Endoscope-Assisted Extreme Lateral Supracerebellar Infratentorial Approach for Resection of Superior Cerebellar Peduncle Pilocytic Astrocytoma: Technical Note
Abstract
:1. Introduction
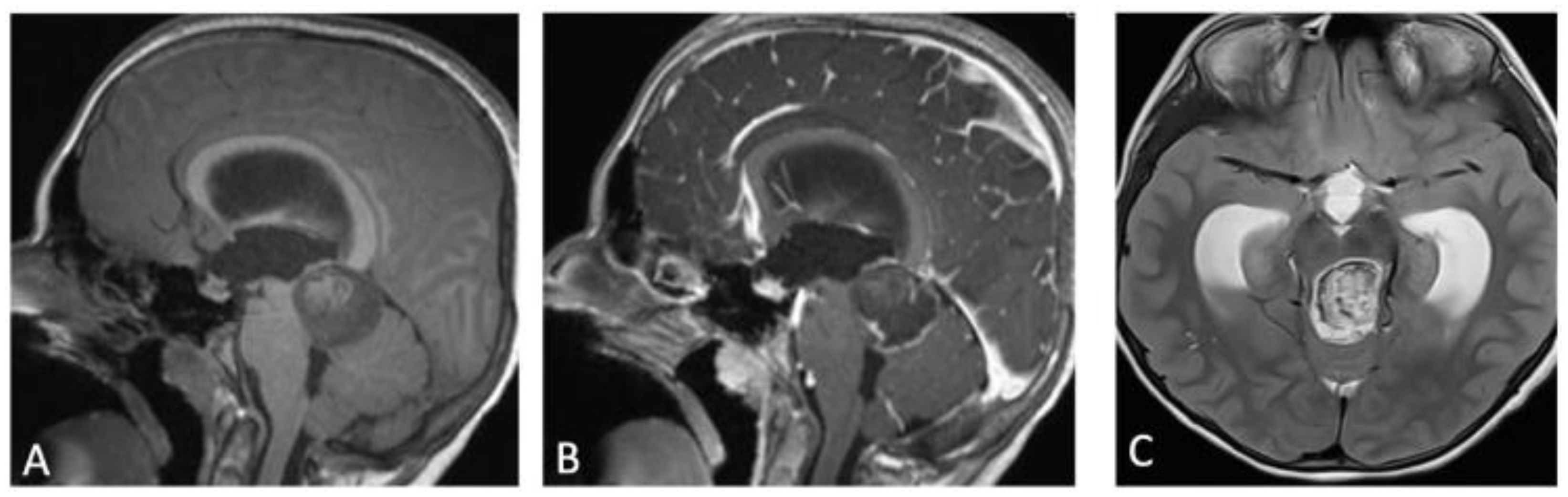
2. Case Presentation
3. Operative Technique
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dodgshun, A.J.; Maixner, W.J.; Hansford, J.R.; Sullivan, M.J. Low rates of recurrence and slow progression of pediatric pilocytic astrocytoma after gross-total resection: Justification for reducing surveillance imaging. J. Neurosurg. Pediatr. 2016, 17, 569–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knight, J.; De Jesus, O. Pilocytic Astrocytoma; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK560614/ (accessed on 27 March 2022).
- Park, J.H.; Jung, N.; Kang, S.J.; Kim, H.S.; Kim, E.; Lee, H.J.; Jung, H.R.; Choe, M.; Shim, Y.J. Survival and Prognosis of Patients with Pilocytic Astrocytoma: A Single-Center Study. Brain Tumor Res. Treat. 2019, 7, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Lieu, A.S.; Hwang, S.L.; Howng, S.L.; Chai, C.Y. Brain tumors with hemorrhage. J. Formos. Med. Assoc. 1999, 98, 365–367. [Google Scholar] [PubMed]
- White, J.B.; Piepgras, D.G.; Scheithauer, B.W.; Parisi, J.E. Rate of spontaneous hemorrhage in histologically proven cases of pilocytic astrocytoma. J. Neurosurg. 2008, 108, 223–226. [Google Scholar] [CrossRef] [Green Version]
- Voigt, K.; Yaşargil, M.G. Cerebral cavernous haemangiomas or cavernomas. Incidence, pathology, localization, diagnosis, clinical features and treatment. Review of the literature and report of an unusual case. Neurochirurgia 1976, 19, 59–68. [Google Scholar] [CrossRef]
- Vishteh, A.G.; David, C.A.; Marciano, F.F.; Coscarella, E.; Spetzler, R.F. Extreme lateral supracerebellar infratentorial approach to the posterolateral mesencephalon: Technique and clinical experience. Neurosurgery 2000, 46, 384–388, discussion 388–389. [Google Scholar] [CrossRef]
- Giammattei, L.; Starnoni, D.; Benes, V.; Froelich, S.; Cossu, G.; Borsotti, F.; Májovsky, M.; Sufianov, A.A.; Fava, A.; di Russo, P.; et al. Extreme Lateral Supracerebellar Infratentorial Approach: Surgical Anatomy and Review of the Literature. World Neurosurg. 2021, 147, 89–104. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Belouaer, A.; Starnoni, D.; Daniel, R.T. Extreme lateral supracerebellar infratentorial approach for tentorial arteriovenous fistula associated with a giant venous ectasia: How I do it. Acta Neurochir. 2021. [Google Scholar] [CrossRef]
- Klironomos, G.; Chiluwal, A.K.; Dehdashti, A.R. Technical Note: Extreme Lateral Supracerebellar Approach for Resection of Superior Cerebellar Peduncle Arteriovenous Malformations. Oper. Neurosurg. 2021, 20, E334–E339. [Google Scholar] [CrossRef]
- Matsushima, K.; Yagmurlu, K.; Kohno, M.; Rhoton, A.L. Anatomy and approaches along the cerebellar-brainstem fissures. J. Neurosurg. 2016, 124, 248–263. [Google Scholar] [CrossRef] [Green Version]
- Sievert, A.J.; Fisher, M.J. Pediatric low-grade gliomas. J. Child Neurol. 2009, 24, 1397–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beneš, V.; Zápotocký, M.; Libý, P.; Táborský, J.; Blažková, J.; Blažková, J.; Sumerauer, D.; Mišove, A.; Perníková, I.; Kynčl, M.; et al. Survival and functional outcomes in paediatric thalamic and thalamopeduncular low grade gliomas. Acta Neurochir. 2022. [Google Scholar] [CrossRef] [PubMed]
- Prasad, G.L.; Nandeesh, B.N.; Menon, G.R. Hemorrhagic presentation of intracranial pilocytic astrocytomas: Literature review. Neurosurg. Rev. 2019, 42, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Donofrio, C.A.; Gagliardi, F.; Callea, M.; da Passano, C.F.; Terreni, M.R.; Cavalli, A.; Spina, A.; Acerno, S.; Bailo, M.; Elbabaa, S.K.; et al. Pediatric cerebellar pilocytic astrocytoma presenting with spontaneous intratumoral hemorrhage. Neurosurg. Rev. 2020, 43, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Tomita, T. Surgical management of cerebellar peduncle lesions in children. Neurosurgery 1986, 18, 568–575. [Google Scholar] [CrossRef]
- Rady, M.R.; Enayet, A.E.; Refaat, A.; Taha, H.; Said, W.; Maher, E.; Beltagy, M.A.E. Management and outcome of pediatric brainstem and cerebellar peduncular low-grade gliomas: A retrospective analysis of 62 cases. Childs Nerv. Syst. 2022, 38, 565–575. [Google Scholar] [CrossRef]
- Sie, M.; de Bont, E.S.J.M.; Scherpen, F.J.G.; Hoving, E.W.; den Dunnen, W.F.A. Tumour vasculature and angiogenic profile of paediatric pilocytic astrocytoma; is it much different from glioblastoma? Neuropathol. Appl. Neurobiol. 2010, 36, 636–647. [Google Scholar] [CrossRef]
- Shibahara, I.; Kanamori, M.; Kumabe, T.; Endo, H.; Sonoda, Y.; Ogawa, Y.; Watanabe, M.; Tominaga, T. Hemorrhagic onset of pilocytic astrocytoma and pilomyxoid astrocytoma. Brain Tumor Pathol. 2009, 26, 1–5. [Google Scholar] [CrossRef]
- Baran, O.; Baydin, S.; Mirkhasilova, M.; Bayramli, N.; Bilgin, B.; Middlebrooks, E.; Ozlen, F.; Tanriover, N. Microsurgical anatomy and surgical exposure of the cerebellar peduncles. Neurosurg. Rev. 2022. [Google Scholar] [CrossRef]
- Malcolm, B. Carpenter, Core Text of Neuroanatomy, 4th ed.; Williams & Wilkins: Philadelphia, PA, USA, 1991; Chapter 8; pp. 239–242. [Google Scholar]
- Kim, M.-S.; Tak, H.J.; Son, S.M. Recovery of cerebellar peduncle injury in a patient with a cerebellar tumor: Validation by diffusion tensor tractography. Neural Regen. Res. 2014, 9, 1929–1932. [Google Scholar] [CrossRef]
- Rhoton, A.L. Tentorial incisura. Neurosurgery 2000, 47, S131–S153. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, C.; Raper, D.M.S.; Rubio, R.R.; Winkler, E.A.; Abla, A.A. Supracerebellar Infratentorial Infratrochlear Trans-Quadrangular Lobule Approach to Pontine Cavernous Malformations. Oper. Neurosurg. 2021, 20, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Hopf, N.J.; Perneczky, A. Endoscopic neurosurgery and endoscope-assisted microneurosurgery for the treatment of intracranial cysts. Neurosurgery 1998, 43, 1330–1336, discussion 1336–1337. [Google Scholar] [CrossRef] [PubMed]
- el Beltagy, M.A.; Atteya, M.M.E. Benefits of endoscope-assisted microsurgery in the management of pediatric brain tumors. Neurosurg. Focus 2021, 50, E7. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadimitriou, K.; Cossu, G.; Hewer, E.; Diezi, M.; Daniel, R.T.; Messerer, M. Endoscope-Assisted Extreme Lateral Supracerebellar Infratentorial Approach for Resection of Superior Cerebellar Peduncle Pilocytic Astrocytoma: Technical Note. Children 2022, 9, 640. https://doi.org/10.3390/children9050640
Papadimitriou K, Cossu G, Hewer E, Diezi M, Daniel RT, Messerer M. Endoscope-Assisted Extreme Lateral Supracerebellar Infratentorial Approach for Resection of Superior Cerebellar Peduncle Pilocytic Astrocytoma: Technical Note. Children. 2022; 9(5):640. https://doi.org/10.3390/children9050640
Chicago/Turabian StylePapadimitriou, Kyriakos, Giulia Cossu, Ekkehard Hewer, Manuel Diezi, Roy Thomas Daniel, and Mahmoud Messerer. 2022. "Endoscope-Assisted Extreme Lateral Supracerebellar Infratentorial Approach for Resection of Superior Cerebellar Peduncle Pilocytic Astrocytoma: Technical Note" Children 9, no. 5: 640. https://doi.org/10.3390/children9050640
APA StylePapadimitriou, K., Cossu, G., Hewer, E., Diezi, M., Daniel, R. T., & Messerer, M. (2022). Endoscope-Assisted Extreme Lateral Supracerebellar Infratentorial Approach for Resection of Superior Cerebellar Peduncle Pilocytic Astrocytoma: Technical Note. Children, 9(5), 640. https://doi.org/10.3390/children9050640





