A Simple Flow Injection Analysis–Tandem Mass Spectrometry Method to Reduce False Positives of C5-Acylcarnitines Due to Pivaloylcarnitine Using Reference Ions †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Participants
2.2. Regents
2.3. Sample Preparation Procedure
2.4. Mass Spectrometric Analysis
3. Results
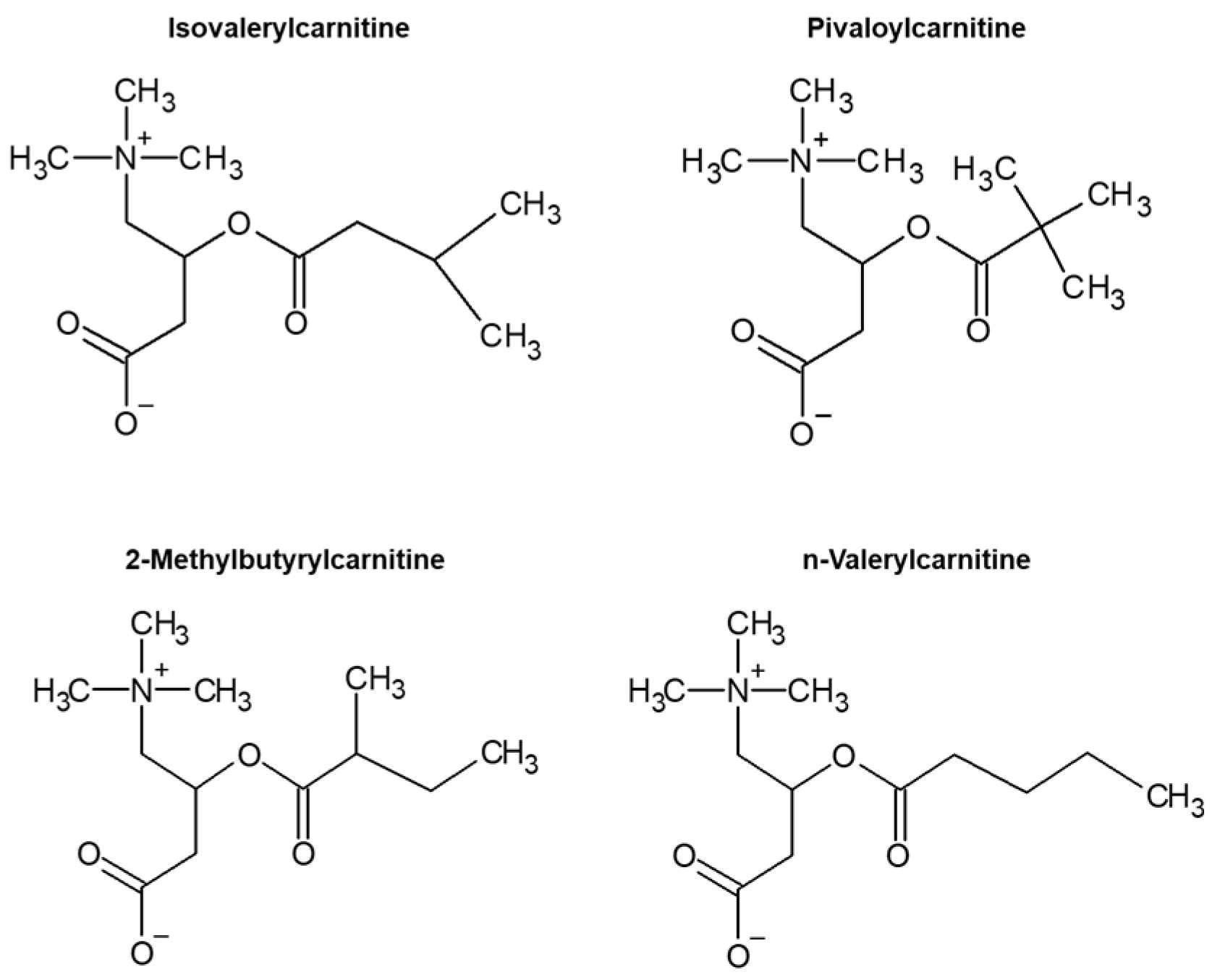
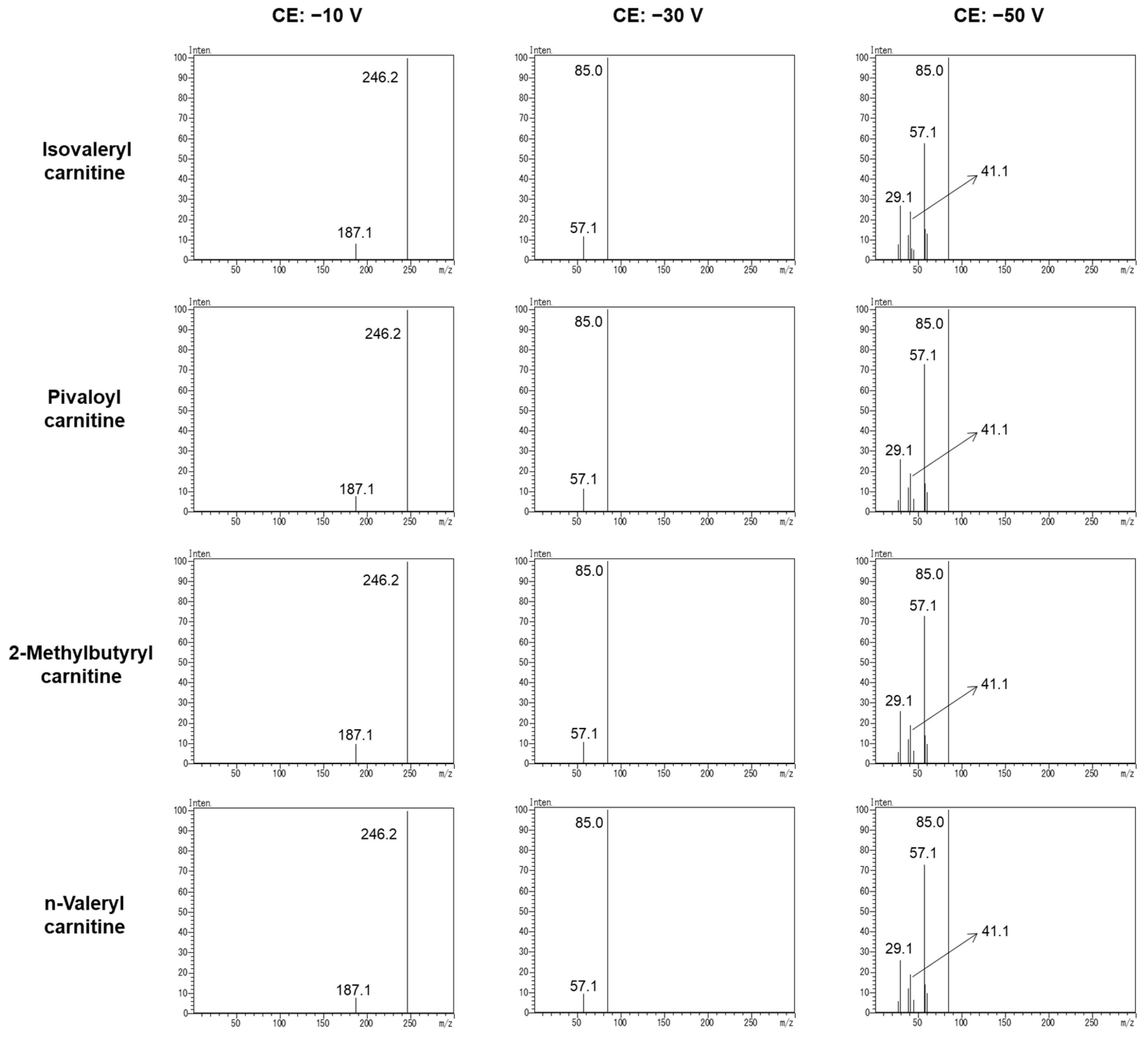
3.1. Specific Product Ion of C5-Acylcarnitine
3.2. Reference Ion Ratio
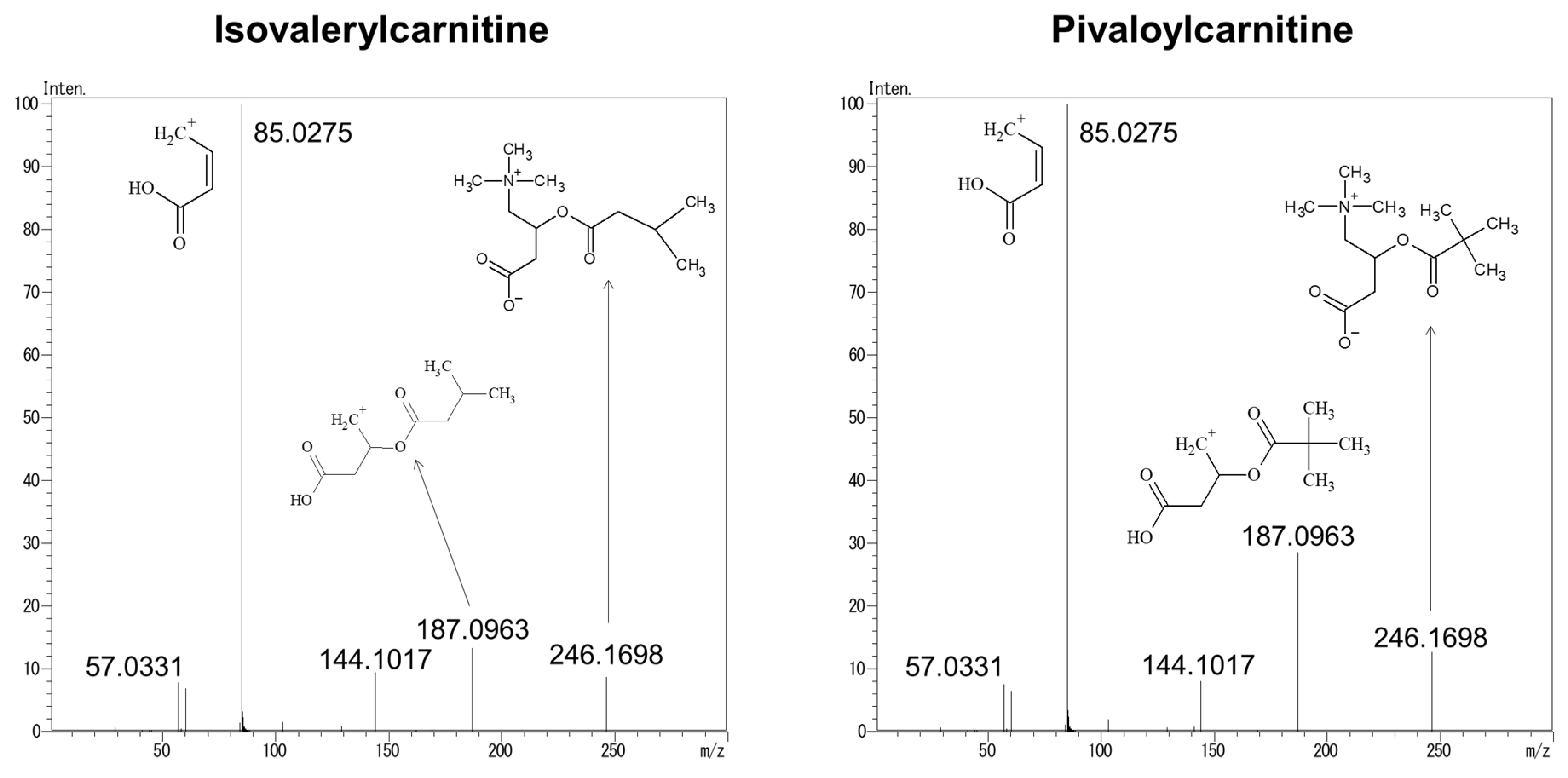
3.3. Structural Analysis of Product Ions Using LC–QTOFMS
3.4. Identification of i-C5 and p-C5 in DBSs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mak, C.M.; Lee, H.C.; Chan, A.Y.; Lam, C.W. Inborn errors of metabolism and expanded newborn screening: Review and update. Crit. Rev. Clin. Lab. Sci. 2013, 50, 142–162. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.M.; Chien, Y.H.; Chiang, C.C.; Ho, H.C.; Hwu, W.L.; Kao, S.M.; Chiang, S.H.; Kao, C.H.; Liu, T.T.; Chiang, H.; et al. Nationwide survey of extended newborn screening by tandem mass spectrometry in Taiwan. J. Inherit. Metab. Dis. 2010, 33, S295–S305. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, F. Updates in Newborn Screening. Pediatric Ann. 2018, 47, e187–e190. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.H.; Matern, D. Acylcarnitine analysis by tandem mass spectrometry. Curr. Protoc. Hum. Genet. 2010, 64, 17.8.1–17.8.20. [Google Scholar] [CrossRef]
- Vilarinho, L.; Rocha, H.; Sousa, C.; Marcão, A.; Fonseca, H.; Bogas, M.; Osório, R.V. Four years of expanded newborn screening in Portugal with tandem mass spectrometry. J. Inherit. Metab. Dis. 2010, 33, 133–138. [Google Scholar] [CrossRef]
- Wilcken, B.; Haas, M.; Joy, P.; Wiley, V.; Bowling, F.; Carpenter, K.; Christodoulou, J.; Cowley, D.; Ellaway, C.; Fletcher, J.; et al. Expanded newborn screening: Outcome in screened and unscreened patients at age 6 years. Pediatrics 2009, 124, e241–e248. [Google Scholar] [CrossRef]
- Tanaka, K.; Budd, M.A.; Efron, M.L.; Isselbacher, K.J. Isovaleric acidemia: A new genetic defect of leucine metabolism. Proc. Natl. Acad. Sci. USA 1966, 56, 236–242. [Google Scholar] [CrossRef] [Green Version]
- Vockley, J.; Ensenauer, R. Isovaleric acidemia: New aspects of genetic and phenotypic heterogeneity. Am. J. Med. Genet. Part C Semin. Med. Genet. 2006, 142C, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Ensenauer, R.; Fingerhut, R.; Maier, E.M.; Polanetz, R.; Olgemöller, B.; Röschinger, W.; Muntau, A.C. Newborn screening for isovaleric acidemia using tandem mass spectrometry: Data from 1.6 million newborns. Clin. Chem. 2011, 57, 623–626. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Chen, D.; Peng, W.; Wang, K.; Lin, W.; Zhuang, J.; Zheng, Z.; Li, M.; Fu, Q. Newborn screening for isovaleric acidemia in Quanzhou, China. Clin. Chim. Acta 2020, 509, 25–29. [Google Scholar] [CrossRef]
- Schlune, A.; Riederer, A.; Mayatepek, E.; Ensenauer, R. Aspects of Newborn Screening in Isovaleric Acidemia Amino acid and acylcarnitine analysis in dried blood spots (DBS) using flow injection analysis-tandem mass spectrometry (FIA-TMS) is widely used in NBS. Int. J. Neonatal Screen. 2018, 4, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Calcar, S.C.; Baker, M.W.; Williams, P.; Jones, S.A.; Xiong, B.; Thao, M.C.; Lee, S.; Yang, M.K.; Rice, G.M.; Rhead, W.; et al. Prevalence and mutation analysis of short/branched chain acyl-CoA dehydrogenase deficiency (SBCADD) detected on newborn screening in Wisconsin. Mol. Genet. Metab. 2013, 110, 111–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Calcar, S.C.; Gleason, L.A.; Lindh, H.; Hoffman, G.; Rhead, W.; Vockley, G.; Wolff, J.A.; Durkin, M.S. 2-methylbutyryl-CoA dehydrogenase deficiency in Hmong infants identified by expanded newborn screen. WMJ 2007, 106, 12–15. [Google Scholar] [PubMed]
- Maeda, Y.; Ito, T.; Suzuki, A.; Kurono, Y.; Ueta, A.; Yokoi, K.; Sumi, S.; Togari, H.; Sugiyama, N. Simultaneous quantification of acylcarnitine isomers containing dicarboxylic acylcarnitines in human serum and urine by high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 799–806. [Google Scholar] [CrossRef]
- Abdenur, J.E.; Chamoles, N.A.; Guinle, A.E.; Schenone, A.B.; Fuertes, A.N. Diagnosis of isovaleric acidaemia by tandem mass spectrometry: False positive result due to pivaloylcarnitine in a newborn screening programme. J. Inherit. Metab. Dis. 1998, 21, 624–630. [Google Scholar] [CrossRef]
- Boemer, F.; Schoos, R.; de Halleux, V.; Kalenga, M.; Debray, F.G. Surprising causes of C5-carnitine false positive results in newborn screening. Mol. Genet. Metab. 2014, 111, 52–54. [Google Scholar] [CrossRef]
- Bonham, J.R.; Carling, R.S.; Lindner, M.; Franzson, L.; Zetterstrom, R.; Boemer, F.; Cerone, R.; Eyskens, F.; Vilarinho, L.; Hougaard, D.M.; et al. Raising Awareness of False Positive Newborn Screening Results Arising from Pivalate-Containing Creams and Antibiotics in Europe When Screening for Isovaleric Acidaemia. Int. J. Neonatal Screen. 2018, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Fernández Ruano, M.L.; Besga García, B.; Montero Plata, A.; Dulín Íñiguez, E. False positive on neonatal screening: C5-carnitin increase in newborns due to pre-labour treatment with cefditoren pivoxil. Rev. Esp. Salud Publica 2018, 92, e201806025. [Google Scholar]
- Malvagia, S.; Forni, G.; Ombrone, D.; la Marca, G. Development of Strategies to Decrease False Positive Results in Newborn Screening. Int. J. Neonatal Screen. 2020, 6, 84. [Google Scholar] [CrossRef]
- Yamada, K.; Kobayashi, H.; Bo, R.; Takahashi, T.; Hasegawa, Y.; Nakamura, M.; Ishige, N.; Yamaguchi, S. Elevation of pivaloylcarnitine by sivelestat sodium in two children. Mol. Genet. Metab. 2015, 116, 192–194. [Google Scholar] [CrossRef]
- Gurian, E.A.; Kinnamon, D.D.; Henry, J.J.; Waisbren, S.E. Expanded newborn screening for biochemical disorders: The effect of a false-positive result. Pediatrics 2006, 117, 1915–1921. [Google Scholar] [CrossRef] [PubMed]
- Hewlett, J.; Waisbren, S.E. A review of the psychosocial effects of false-positive results on parents and current communication practices in newborn screening. J. Inherit. Metab. Dis. 2006, 29, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.J.; He, J.; Chen, H.; Shi, X.D.; Li, Y. Psychological effects of false-positive results in expanded newborn screening in China. PLoS ONE 2012, 7, e36235. [Google Scholar]
- Waisbren, S.E.; Albers, S.; Amato, S.; Ampola, M.; Brewster, T.G.; Demmer, L.; Eaton, R.B.; Greenstein, R.; Korson, M.; Larson, C.; et al. Effect of expanded newborn screening for biochemical genetic disorders on child outcomes and parental stress. JAMA 2003, 290, 2564–2572. [Google Scholar] [CrossRef] [Green Version]
- Carling, R.S.; Burden, D.; Hutton, I.; Randle, R.; John, K.; Bonham, J.R. Introduction of a Simple Second Tier Screening Test for C5 Isobars in Dried Blood Spots: Reducing the False Positive Rate for Isovaleric Acidaemia in Expanded Newborn Screening. JIMD Rep. 2018, 38, 75–80. [Google Scholar]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Cloppenborg, T.; Janzen, N.; Wagner, H.; Steuerwald, U.; Peter, M.; Das, A. Application of a second-tier newborn screening assay for c5 isoforms. JIMD Rep. 2014, 13, 23–26. [Google Scholar]
- Forni, S.; Fu, X.; Palmer, S.E.; Sweetman, L. Rapid determination of C4-acylcarnitine and C5-acylcarnitine isomers in plasma and dried blood spots by UPLC-MS/MS as a second tier test following flow-injection MS/MS acylcarnitine profile analysis. Mol. Genet. Metab. 2010, 101, 25–32. [Google Scholar] [CrossRef]
- Janzen, N.; Steuerwald, U.; Sander, S.; Terhardt, M.; Peter, M.; Sander, J. UPLC-MS/MS analysis of C5-acylcarnitines in dried blood spots. Clin. Chim. Acta 2013, 421, 41–45. [Google Scholar] [CrossRef]
- Minkler, P.E.; Stoll, M.S.K.; Ingalls, S.T.; Hoppel, C.L. Selective and accurate C5 acylcarnitine quantitation by UHPLC-MS/MS: Distinguishing true isovaleric acidemia from pivalate derived interference. J. Chromatogr. B 2017, 1061–1062, 128–133. [Google Scholar] [CrossRef]
- Poggiali, S.; Ombrone, D.; Forni, G.; Malvagia, S.; Funghini, S.; Mura, M.; Pasquini, E.; Santoro, L.; Bellavia, V.; Granata, O.M.; et al. Reducing the False-Positive Rate for Isovalerylcarnitine in Expanded Newborn Screening: The Application of a Second-Tier Test. J. Inborn Errors Metab. Screen. 2016, 4, 2326409816661355. [Google Scholar] [CrossRef] [Green Version]
- Shigematsu, Y.; Hata, I.; Tajima, G. Useful second-tier tests in expanded newborn screening of isovaleric acidemia and methylmalonic aciduria. J. Inherit. Metab. Dis. 2010, 33, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Shigematsu, Y.; Hata, I.; Tanaka, Y. Stable-isotope dilution measurement of isovalerylglycine by tandem mass spectrometry in newborn screening for isovaleric acidemia. Clin. Chim. Acta 2007, 389, 82–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, Y.; Hakajima, Y.; Yamaguchi, T.; Ito, T. Second-tier test in newborn screening to discriminate C5-acylcarnitines using carnitine esterase and flow-injection tandem mass spectrometry. Jpn. J. Neonatal Screen. 2020, 30, 35–41. [Google Scholar]

| Case | % of Total C5-Acylcarnitine Isomers | C5 Conc. (μmol/L) | i-C5 Score | p-C5 Score | |||
|---|---|---|---|---|---|---|---|
| i-C5 | p-C5 | m-C5 | v-C5 | ||||
| Samples from cases who had pivalate-containing antibiotics | |||||||
| 1 | 12.2 ± 1.1 | 79.7 ± 1.4 | 8.1 ± 0.7 | 0 | 0.66 ± 0.01 | 62.5 ± 5.5 | 97.1 ± 2.3 |
| 2 | 9.6 ± 0.5 | 90.4 ± 2.7 | 0 | 0 | 0.96 ± 0.02 | 37.1 ± 5.7 | 82.0 ± 4.1 |
| 3 | 0 | 100 | 0 | 0 | 1.31 ± 0.01 | 57.9 ± 6.7 | 96.5 ± 4.4 |
| 4 | 0 | 100 | 0 | 0 | 1.33 ± 0.01 | 58.8 ± 2.1 | 97.7 ± 1.5 |
| 5 | 0 | 100 | 0 | 0 | 1.37 ± 0.01 | 50.0 ± 2.4 | 91.3 ± 1.8 |
| 6 | 0 | 100 | 0 | 0 | 1.64 ± 0.01 | 55.6 ± 0.5 | 95.4 ± 0.4 |
| 7 | 0 | 100 | 0 | 0 | 1.67 ± 0.01 | 55.3 ± 1.8 | 95.2 ± 1.3 |
| 8 | 0 | 100 | 0 | 0 | 1.91 ± 0.02 | 55.6 ± 3.3 | 95.4 ± 2.4 |
| 9 | 0 | 100 | 0 | 0 | 3.73 ± 0.03 | 58.5 ± 1.3 | 97.5 ± 0.9 |
| 10 | 0 | 100 | 0 | 0 | 3.84 ± 0.03 | 41.7 ± 1.5 | 85.3 ± 1.1 |
| 11 | 0 | 100 | 0 | 0 | 10.98 ± 0.08 | 60.9 ± 0.9 | 99.2 ± 0.5 |
| Samples from cases with isovaleric acidemia | |||||||
| 12 | 100 | 0 | 0 | 0 | 2.57 ± 0.02 | 91.1 ± 1.9 | 78.9 ± 1.4 |
| 13 | 100 | 0 | 0 | 0 | 2.67 ± 0.03 | 93.6 ± 4.7 | 77.1 ± 3.4 |
| 14 | 100 | 0 | 0 | 0 | 5.31 ± 0.04 | 97.3 ± 1.7 | 73.4 ± 2.3 |
| 15 | 99.9 ± 7.0 | 0.1 ± 0.0 | 0 | 0 | 13.72 ± 0.10 | 99.0 ± 0.5 | 72.9 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hattori, T.; Notsu, Y.; Tanaka, M.; Matsui, M.; Iida, T.; Watanabe, J.; Osawa, Y.; Yamaguchi, S.; Yano, S.; Taketani, T.; et al. A Simple Flow Injection Analysis–Tandem Mass Spectrometry Method to Reduce False Positives of C5-Acylcarnitines Due to Pivaloylcarnitine Using Reference Ions. Children 2022, 9, 694. https://doi.org/10.3390/children9050694
Hattori T, Notsu Y, Tanaka M, Matsui M, Iida T, Watanabe J, Osawa Y, Yamaguchi S, Yano S, Taketani T, et al. A Simple Flow Injection Analysis–Tandem Mass Spectrometry Method to Reduce False Positives of C5-Acylcarnitines Due to Pivaloylcarnitine Using Reference Ions. Children. 2022; 9(5):694. https://doi.org/10.3390/children9050694
Chicago/Turabian StyleHattori, Takanari, Yoshitomo Notsu, Misa Tanaka, Miki Matsui, Tetsuo Iida, Jun Watanabe, Yoshimitsu Osawa, Seiji Yamaguchi, Shozo Yano, Takeshi Taketani, and et al. 2022. "A Simple Flow Injection Analysis–Tandem Mass Spectrometry Method to Reduce False Positives of C5-Acylcarnitines Due to Pivaloylcarnitine Using Reference Ions" Children 9, no. 5: 694. https://doi.org/10.3390/children9050694






