Echocardiographic Determination of Percutaneous Central Venous Catheters in the Superior Vena Cava: A Prospective Cohort Study
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Design and Sample
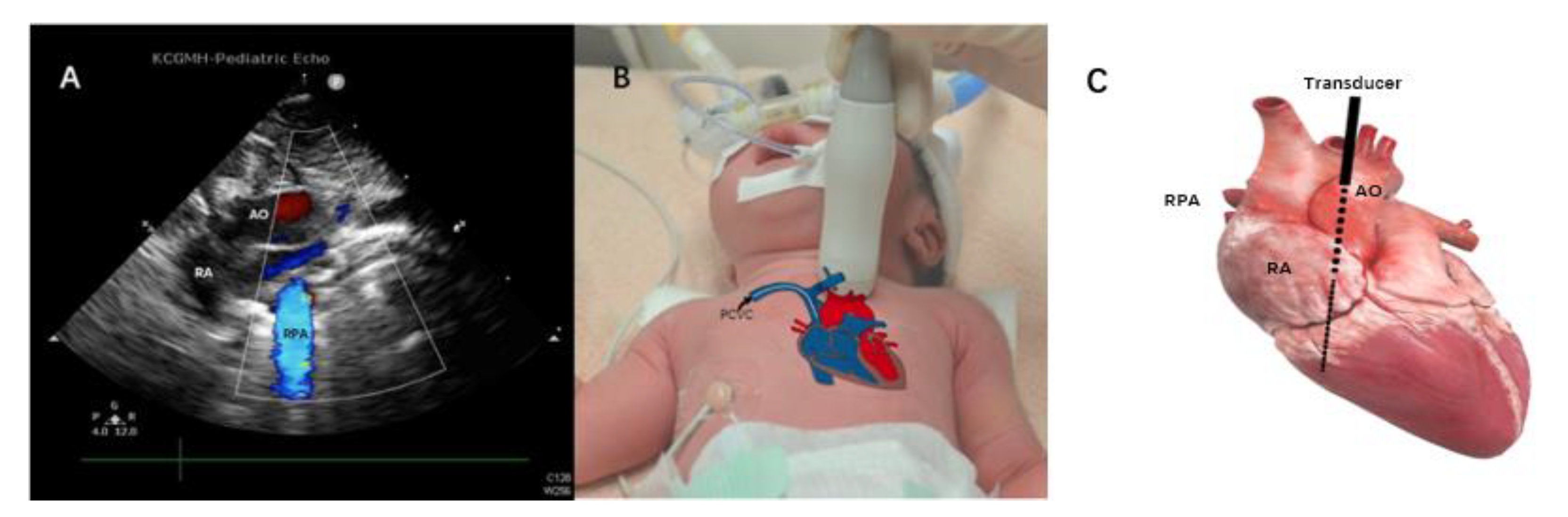
2.2. Methods
2.3. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soares, B.N.; Pissarra, S.; Rouxinol-Dias, A.L.; Costa, S.; Guimaraes, H. Complications of central lines in neonates admitted to a level III Neonatal Intensive Care Unit. J. Matern. Fetal Neonatal Med. 2018, 31, 2770–2776. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S.S.; Connolly, B.; Shearkhani, O.; Brown, M.; Hamilton, R. Arrhythmias in Children with Peripherally Inserted Cen-tral Catheters (PICCs). Pediatr. Cardiol. 2020, 41, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Liem, T.K.; Yanit, K.E.; Moseley, S.E.; Landry, G.J.; Deloughery, T.G.; Rumwell, C.A.; Mitchell, E.L.; Moneta, G.L. Peripheral-ly inserted central catheter usage patterns and associated symptomatic upper extremity venous thrombosis. J. Vasc. Surg. 2012, 55, 761–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atmawidjaja, R.W.; Azri, M.; Ismail, I.H. Cardiac tamponade: A rare but preventable complication of central venous catheter in neonates. Med. J. Malaysia 2016, 71, 147–148. [Google Scholar] [PubMed]
- Sertic, A.J.; Connolly, B.L.; Temple, M.J.; Parra, D.A.; Amaral, J.G.; Lee, K.S. Perforations associated with peripherally insert-ed central catheters in a neonatal population. Pediatr. Radiol. 2018, 48, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Alhatem, A.; Estrella, Y.; Jones, A.; Algarrahi, K.; Fofah, O.; Heller, D.S. Percutaneous Route of Life: Chylothorax or Total Parenteral Nutrition-Related Bilateral Pleural Effusion in a Neonate? Fetal Pediatr. Pathol. 2021, 40, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Franklin, I.; Gilmore, C. Placement of a peripherally inserted central catheter into the azygous vein. J. Med. Radiat. Sci. 2015, 62, 160–162. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.L.; Ou-Yang, M.C.; Chen, F.S.; Chung, M.Y.; Chen, C.C.; Liu, Y.C.; Lin, K.H.; Huang, H.C. The equations of the in-serted length of percutaneous central venous catheters on neonates in NICU. Pediatr. Neonatol. 2019, 60, 305–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohki, Y.; Tabata, M.; Kuwashima, M.; Takeuchi, H.; Nako, Y.; Morikawa, A. Ultrasonographic detection of very thin percu-taneous central venous catheter in neonates. Acta Paediatr. 2000, 89, 1381–1384. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Su, L.T.; Liu, Y.C.; Chang, H.Y.; Ou-Yang, M.C.; Chung, M.Y.; Chen, F.S.; Chen, C.C.; Chen, I.L. The role of ultrasonography for detecting tip location of percutaneous central venous catheters in neonates-a single-center, prospective cohort study. Pediatr. Neonatol. 2021, 62, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Jiao, J.; Zhang, Y.; Tian, L.; Miao, J.; Hao, X.; Sun, Z.; Sun, Q. A randomized controlled study of bedside electrocardio-graph-guided tip location technique & the traditional chest radiography tip location technique for peripherally inserted cen-tral venous catheter in cancer patients. Indian J. Med. Res. 2018, 147, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Farahbakhsh, N.; Tabatabaii, S.A. Role of ultrasound for central catheter tip localization in neonates: A review of the current evidence. J. Matern. Fetal Neonatal Med. 2019, 32, 2429–2437. [Google Scholar] [CrossRef]
- Su, L.T.; Huang, H.C.; Liu, Y.C.; Chang, H.Y.; Ou-Yang, M.C.; Chen, C.C.; Chen, F.S.; Chung, M.Y.; Chen, I.L. The appro-priate frequency of dressing for percutaneous central venous catheters in preventing catheter-related blood stream infection in NICU-A randomized controlled trial. Pediatr. Neonatol. 2021, 62, 292–297. [Google Scholar] [CrossRef]
- Acun, C.; Baker, A.; Brown, L.S.; Iglesia, K.A.; Sisman, J. Peripherally inserted central cathether migration in neonates: Inci-dence, timing and risk factors. J. Neonatal Perinat. Med. 2021, 14, 411–417. [Google Scholar] [CrossRef]
- Shih, C.C.; Chen, S.J.; Hsu, Y.P. Timely identified early migration of peripherally inserted central catheter by focused ultra-sound. J. Med. Ultrasound 2018, 26, 215–217. [Google Scholar] [CrossRef]
- Cortes-Puentes, G.A.; Oeckler, R.A.; Marini, J.J. Physiology-guided management of hemodynamics in acute respiratory dis-tress syndrome. Ann. Transl. Med. 2018, 6, 353. [Google Scholar] [CrossRef]
- Nadroo, A.M.; Glass, R.B.; Lin, J.; Green, R.S.; Holzman, I.R. Changes in upper extremity position cause migration of periph-erally inserted central catheters in neonates. Pediatrics 2002, 110, 131–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Povoski, S.P.; Khabiri, H. Persistent left superior vena cava: Review of the literature, clinical implications, and relevance of alterations in thoracic central venous anatomy as pertaining to the general principles of central venous access device place-ment and venography in cancer patients. World J. Surg. Oncol. 2011, 9, 173. [Google Scholar] [CrossRef] [Green Version]


| IVC (n = 50) | SVC (n = 50) | p-Value | ||||
|---|---|---|---|---|---|---|
| Invasive Ventilation (n = 13) | Non-Invasive Ventilation (n = 37) | p-Value | Total | (IVC vs. SVC) | ||
| Gestational age (wk) | 31.43 ± 5.11 | 30.87 ± 5.10 | 32.63 ± 3.83 | 0.27 | 32.16 ± 4.21 | 0.43 |
| BW (g) | 1642.18 ± 984.97 | 1725.77 ± 847.87 | 1815.27 ±853.96 | 0.75 | 1792.00 ± 844.63 | 0.42 |
| Gender (M/F) | 33/17 | 9/4 | 26/11 | 1.00 | 35/15 | 0.41 |
| Age at the moment of intervention (days) | 14.60 ± 23.90 | 25.46 ± 29.86 | 17.54 ± 26.74 | 0.41 | 19.60 ± 27.49 | 0.33 |
| BW at the moment of intervention (g) | 2035.60 ± 1036.72 | 2092.85 ± 898.87 | 2015.49 ± 1091.78 | 0.80 | 1819.40 ± 1210.06 | 0.34 |
| Duration of intervention time (min) | 10.13 ± 8.07 | 14.38 ± 10.29 | 12.49 ±11.34 | 0.58 | 12.98 ± 11.00 | 0.15 |
| Duration of echo examination (min) | 3.17 ± 1.72 | 13.62 ± 11.10 | 12.11 ± 8.02 | 0.60 | 12.50 ± 8.82 | < 0.001 |
| Duration of X-ray examination (min) | 149.32 ± 115.30 | 165.46 ± 71.06 | 159.10 ± 87.79 | 0.80 | 160.80 ± 83.10 | 0.57 |
| Repositioning rate (%) | 4.00% | 0 | 21.62% | 0.09 | 16.00% | 0.09 |
| Unexpected removal | 20.00% | 30.77% | 29.73% | 1.00 | 30.00% | 0.20 |
| CRBSI (‰) | 3.20‰ | 0 | 6.90‰ | 0.19 | 4.77‰ | 0.53 |
| Occlusion (%) | 14.00% | 7.69% | 13.51% | 1.00 | 12.00% | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-S.; Huang, H.-C.; Liu, Y.-C.; Chen, I.-L. Echocardiographic Determination of Percutaneous Central Venous Catheters in the Superior Vena Cava: A Prospective Cohort Study. Children 2022, 9, 624. https://doi.org/10.3390/children9050624
Wang Y-S, Huang H-C, Liu Y-C, Chen I-L. Echocardiographic Determination of Percutaneous Central Venous Catheters in the Superior Vena Cava: A Prospective Cohort Study. Children. 2022; 9(5):624. https://doi.org/10.3390/children9050624
Chicago/Turabian StyleWang, Yao-Sheng, Hsin-Chun Huang, Yu-Chen Liu, and I-Lun Chen. 2022. "Echocardiographic Determination of Percutaneous Central Venous Catheters in the Superior Vena Cava: A Prospective Cohort Study" Children 9, no. 5: 624. https://doi.org/10.3390/children9050624







