ALARA in Pediatric Electrophysiology Laboratory
Abstract
:1. Introduction
2. Background
3. Historical Landmarks
4. Strategies
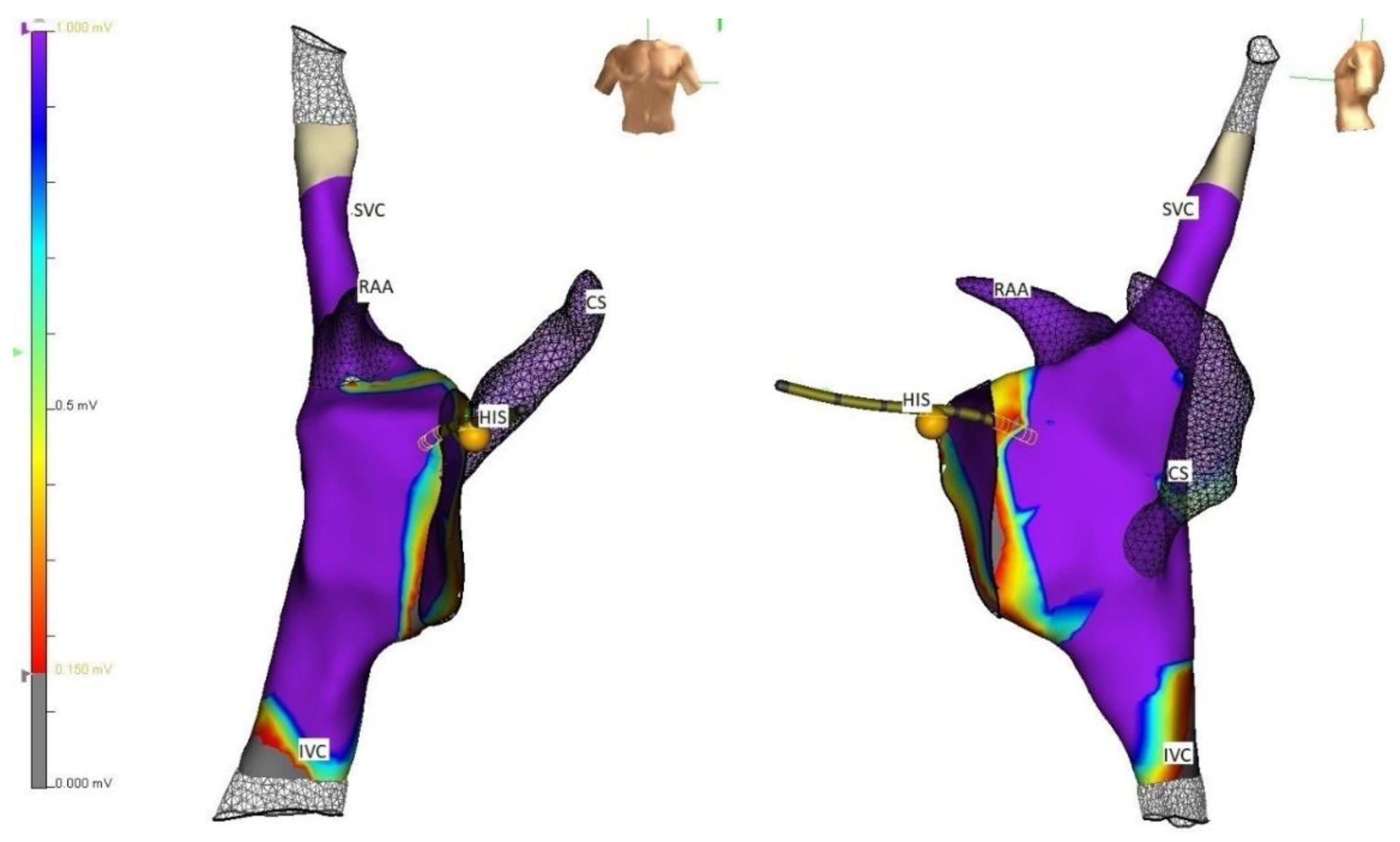
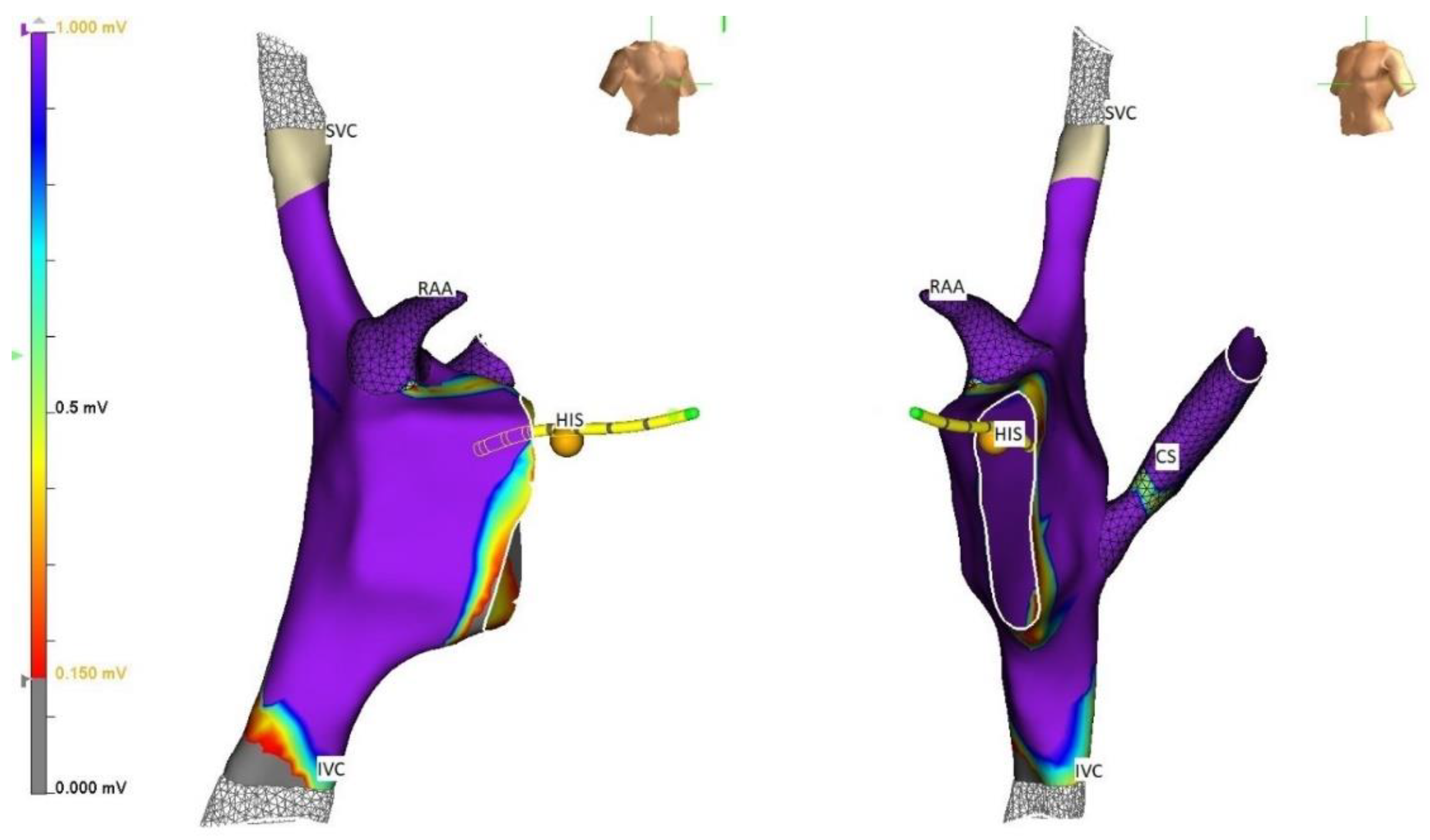
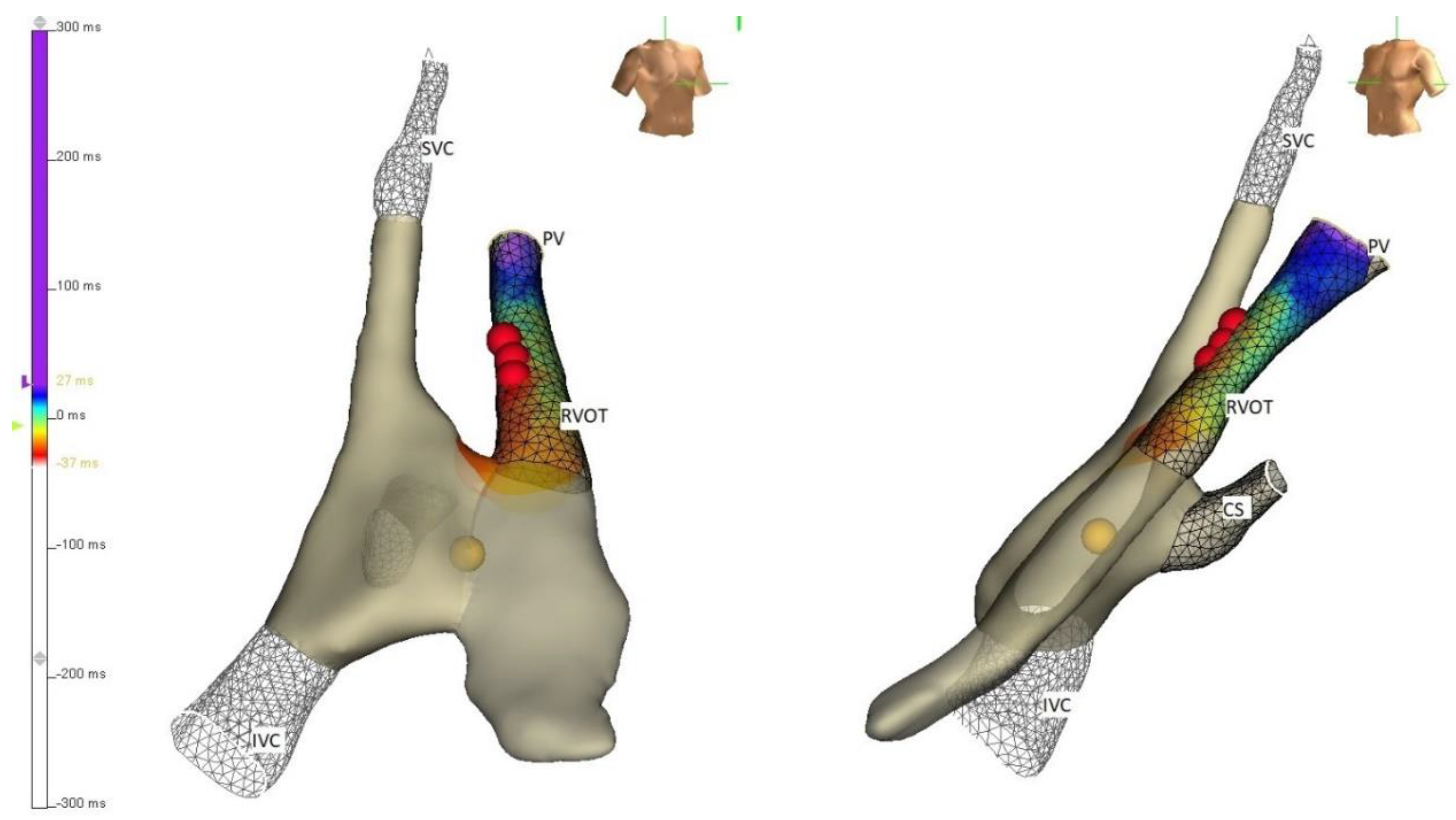
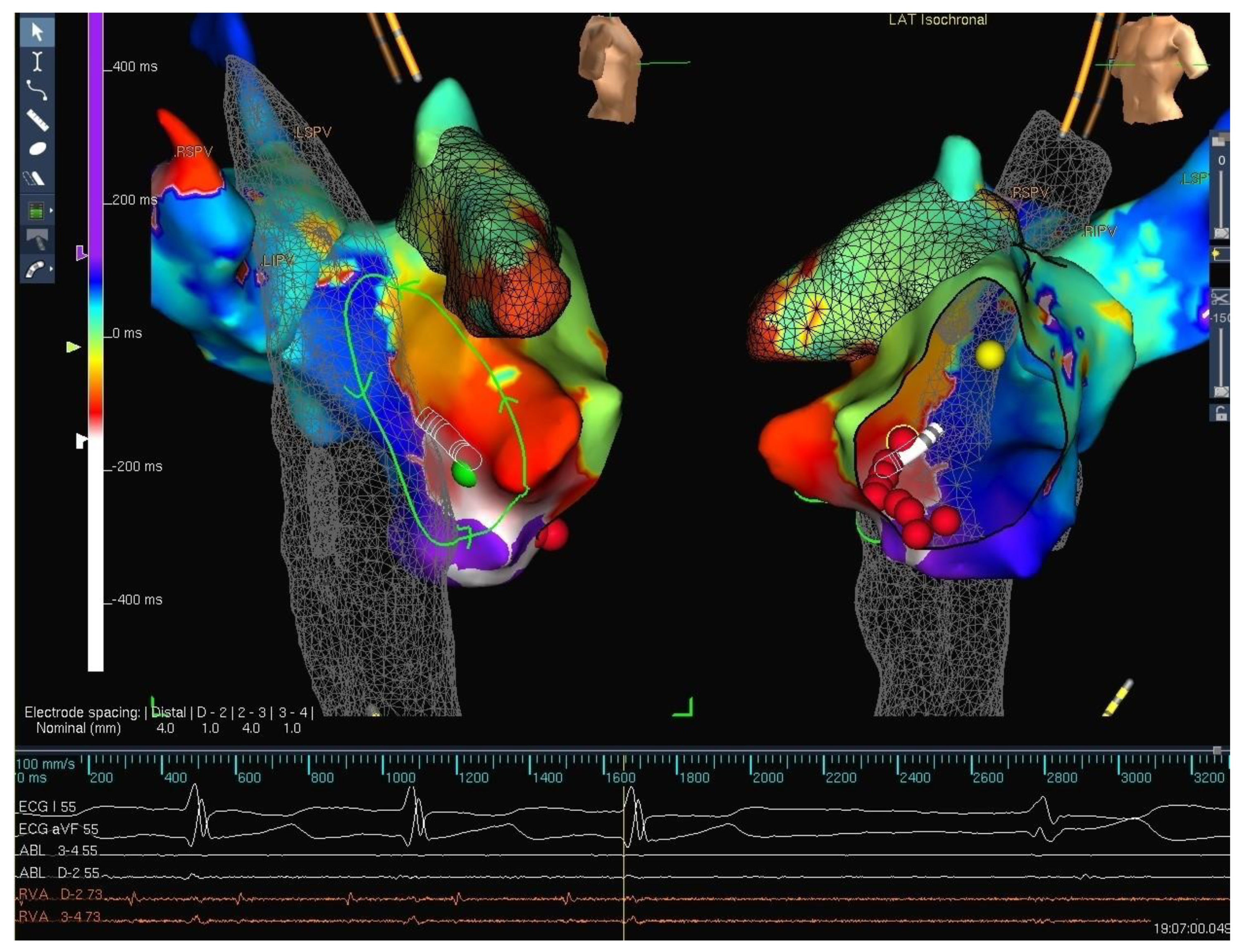
5. Techniques
6. Outcomes
7. Adverse Effects
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klein, L.W.; Miller, D.L.; Balter, S.; Laskey, W.; Haines, D.; Norbash, A.; Mauro, M.A.; Goldstein, J.A. Occupational health hazards in the interventional laboratory: Time for a safer environment. Radiology 2009, 250, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.P.; Miller, D.L. Minimising radiation exposure to physicians performing fluoroscopically guided cardiac catheterisation procedures: A review. Radiat. Prot. Dosim. 2009, 133, 227–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, T.; Rothschild, M., IV. The excitatory process in the dog’s heart. Part II.-The ventricles. Philos. Trans. R. Soc. London. Ser. B Contain. Pap. A Biol. Character 1915, 206, 181–226. [Google Scholar]
- Gepstein, L.; Hayam, G.; Ben-Haim, S.A. A novel method for nonfluoroscopic catheter-based electroanatomical mapping of the heart: In vitro and in vivo accuracy results. Circulation 1997, 95, 1611–1622. [Google Scholar] [CrossRef]
- Smeets, J.L.; Ben-Haim, S.A.; Rodriguez, L.-M.; Timmermans, C.; Wellens, H.J. New method for nonfluoroscopic endocardial mapping in humans: Accuracy assessment and first clinical results. Circulation 1998, 97, 2426–2432. [Google Scholar] [CrossRef] [Green Version]
- Drago, F.; Silvetti, M.S.; Di Pino, A.; Grutter, G.; Bevilacqua, M.; Leibovich, S. Exclusion of fluoroscopy during ablation treatment of right accessory pathway in children. J. Cardiovasc. Electrophysiol. 2002, 13, 778–782. [Google Scholar] [CrossRef]
- Smith, G.; Clark, J.M. Elimination of fluoroscopy use in a pediatric electrophysiology laboratory utilizing three-dimensional mapping. Pacing Clin. Electrophysiol. 2007, 30, 510–518. [Google Scholar] [CrossRef]
- Bharmanee, A.; Gowda, S.; Singh, H.R. Feasibility, accuracy, and safety of 3-dimensional electroanatomic mapping without fluoroscopy in patients with congenital heart defects. Heart Rhythm. 2016, 13, 1667–1673. [Google Scholar] [CrossRef]
- Chambers, E.C.; Fetterly, K.A.; Holzer, R.; Paul Lin, P.J.; Blankenship, J.C.; Balter, S.; Laskey, W.K. Radiation safety program for the cardiac catheterization laboratory. Catheter. Cardiovasc. Interv. 2011, 77, 546–556. [Google Scholar] [CrossRef]
- Cardiac Rhythm News. Biosense Webster Launches Latest Generation of Carto Heart Mapping System. Available online: https://cardiacrhythmnews.com/biosense-webster-launches-latest-generation-of-carto-heart-mapping-system/ (accessed on 6 January 2022).
- Abbott. Ensite Precision Cardiac Mapping System. Available online: https://www.cardiovascular.abbott/us/en/hcp/products/electrophysiology/mapping-systems/ensite/about/how-it-works.html (accessed on 6 January 2022).
- Boston Scientific. Rhythmia HDx Mapping System. Available online: https://www.bostonscientific.com/content/dam/bostonscientific/ep/portfolio-group/EP%20Systems/Mapping%20Systems/RHYTHMIA/RHYTHMIA%20HDx/EP-436505-AD+Rhythmia+HDx+Brochure_NEW-FINAL.PDF (accessed on 6 January 2022).
- Schwagten, B.; Jordaens, L.; Witsenburg, M.; Duplessis, F.; Thornton, A.; Van Belle, Y.; Szili-Torok, T. Initial experience with catheter ablation using remote magnetic navigation in adults with complex congenital heart disease and in small children. Pacing Clin. Electrophysiol. 2009, 32, S198–S201. [Google Scholar] [CrossRef]
- Clark, B.C.; Sumihara, K.; Berul, C.I.; Moak, J.P. Off the pedal: Fluoroless transseptal puncture in pediatric supraventricular tachycardia ablation. Pacing Clin. Electrophysiol. 2017, 40, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Baykaner, T.; Quadros, K.K.; Thosani, A.; Yasmeh, B.; Mitra, R.; Liu, E.; Belden, W.; Liu, Z.; Costea, A.; Brodt, C.R. Safety and efficacy of zero fluoroscopy transseptal puncture with different approaches. Pacing Clin. Electrophysiol. 2020, 43, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.; Bockoven, J.; Lane, J.; Patel, C.; Smith, G. Use of three-dimensional catheter guidance and trans-esophageal echocardiography to eliminate fluoroscopy in catheter ablation of left-sided accessory pathways. Pacing Clin. Electrophysiol. 2008, 31, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Miyake, C.Y.; Mah, D.Y.; Atallah, J.; Oikle, H.P.; Melgar, M.L.; Alexander, M.E.; Berul, C.I.; Cecchin, F.; Walsh, E.P.; Triedman, J.K. Nonfluoroscopic imaging systems reduce radiation exposure in children undergoing ablation of supraventricular tachycardia. Heart Rhythm. 2011, 8, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Papagiannis, J.; Avramidis, D.; Alexopoulos, C.; Kirvassilis, G. Radiofrequency ablation of accessory pathways in children and congenital heart disease patients: Impact of a nonfluoroscopic navigation system. Pacing Clin. Electrophysiol. 2011, 34, 1288–1396. [Google Scholar] [CrossRef]
- Tuzcu, V. Significant reduction of fluoroscopy in pediatric catheter ablation procedures: Long-term experience from a single center. Pacing Clin. Electrophysiol. 2012, 35, 1067–1073. [Google Scholar] [CrossRef]
- Mah, D.Y.; Miyake, C.Y.; Sherwin, E.D.; Walsh, A.; Anderson, M.J.; Western, K.; Abrams, D.J.; Alexander, M.E.; Cecchin, F.; Walsh, E.P. The use of an integrated electroanatomic mapping system and intracardiac echocardiography to reduce radiation exposure in children and young adults undergoing ablation of supraventricular tachycardia. Europace 2014, 16, 277–283. [Google Scholar] [CrossRef] [Green Version]
- Papagiannis, J.; Tsoutsinos, A.; Kirvassilis, G.; Sofianidou, I.; Koussi, T.; Laskari, C.; Kiaffas, M.; Apostolopoulou, S.; Rammos, S. Nonfluoroscopic catheter navigation for radiofrequency catheter ablation of supraventricular tachycardia in children. Pacing Clin. Electrophysiol. 2006, 29, 971–978. [Google Scholar] [CrossRef]
- Pass, R.H.; Gates, G.G.; Gellis, L.A.; Nappo, L.; Ceresnak, S.R. Reducing patient radiation exposure during paediatric SVT ablations: Use of CARTO® 3 in concert with “ALARA” principles profoundly lowers total dose. Cardiol. Young 2015, 25, 963–968. [Google Scholar] [CrossRef]
- Nagaraju, L.; Menon, D.; Aziz, P.F. Use of 3D electroanatomical navigation (CARTO-3) to minimize or eliminate fluoroscopy use in the ablation of pediatric supraventricular tachyarrhythmias. Pacing Clin. Electrophysiol. 2016, 39, 574–580. [Google Scholar] [CrossRef]
- Gellis, L.A.; Ceresnak, S.R.; Gates, G.J.; Nappo, L.; Pass, R.H. Reducing patient radiation dosage during pediatric SVT ablations using an “ALARA” radiation reduction protocol in the modern fluoroscopic era. Pacing Clin. Electrophysiol. 2013, 36, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Bigelow, A.M.; Smith, P.C.; Timberlake, D.T.; McNinch, N.L.; Smith, G.L.; Lane, J.R.; Clark, J.M. Procedural outcomes of fluoroless catheter ablation outside the traditional catheterization lab. Ep Eur. 2017, 19, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Mar, P.L.; Chong, L.; Perez, A.; Lakkireddy, D.; Gopinathannair, R. Entrapment of diagnostic catheter within Advisor HD grid mapping catheter. J. Cardiovasc. Electrophysiol. 2021, 32, 860–861. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N.; Kobayashi, D.; Karpawich, P.P. Pulmonary damage following right ventricular outflow tachycardia ablation in a child: When electroanatomical mapping isn’t good enough. Pacing Clin. Electrophysiol. 2018, 41, 561–565. [Google Scholar] [CrossRef]
- Saul, J.P.; Kanter, R.J.; Abrams, D.; Asirvatham, S.; Bar-Cohen, Y.; Blaufox, A.D.; Cannon, B.; Clark, J.; Dick, M.; Freter, A. PACES/HRS expert consensus statement on the use of catheter ablation in children and patients with congenital heart disease. Heart Rhythm. 2016, 13, e251–e289. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, H.R. ALARA in Pediatric Electrophysiology Laboratory. Children 2022, 9, 866. https://doi.org/10.3390/children9060866
Singh HR. ALARA in Pediatric Electrophysiology Laboratory. Children. 2022; 9(6):866. https://doi.org/10.3390/children9060866
Chicago/Turabian StyleSingh, Harinder R. 2022. "ALARA in Pediatric Electrophysiology Laboratory" Children 9, no. 6: 866. https://doi.org/10.3390/children9060866
APA StyleSingh, H. R. (2022). ALARA in Pediatric Electrophysiology Laboratory. Children, 9(6), 866. https://doi.org/10.3390/children9060866




