3 (0.1–8.1) | 0.797 |
| Parameter | Variable | Estimate | 95% CI | Odds Ratio | 95% CI |
| β1 | Eos | 0.017 | 0.003 to 0.053 | 1.017 | 1.003 to 1.054 |
| β2 | HDM IgE | −0.028 | −0.078 to −0.002 | 0.973 | 0.925 to 0.998 |
| Statistic | 95% CI | p value | |||
| Area under the ROC curve | 0.938 | 0.822 to 1.000 | 0.003 | ||
| Hosmer–Lemeshow test | 5.506 | 0.702 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogami, K.; Nagao, M.; Takase, T.; Yasuda, Y.; Yamada, S.; Matsunaga, M.; Hoshi, M.; Hamada, K.; Kuwabara, Y.; Tsugawa, T.; et al. House Dust Mite Subcutaneous Immunotherapy and Lung Function Trajectory in Children and Adolescents with Asthma. Children 2022, 9, 487. https://doi.org/10.3390/children9040487
Nogami K, Nagao M, Takase T, Yasuda Y, Yamada S, Matsunaga M, Hoshi M, Hamada K, Kuwabara Y, Tsugawa T, et al. House Dust Mite Subcutaneous Immunotherapy and Lung Function Trajectory in Children and Adolescents with Asthma. Children. 2022; 9(4):487. https://doi.org/10.3390/children9040487
Chicago/Turabian StyleNogami, Kazutaka, Mizuho Nagao, Takafumi Takase, Yasuaki Yasuda, Shingo Yamada, Mayumi Matsunaga, Miyuki Hoshi, Kana Hamada, Yu Kuwabara, Takeshi Tsugawa, and et al. 2022. "House Dust Mite Subcutaneous Immunotherapy and Lung Function Trajectory in Children and Adolescents with Asthma" Children 9, no. 4: 487. https://doi.org/10.3390/children9040487
APA StyleNogami, K., Nagao, M., Takase, T., Yasuda, Y., Yamada, S., Matsunaga, M., Hoshi, M., Hamada, K., Kuwabara, Y., Tsugawa, T., & Fujisawa, T. (2022). House Dust Mite Subcutaneous Immunotherapy and Lung Function Trajectory in Children and Adolescents with Asthma. Children, 9(4), 487. https://doi.org/10.3390/children9040487






