Epidemiology, Microbiology and Severity of Bronchiolitis in the First Post-Lockdown Cold Season in Three Different Geographical Areas in Italy: A Prospective, Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
2.3. Outcomes
2.3.1. Primary Outcome
2.3.2. Secondary Outcome
- -
- To define overall disease severity (defined in terms of need of intensive care unit or different type of ventilation support) in children with bronchiolitis during the first cold season where lockdowns (full or partial) were not implemented in Italy;
- -
- To define the main etiological agents in this cohort of children with bronchiolitis;
- -
- To compare disease severity in children with RSV versus non-RSV bronchiolitis;
- -
- To understand the impact of SARS-CoV-2 on a large cohort of children with bronchiolitis;
- -
- To compare disease severity in children with bronchiolitis due to a single etiology versus those with multiple viruses detected; specifically, we defined as “co-infection group” those children that had more than one virus detected simultaneously at the nasopharyngeal swab;
- -
- To compare disease severity and main microbiological agents in children with bronchiolitis younger or older than 12 months of age.
- -
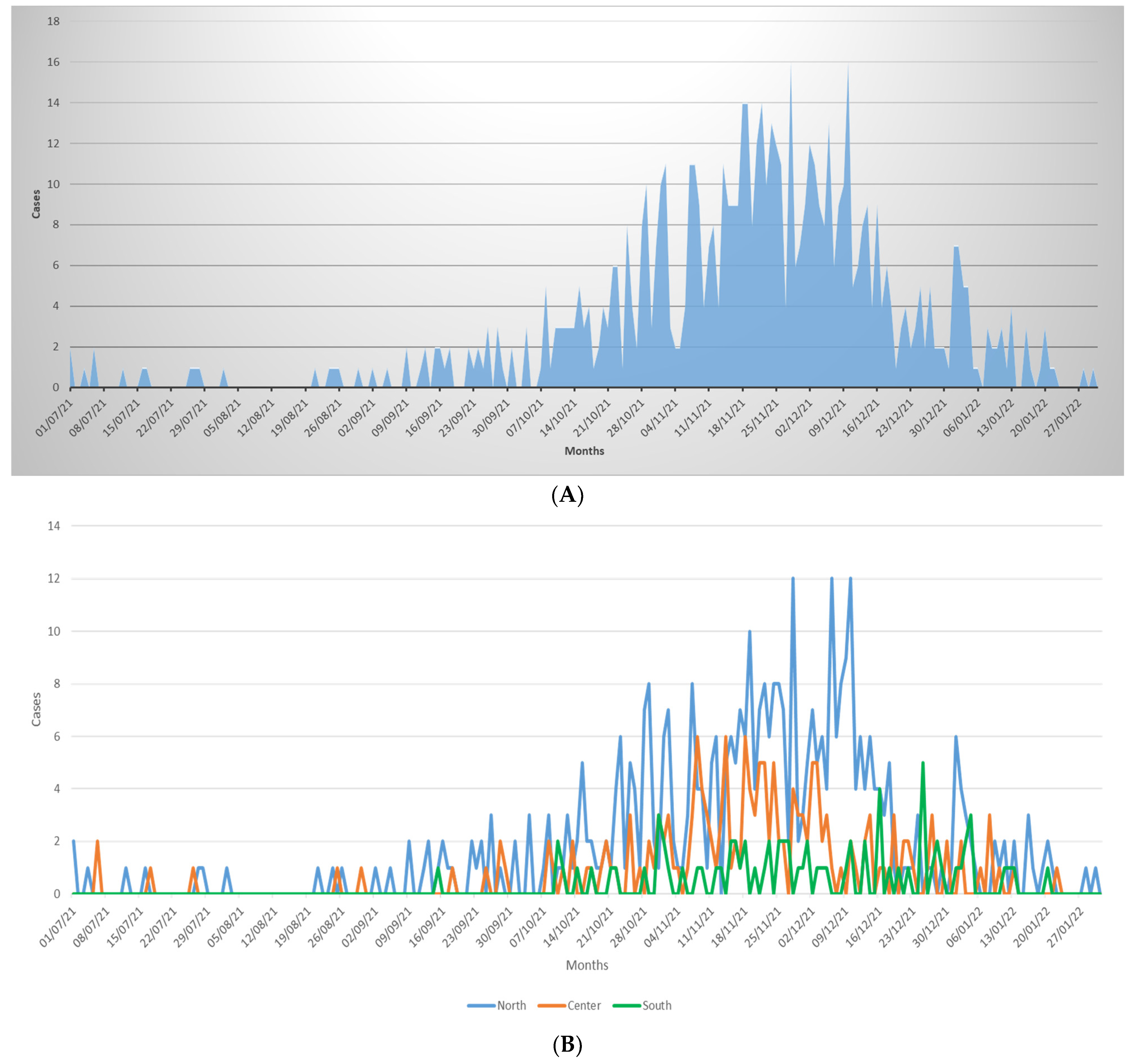
- To compare the temporal distribution of bronchiolitis cases during the 2021/22 season with pre-pandemic seasons (data available only for Bologna, Rome, and Catania).
2.4. Data Collection
2.5. Statistical Analyses
3. Results
3.1. Study Population
3.2. Aetiologies of Bronchiolitis and Impact on Disease Severity
3.3. Disease Severity According to Age Groups or Prematurity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Available online: https://ourworldindata.org/grapher/covid-deaths-income (accessed on 31 January 2022).
- Available online: https://www.undp.org/coronavirus/socio-economic-impact-covid-19 (accessed on 31 January 2022).
- Buonsenso, D.; Roland, D.; De Rose, C.; Vásquez-Hoyos, P.; Ramly, B.; Chakakala-Chaziya, J.N.; Munro, A.; González-Dambrauskas, S. Schools Closures During the COVID-19 Pandemic: A Catastrophic Global Situation. Pediatr. Infect Dis. J. 2021, 40, e146–e150. [Google Scholar] [CrossRef] [PubMed]
- Kadambari, S.; Goldacre, R.; Morris, E.; Goldacre, M.J.; Pollard, A.J. Indirect effects of the covid-19 pandemic on childhood infection in England: Population based observational study. BMJ 2022, 376, e067519. [Google Scholar] [CrossRef] [PubMed]
- Binns, E.; Koenraads, M.; Hristeva, L.; Flamant, A.; Baier-Grabner, S.; Loi, M.; Lempainen, J.; Osterheld, E.; Ramly, B.; Chakakala-Chaziya, J.; et al. Influenza and respiratory syncytial virus during the COVID-19 pandemic: Time for a new paradigm? Pediatr. Pulmonol. 2021, 57, 38–42. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Surveillance Atlas of Infectious Diseases. European Centre for Disease Prevention and Control. 2020. Available online: https://www.ecdc.europa.eu/en/surveillance-atlas-infectious-diseases (accessed on 31 December 2020).
- Guitart, C.; Bobillo-Perez, S.; Alejandre, C.; Armero, G.; Launes, C.; Cambra, F.J.; Balaguer, M.; Jordan, I.; Hospital Network for R. S. V. surveillance in Catalonia. Bronchiolitis, epidemiological changes during the SARS-CoV-2 pandemic. BMC Infect Dis. 2022, 24, 84. [Google Scholar] [CrossRef]
- Olsen, S.J.; Winn, A.K.; Budd, A.P.; Prill, M.M.; Steel, J.; Midgley, C.M.; Kniss, K.; Burns, E.; Rowe, T.; Foust, A.; et al. Changes in influenza and other respiratory virus activity during the COVID-19 pandemic—United States, 2020–2021. Morb. Mortal. Wkly. Rep. 2021, 70, 1013–1019. [Google Scholar] [CrossRef]
- Vásquez-Hoyos, P.; Diaz-Rubio, F.; Monteverde-Fernandez, N.; Jaramillo-Bustamante, J.C.; Carvajal, C.; Serra, A.; Karsies, T.; Rotta, A.T.; González-Dambrauskas, S. Reduced PICU respiratory admissions during COVID-19. Arch. Dis. Child. 2021, 106, 808–811. [Google Scholar] [CrossRef]
- Yeoh, D.K.; Foley, D.A.; Minney-Smith, C.A.; Martin, A.C.; Mace, A.O.; Sikazwe, C.T.; Le, H.; Levy, A.; Blyth, C.C.; Moore, H.C. The impact of COVID-19 public health measures on detections of influenza and respiratory syncytial virus in children during the 2020 Australian winter. Clin. Infect. Dis. 2020, 72, 2199–2202. [Google Scholar] [CrossRef]
- Britton, P.N.; Hu, N.; Saravanos, G.; Shrapnel, J.; Davis, J.; Snelling, T.; Dalby-Payne, J.; Kesson, A.M.; Wood, N.; Macartney, K.; et al. COVID-19 public health measures and respiratory syncytial virus. Lancet Child Adolesc. Health 2020, 4, e42–e43. [Google Scholar] [CrossRef]
- Trenholme, A.; Webb, R.; Lawrence, S.; Arrol, S.; Taylor, S.; Ameratunga, S.; Byrnes, C.A. COVID-19 and Infant Hospitalizations for Seasonal Respiratory Virus Infections, New Zealand, 2020. Emerg. Infect. Dis. 2021, 27, 641–643. [Google Scholar] [CrossRef]
- Pata, D.; Gatto, A.; Buonsenso, D.; Chiaretti, A. A COVID-19 outbreak’s lesson: Best use of the paediatric emergency department. Acta Paediatr. 2020, 109, 1903–1904. [Google Scholar] [CrossRef]
- Available online: https://ourworldindata.org/policy-responses-covid (accessed on 31 January 2022).
- Foley, D.A.; Yeoh, D.K.; Minney-Smith, C.A.; Martin, A.C.; Mace, A.O.; Sikazwe, C.T.; Le, H.; Levy, A.; Moore, H.C.; Blyth, C.C. The Interseasonal Resurgence of Respiratory Syncytial Virus in Australian Children Following the Reduction of Coronavirus Disease 2019-Related Public Health Measures. Clin. Infect. Dis. 2021, 73, e2829–e2830. [Google Scholar] [CrossRef] [PubMed]
- ESR. Laboratory-Based Virology Weekly Report. 2021. Available online: https://www.esr.cri.nz/our-research/research-projects/shivers-vstudy/shivers-v-reports/laboratory-basedvirology-weekly-report/ (accessed on 7 January 2021).
- Public Health Surveillance Inforamtion for New Zealands Public Health Action. Laboratory-Based Virology Weekly Report, 2004–2019. 2021. Available online: https://surv.esr.cri.nz/virology/virology_weekly_report.php (accessed on 7 January 2021).
- Saravanos, G.L.; Hu, N.; Homaira, N.; Muscatello, D.J.; Jaffe, A.; Bartlett, A.W.; Wood, N.J.; Rawlinson, W.; Kesson, A.; Lingam, R.; et al. RSV Epidemiology in Australia Before and During COVID-19. Pediatrics 2022, 26, e2021053537. [Google Scholar] [CrossRef] [PubMed]
- Hatter, L.; Eathorne, A.; Hills, T.; Bruce, P.; Beasley, R. Respiratory syncytial virus: Paying the immunity debt with interest. Lancet Child Adolesc. Health 2021, 5, e44–e45. [Google Scholar] [CrossRef]
- Bem, R.A.; Bont, L.J.; Van Woensel, J.B.M. Life-threatening bronchiolitis in children: Eight decades of critical care. Lancet Respir. Med. 2020, 8, 142–144. [Google Scholar] [CrossRef] [Green Version]
- Cohen, R.; Pettoello-Mantovani, M.; Somekh, E.; Levy, C. European Pediatric Societies Call for an Implementation of Regular Vaccination Programs to Contrast the Immunity Debt Associated to Coronavirus Disease 2019 Pandemic in Children. J. Pediatr. 2021, 27, S0022-3476(21)01159-8. [Google Scholar] [CrossRef]
- Baraldi, E.; Lanari, M.; Manzoni, P.; Rossi, G.A.; Vandini, S.; Rimini, A.; Romagnoli, C.; Colonna, P.; Biondi, A.; Biban, P.; et al. Inter-society consensus document on treatment and prevention of bronchiolitis in newborns and infants. Ital. J. Pediatr. 2014, 40, 65. [Google Scholar] [CrossRef]
- Team, B.G. Cincinnati Children’s Hospital Medical Center: Evidence Based Clinical Practice Guideline for Medical Management of Bronchiolitis in Infants 1 Year of Age or Less Presenting with a First Time Episode. Cincinnati Child. Hosp. Med. Cent. 2010, 1, 1–13. [Google Scholar]
- Management of Bronchiolitis in Infants: The Hospital for Sick Children, Clinical Practice Guideline, Last Modified. 2011. Available online: https://www.seattlechildrens.org/pdf/bronchiolitis-pathway.pdf (accessed on 30 September 2021).
- National Collaborating Centre for Women’s and Children’s Health (UK). Bronchiolitis: Diagnosis and Management of Bronchiolitis in Children; National Institute for Health and Care Excellence: London, UK, 2015. [Google Scholar]
- Ralston, S.L.; Lieberthal, A.S.; Meissner, H.C.; Alverson, B.K.; Baley, J.E.; Gadomski, A.M.; Johnson, D.W.; Light, M.J.; Maraqa, N.F.; Mendonca, E.A.; et al. Clinical Practice Guideline: The Diagnosis, Management, and Prevention of Bronchiolitis. Pediatrics 2014, 134, e1474–e1502. [Google Scholar] [CrossRef] [Green Version]
- Korppi, M. Virus-induced wheezing in infants aged 12-24 months and bronchiolitis in infants under 6 months are different clinical entities. Acta Paediatr. 2015, 104, e539. [Google Scholar] [CrossRef]
- Azzari, C.; Baraldi, E.; Bonanni, P.; Bozzola, E.; Coscia, A.; Lanari, M.; Manzoni, P.; Mazzone, T.; Sandri, F.; Lisi, G.C.; et al. Epidemiology and prevention of respiratory syncytial virus infections in children in Italy. Ital. J. Pediatr. 2021, 47, 198. [Google Scholar] [CrossRef]
- Broberg, E.K.; Waris, M.; Johansen, K.; Snacken, R.; Penttinen, P. European Influenza Surveillance Network. Seasonality and geographical spread of respiratory syncytial virus epidemics in 15 European countries, 2010 to 2016. Euro. Surveill. 2018, 23, 17–00284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curatola, A.; Lazzareschi, I.; Bersani, G.; Covino, M.; Gatto, A.; Chiaretti, A. Impact of COVID-19 outbreak in acute bronchiolitis: Lesson from a tertiary Italian Emergency Department. Pediatr. Pulmonol. 2021, 56, 2484–2488. [Google Scholar] [CrossRef] [PubMed]
- Stera, G.; Pierantoni, L.; Masetti, R.; Leardini, D.; Biagi, C.; Buonsenso, D.; Pession, A.; Lanari, M. Impact of SARS-CoV-2 Pandemic on Bronchiolitis Hospitalizations: The Experience of an Italian Tertiary Center. Children 2021, 8, 556. [Google Scholar] [CrossRef] [PubMed]
- Fourgeaud, J.; Toubiana, J.; Chappuy, H.; Delacourt, C.; Moulin, F.; Parize, P.; Scemla, A.; Abid, H.; Leruez-Ville, M.; Frange, P. Impact of public health measures on the post-COVID-19 respiratory syncytial virus epidemics in France. Eur. J. Clin. Microbiol. 2021, 40, 2389–2395. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Pitzer, V.E.; Shapiro, E.D.; Bont, L.J.; Weinberger, D.M. Estimation of the Timing and Intensity of Reemergence of Respiratory Syncytial Virus Following the COVID-19 Pandemic in the US. JAMA Netw. Open 2021, 4, e2141779. [Google Scholar] [CrossRef]
- Simoes, E.A. Respiratory syncytial virus infection. Lancet 1999, 354, 847–852. [Google Scholar] [CrossRef]
- Nair, H.; Nokes, D.J.; Gessner, B.D.; Dherani, M.; Madhi, S.A.; Singleton, R.J.; O’Brien, K.L.; Roca, A.; Wright, P.F.; Bruce, N.; et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet 2010, 375, 1545–1555. [Google Scholar] [CrossRef] [Green Version]
- Bastos, J.C.S.; Simas, P.V.M.; Caserta, L.C.; Bragunde, A.E.A.; Marson, F.A.D.L.; Martini, M.C.; Padilla, M.A.; Ribeiro, J.D.; dos Santos, M.M.A.B.; Arns, C.W. Rhinoviruses as critical agents in severe bronchiolitis in infants. J. Pediatr. 2021, S0021-7557(21)00167-4. [Google Scholar] [CrossRef]
- Bizot, E.; Bousquet, A.; Charpié, M.; Coquelin, F.; Lefevre, S.; Le Lorier, J.; Patin, M.; Sée, P.; Sarfati, E.; Walle, S.; et al. Rhinovirus: A Narrative Review on Its Genetic Characteristics, Pediatric Clinical Presentations, and Pathogenesis. Front. Pediatr. 2021, 9, 643219. [Google Scholar] [CrossRef]
- Andina-Martinez, D.; Alonso-Cadenas, J.A.; Cobos-Carrascosa, E.; Bodegas, I.; Oltra-Benavent, M.; Plazaola, A.; Epalza, C.; Jimenez-García, R.; Moraleda, C.; Tagarro, A. EPICO-AEP Working Group. SARS-CoV-2 acute bronchiolitis in hospitalized children: Neither frequent nor more severe. Pediatr. Pulmonol. 2022, 57, 57–65. [Google Scholar] [CrossRef]
- Available online: https://ourworldindata.org/coronavirus/country/italy (accessed on 18 February 2022).
- Wu, A.; Mihaylova, V.T.; Landry, M.L.; Foxman, E.F. Interference between rhinovirus and influenza a virus: A clinical data analysis and experimental infection study. Lancet Microbe 2020, 1, e254–e262. [Google Scholar] [CrossRef]
- Ånestad, G.; Nordbø, S.A. Virus interference. Did rhinoviruses activity hamper the progress of the 2009 influenza A (H1N1) pandemic in Norway? Med. Hypotheses 2011, 77, 1132–1134. [Google Scholar] [CrossRef] [PubMed]
- Casalegno, J.S.; Ottmann, M.; Duchamp, M.B.; Escuret, V.; Billaud, G.; Frobert, E.; Morfin, F.; Lina, B. Rhinoviruses delayed the circulation of the pandemic influenza A (H1N1) 2009 virus in France. Clin. Microbiol. Infect. 2010, 16, 326–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linde, A.; Rotzén-Östlund, M.; Zweygberg-Wirgart, B.; Rubinova, S.; Brytting, M. Does viral interference affect spread of influenza? Eurosurveillance 2009, 14, 19354. [Google Scholar] [CrossRef]
- Halfmann, P.J.; Nakajima, N.; Sato, Y.; Takahashi, K.; Accola, M.; Chiba, S.; Fan, S.; Neumann, G.; Rehrauer, W.; Suzuki, T.; et al. SARS-CoV-2 Interference of Influenza Virus Replication in Syrian Hamsters. J. Infect. Dis. 2022, 225, 282–286. [Google Scholar] [CrossRef]

| Study Population | North | Middle | South | p-Value | |
|---|---|---|---|---|---|
| n = 657 | n = 410 | n = 170 | n = 77 | ||
| Sex, n (%) | |||||
| • Female | 368 (56.01) | 170 (41.46) | 77 (45.29) | 42 (54.55) | 0.1 |
| • Male | 289 (43.99) | 240 (58.54) | 93 (54.71) | 35 (45.45) | |
| Age (months), median (IQR) | 4 (2–8.5) | 4 (2–9) | 3 (2–8) | 3.4 (1.6–6) | 0.18 |
| Co-morbidities, n(%) n (%) | 94 (14.31) | 56 (13.66) | 28 (16.47) | 10 (12.99) | 0.64 |
| Gestazional age, n (%) | |||||
| • <34 weeks | 18/636 (2.83) | 8/391 (2.05) | 8/168 (4.76) | 2/77 (2.60) | 0.24 |
| • 34–36 weeks | 42/636 (6.60) | 25/391 (6.39) | 9/168 (5.36) | 8/77 (10.39) | |
| • ≥37 weeks | 576/636 (90.57) | 358/391 (91.56) | 151/168 (89.88) | 67/77 (87.01) | |
| Chronic lung disease of prematurity, n (%) | 7 (1.01) | 2 (0.49) | 5 (2.94) | 0 | 0.02 |
| Congenital heart disease, n (%) | 16 (2.44) | 13 (3.17) | 2 (1.18) | 1 (1.30) | 0.42 |
| Neuromuscolar Disease, n (%) | 6 (0.91) | 2 (0.49 | 4 (2.35) | 0 | 0.07 |
| Other comorbidities, n (%) | 34 (5.18) | 20 (4.88) | 13 (7.65) | 1 (1.30) | 0.1 |
| Siblings n (%) | 292(44.44) | 147(35.85) | 98 (57.65) | 47 (61.04) | <0.001 |
| Palivizumab | 3 (0.46) | 3 (0.73) | 0 | 0 | 0.67 |
| Admitted to hospital, n (%) | 368 (56.01) | 231 (56.34) | 71 (41.76) | 66 (85.71) | <0.001 |
| Admitted to PICU, n (%) | 26 (3.96) | 21 (5.12) | 3 (1.76) | 2 (2.60) | 0.15 |
| Admitted to PICU within the first 7 days since initial evaluation, n (%) | 39 (5.94) | 24 (5.85) | 13 (7.65) | 2 (2.60) | 0.31 |
| Need for nasogastric fluids *, n (%) | 4 (0.61) | 1 (0.24) | 3 (1.76) | 0 | 0.12 |
| Intravenous fluids *, n (%) | 221 (33.64) | 105 (25.61) | 59 (34.71) | 57 (74.03) | <0.001 |
| Need for Oxygen Low Flow *, n (%) | 167 (25.42) | 119 (29.02) | 47 (27.65) | 1 (1.30) | <0.001 |
| Need for High Flow Oxygen *, n (%) | 128 (19.48) | 45 (10.98) | 39 (22.94) | 44 (57.14) | <0.001 |
| CPAP *, n (%) | 43 (6.54) | 31 (7.56) | 12 (7.06) | 0 | 0.05 |
| Mechanical ventilation *, n (%) | 2 (0.30) | 1 (0.24) | 1 (0.59) | 0 | 0.61 |
| Salbutamol nebulization, n (%) | 262 (39.88) | 175 (42.68) | 34 (20.00) | 53 (68.83) | <0.001 |
| Other bronchodilator, n (%) | 55 (8.37) | 52 (12.68) | 3 (1.76) | 0 | <0.001 |
| Corticosteroid treatment, n (%) | 118 (17.96) | 80 (19.51) | 4 (2.35) | 34 (44.16) | <0.001 |
| Antibiotic treatment, n (%) | 148 (22.53) | 87 (21.22) | 17 (10) | 44 (57.14) | <0.001 |
| Detection of Virus on RT-PCR (Based on 264 Children Tested with PCR, of Which 216 Had at Least One Positive Virus) * | Study Population | North n = 110 | Middle n = 83 | South n = 23 | p-Value |
|---|---|---|---|---|---|
| RSV, n (%) | 162 (75) | 85 (77.27) | 58 (69.88) | 19 (82.61) | 0.4 |
| Rhinovirus, n (%) | 48 (22.22) | 8 (8.18) | 34 (40.96) | 5 (21.74) | <0.001 |
| SARS-CoV-2 *, n (%) | 18 (8.33) | 16 (14.55) | 2 (2.41) | 0 | 0.003 |
| Human-Metapneumovirus, n (%)) | 12 (5.56) | 3 (2.73) | 7 (8.43) | 2 (8.70) | 0.122 |
| Parainfluenza, n (%) | 8 (4.17) | 6 (5.45) | 2 (2.41) | 1 (4.35) | 0.61 |
| Adenovirus, n (%) | 2 (0.93) | 0 | 2 (2.41) | 0 | 0.35 |
| Other viruses, n (%) | 14 (6.48) | 4 (3.64) | 7 (7.23) | 4 (17.39) | 0.05 |
| Coinfection, n (%) | 43 (19.91) | 13 (11.81) | 25 (30.12) | 5 (21.74) | 0.006 |
| RSV | Not-RSV | p-Value | |
|---|---|---|---|
| n = 162 | n = 54 # | ||
| Sex, n (%) | 0.43 | ||
| • Female | 76 (46.91) | 22 (40.74) | |
| • Male | 86 (53.09) | 32 (59.26) | |
| Age (months), median (IQR) | 2 (1–5) | 4 (1.69–11) | 0.004 |
| Co-morbidities, n (%) n (%) | 27 (16.67) | 14 (25.93) | 0.13 |
| Gestazional age, n (%) | 0.77 | ||
| • <34 weeks | 6/161 (3.73) | 2/53 (3.77) | |
| • 34–36 weeks | 13/161 (8.07) | 6/53 (11.32) | |
| • ≥37 weeks | 142/161 (88.20) | 45/53 (84.91) | |
| Chronic lung disease of prematurity, n (%) | 1 (0.62) | 2 (3.70) | 0.09 |
| Congenital heart disease, n (%) | 5 (3.09) | 1 (1.85) | 0.53 |
| Neuromuscolar Disease, n (%) | 3 (1.85) | 2 (3.70) | 0.68 |
| Other comorbidities, n (%) | 10 (6.17) | 2 (3.70) | 0.38 |
| Siblings n (%) | 96 (59.26) | 27 (50) | 0.23 |
| Palivizumab | 0 | 0 | |
| Admitted to hospital, n (%) | 130 (80.25) | 37 (68.52) | 0.06 |
| Admitted to PICU, n (%) | 9 (5.56) | 3 (5.56) | 0.61 |
| Admitted to PICU within the first 7 days since initial evaluation, n (%) | 22 (13.58) | 4 (7.41) | 0.7 |
| Need for nasogastric fluids *, n (%) | 3 (1.85) | 0 | 0.42 |
| Intravenous fluids *, n (%) | 95 (58.64) | 24 (44.44) | 0.07 |
| Need for Oxygen Low Flow, n (%) | 74 (45.68) | 18 (33.33) | 0.11 |
| Need for High Flow Oxygen *, n (%) | 57 (35.19) | 21 (38.89) | 0.62 |
| CPAP *, n (%) | 24 (14.81) | 3 (5.56) | 0.05 |
| Mechanica ventilation *, n (%) | 1 (0.62) | 1 (1.85) | 0.43 |
| Salbutamol nebulization, n (%) | 65 (40.12) | 15 (27.78) | 0.1 |
| Other bronchodilator, n(%) | 14 (8.64) | 2 (3.70) | 0.19 |
| Corticosteroid treatment, n (%) | 38 (23.46) | 7 (12.96) | 0.1 |
| Antibiotic treatment, n (%) | 51 (31.48) | 9 (16.67) | 0.03 |
| Single Agent | Coinfections | p-Value | |
|---|---|---|---|
| n = 173 | n = 43 | ||
| Sex, n (%) | 0.87 | ||
| • Female | 20 (46.51) | 78 (45.09) | |
| • Male | 23 (53.49) | 95 (54.91) | |
| Age (months), median (IQR) | 3 (1–7) | 3 (1–6) | 0.58 |
| Co-morbidities, n(%) n (%) | 32 (18.50) | 9 (20.93) | 0.72 |
| • <34 weeks | 4/171 (2.34) | 4 (9.30) | 0.11 |
| • 34–36 | 16/171 (9.36) | 3 (6.98) | |
| • ≥37 | 151/171 (88.30) | 36 (83.72) | |
| Chronic lung disease of prematurity, n (%) | 1 (0.58) | 2 (4.65) | 0.1 |
| Congenital heart disease, n (%) | 4 (2.31) | 2 (4.65) | 0.34 |
| Neuromuscolar Disease, n (%) | 4 (2.31) | 1 (2.33) | 0.67 |
| Other comorbidities, n (%) | 9 (5.20) | 3 (6.98) | 0.44 |
| Siblings n (%) | 94 (54.34) | 29 (67.44) | 0.12 |
| Palivizumab | 0 | 0 | |
| Admitted to hospital, n (%) | 137 (79.19) | 30 (69.77) | 0.18 |
| Admitted to PICU, n (%) | 9 (5.20) | 3 (6.98) | 0.44 |
| Admitted to PICU within the first 7 days since initial evaluation, n (%) | 19 (10.98) | 7 (16.28) | 0.34 |
| Need for nasogastric fluids *, n (%) | 3 (16.28) | 0 | 0.51 |
| Intravenous fluids *, n (%) | 95 (54.91) | 24 (55.81) | 0.91 |
| Need for Oxygen Low Flow *, n (%) | 72 (41.62) | 20 (46.51) | 0.56 |
| Need for High Flow Oxygen *, n (%) | 60 (34.68) | 18 (41.86) | 0.38 |
| CPAP *, n (%) | 20 (11.56) | 7 (16.28) | 0.4 |
| Mechanical ventilation *, n (%) | 1 (0.58) | 1 (2.33) | 0.36 |
| Salbutamol nebulization, n (%) | 67 (38.73) | 13 (30.23) | 0.3 |
| Other bronchodilator, n (%) | 16 (9.25) | 0 | 0.025 |
| Corticosteroid treatment, n (%) | 34 (19.65) | 11 (25.58) | 0.39 |
| Antibiotic treatment, n (%) | 44 (25.43) | 16 (37.21) | 0.12 |
| 216 Patients with an Identified Virus | 12 Months or Less n = 193 | >12 Months n = 23 | p-Value |
|---|---|---|---|
| RSV, n (%) | 147 (76.17) | 15 (65.22) | 0.25 |
| Rhinovirus, n (%) | 38 (19.69) | 10 (43.48) | 0.009 |
| SARS-CoV-2, n (%) | 18 (9.33) | 0 | 0.12 |
| Human-Metapneumovirus, n (%) | 11 (5.70) | 1(4.35) | 0.79 |
| Parainfluenza, n (%) | 9 (4.66) | 0 | 0.36 |
| Adenovirus, n (%) | 1 (0.50) | 1 (4.35) | 0.2 |
| Other viruses, n (%) | 8 (4.15) | 6 (26.09) | 0.001 |
| Coinfection, n (%) | 37 (19.17) | 6 (26.9) | 0.294 |
| 657 total patients | 12 months or less n = 548 | >12 months n = 109 | p-Value |
| Admitted to hospital, n (%) | 315 (57.48) | 53 (48.62) | 0.09 |
| Admitted to PICU, n (%) | 21 (3.83) | 5 (4.59) | 0.72 |
| Admitted to PICU within the first 7 days since initial evaluation, n (%) | 34 (6.20) | 5 (4.59) | 0.34 |
| Need for High Flow Oxygen *, n (%) | 148 (27.01) | 19 (17.43) | 0.04 |
| CPAP *, n (%) | 35 (6.39) | 8 (7.34) | 0.71 |
| Mechanical ventilation *, n (%) | 1 (0.18) | 1 (0.92) | 0.3 |
| 214 CHILDREN with a Virus Isolated and Known Gestational Age | <34 Weeks n = 8 | 34–36 Weeks n = 19 | 37 or More n = 187 | p-Value |
|---|---|---|---|---|
| RSV, n (%) | 6 (75.00) | 13 (68.42) | 142 (75.94) | 0.77 |
| Rhinovirus, n (%) | 2 (25.00) | 5 (26.32) | 40 (21.39) | 0.65 |
| SARS-CoV-2, n (%) | 0 | 2 (10.53) | 16 (8.56) | 0.84 |
| Human-Metapneumovirus, n (%) | 1 (12.50) | 0 | 11 (5.88) | 0.39 |
| Parainfluenza, n (%) | 1 (12.50) | 2 (10.53) | 6 (3.21) | 0.1 |
| Adenovirus, n (%) | 0 | 0 | 2 (1.07) | 1 |
| Other viruses, n (%) | 2 (25.00) | 0 | 12 (6.42) | 0.08 |
| Coinfection, n (%) | 4 (50.00) | 3 (15.79) | 36 (19.46) | 0.11 |
| 636 children with known gestational age | <34 weeks n = 18 | 34–36 weeks n = 42 | 37 or more n = 576 | p-Value |
| Admitted to hospital, n (%) | 15 (83.33) | 25 (59.52) | 316 (54.86) | 0.05 |
| Admitted to PICU, n (%) | 2 (11.11) | 1 (2.38) | 20 (3.47) | 0.19 |
| Admitted to PICU within the first 7 days since initial evaluation, n (%) | 3 (16.67) | 1 (2.38) | 33 (5.73) | 0.1 |
| Need for High Flow Oxygen *, n (%) | 7 (38.89) | 13 (30.95) | 105 (18.23) | 0.015 |
| CPAP *, n (%) | 3 (16.67) | 3 (7.14) | 35 (6.08) | 0.168 |
| Mechanical ventilation *, n (%) | 1 (5.56) | 0 | 1 (0.17) | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camporesi, A.; Morello, R.; Ferro, V.; Pierantoni, L.; Rocca, A.; Lanari, M.; Trobia, G.L.; Sciacca, T.; Bellinvia, A.G.; De Ferrari, A.; et al. Epidemiology, Microbiology and Severity of Bronchiolitis in the First Post-Lockdown Cold Season in Three Different Geographical Areas in Italy: A Prospective, Observational Study. Children 2022, 9, 491. https://doi.org/10.3390/children9040491
Camporesi A, Morello R, Ferro V, Pierantoni L, Rocca A, Lanari M, Trobia GL, Sciacca T, Bellinvia AG, De Ferrari A, et al. Epidemiology, Microbiology and Severity of Bronchiolitis in the First Post-Lockdown Cold Season in Three Different Geographical Areas in Italy: A Prospective, Observational Study. Children. 2022; 9(4):491. https://doi.org/10.3390/children9040491
Chicago/Turabian StyleCamporesi, Anna, Rosa Morello, Valentina Ferro, Luca Pierantoni, Alessandro Rocca, Marcello Lanari, Gian Luca Trobia, Tiziana Sciacca, Agata Giuseppina Bellinvia, Alessandra De Ferrari, and et al. 2022. "Epidemiology, Microbiology and Severity of Bronchiolitis in the First Post-Lockdown Cold Season in Three Different Geographical Areas in Italy: A Prospective, Observational Study" Children 9, no. 4: 491. https://doi.org/10.3390/children9040491
APA StyleCamporesi, A., Morello, R., Ferro, V., Pierantoni, L., Rocca, A., Lanari, M., Trobia, G. L., Sciacca, T., Bellinvia, A. G., De Ferrari, A., Valentini, P., Roland, D., & Buonsenso, D. (2022). Epidemiology, Microbiology and Severity of Bronchiolitis in the First Post-Lockdown Cold Season in Three Different Geographical Areas in Italy: A Prospective, Observational Study. Children, 9(4), 491. https://doi.org/10.3390/children9040491








