The Phenotypical Profile and Outcomes of Neonates with Congenital Tracheoesophageal Fistula Associated with Congenital Cardiac Anomalies Presenting for Surgery
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sfeir, R.; Bonnard, A.; Khen-Dunlop, N.; Auber, F.; Gelas, T.; Michaud, L.; Podevin, G.; Breton, A.; Fouquet, V.; Piolat, C.; et al. Esophageal atresia: Data from a national cohort. J. Pediatr. Surg. 2013, 48, 1664–1669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lal, D.R.; Gadepalli, S.K.; Downard, C.D.; Ostlie, D.J.; Minneci, P.C.; Swedler, R.M.; Chelius, T.; Cassidy, L.; Rapp, C.T.; Deans, K.J.; et al. Perioperative management and outcomes of esophageal atresia and tracheoesophageal fistula. J. Pediatr. Surg. 2017, 52, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Motshabi, P. Anaesthesia for oesophageal atresia with or without tracheo-oesophageal atresia. S. Afr. J. Anaesth. Analg. 2014, 20, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Diaz, L.K.; Akpek, E.A.; Dinavahi, R.; Andropoulos, D.B. Tracheoesophageal fistula and associated congenital heart disease: Implications for anesthetic management and survival. Pediatr. Anesth. 2005, 15, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Andropoulos, D.; Rowe, R.; Betts, J. Anaesthetic and surgical airway management during tracheo-oesophageal fistula repair. Pediatr. Anesth. 1998, 8, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Broemling, N.; Campbell, F. Anesthetic management of congenital tracheoesophageal fistula. Pediatr. Anesth. 2011, 21, 1092–1099. [Google Scholar] [CrossRef]
- Pedersen, R.N.; Calzolari, E.; Husby, S.; Garne, E. Oesophageal atresia: Prevalence, prenatal diagnosis and associated anomalies in 23 European regions. Arch. Dis. Child. 2012, 97, 227–232. [Google Scholar] [CrossRef]
- Waterston, D.; Carter, R.B.; Aberdeen, E. Oesophageal atresia: Tracheo-oesophageal fistula: A study of survival in 218 infants. Lancet 1962, 279, 819–822. [Google Scholar] [CrossRef]
- Spitz, L.; Kiely, E.; Morecroft, J.; Drake, D. Oesophageal atresia: At-risk groups for the 1990s. J. Pediatr. Surg. 1994, 29, 723–725. [Google Scholar] [CrossRef]
- Sulkowski, J.P.; Cooper, J.N.; Lopez, J.J.; Jadcherla, Y.; Cuenot, A.; Mattei, P.; Deans, K.J.; Minneci, P.C. Morbidity and mortality in patients with esophageal atresia. Surgery 2014, 156, 483–491. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, S.R.; Ashcraft, K.W.; Sharp, R.J.; Murphy, J.P.; Snyder, C.L.; Sigalet, D.L. Survival of patients with esophageal atresia: Influence of birth weight, cardiac anomaly, and late respiratory complications. J. Pediatr. Surg. 1999, 34, 70–74. [Google Scholar] [CrossRef]
- Wang, B.; Tashiro, J.; Allan, B.J.; Sola, J.E.; Parikh, P.P.; Hogan, A.R.; Neville, H.L.; Perez, E.A. A nationwide analysis of clinical outcomes among newborns with esophageal atresia and tracheoesophageal fistulas in the United States. J. Surg. Res. 2014, 190, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Reeves, S.T.; Burt, N.; Smith, C.D. Is it time to re-evaluate the airway management of tracheoesophageal fistula? Anesth. Analg. 1995, 81, 866–869. [Google Scholar] [CrossRef]
- Spitz, L. Esophageal atresia and tracheoesophageal fistula in children. Curr. Opin. Pediatr. 1993, 5, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Houska, N.; Twite, M.; Ing, R. The Importance of the Airway in Children Undergoing Surgery for Congenital Heart Disease. J. Cardiothorac. Vasc. Anesth. 2020, 35, 145–157. [Google Scholar] [CrossRef]
- Foz, C.; Peyton, J.; Staffa, S.J.; Kovatsis, P.; Park, R.; DiNardo, J.A.; Nasr, V.G. Airway Abnormalities in Patients with Congenital Heart Disease: Incidence and Associated Factors. J. Cardiothorac. Vasc. Anesth. 2021, 35, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Jones, M.O.; Couriel, J.M.; Losty, P.D. Oesophageal atresia and tracheo-oesophageal fistula. Arch. Dis. Child. Fetal. Neonatal. Ed. 2006, 91, F381–F384. [Google Scholar] [CrossRef]
- Hammer, G.B. Surgery. In A Practice of Anesthesia for Infants and Children, 6th ed.; Coté, C.J., Lerman, J., Anderson, B.J., Eds.; Elsevier: Philadelphia, PA, USA, 2019; pp. 340–354. [Google Scholar] [CrossRef]
- Zimmer, J.; Eaton, S.; Murchison, L.E.; De Coppi, P.; Ure, B.M.; Dingemann, C. State of Play: Eight Decades of Surgery for Esophageal Atresia. Eur. J. Pediatr. Surg. 2019, 29, 39–48. [Google Scholar] [CrossRef]
- Golianu, B.; Hammer, G.B. Pediatric thoracic anesthesia. Curr. Opin. Anaesthesiol. 2005, 18, 5–11. [Google Scholar] [CrossRef] [Green Version]
- White, M.C.; Peyton, J.M. Anaesthetic management of children with congenital heart disease for non-cardiac surgery. Contin. Educ. Anaesth. Crit. Care Pain 2012, 12, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Junghare, S.W.; Desurkar, V. Congenital heart diseases and anaesthesia. Indian J. Anaesth 2017, 61, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Knottenbelt, G.; Costi, D.; Stephens, P.; Beringer, R.; Davidson, A. An audit of anesthetic management and complications of trachea-oesophageal fistula and esophageal atresia repair. Pediatr. Anesth. 2012, 22, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Malakounides, G.; Lyon, P.; Cross, K.; Pierro, A.; De Coppi, P.; Drake, D.; Kiely, E.; Spitz, L.; Curry, J. Esophageal atresia: Improved outcome in high-risk groups revisited. Eur. J. Pediatr. Surg. 2016, 26, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Keefe, G.; Culbreath, K.; Edwards, E.M.; Morrow, K.A.; Soll, R.F.; Modi, B.P.; Horbar, J.D.; Jaksic, T. Current outcomes of infants with esophageal atresia and tracheoesophageal fistula: A multicentre analysis. J. Pediatr. Surg. 2022. [Google Scholar] [CrossRef]
- Vukadin, M.; Savic, D.; Malikovic, A.; Jovanovic, D.; Milickovic, M.; Bosnic, S.; Vlahovic, A. Analysis of Prognostic Factors and Mortality in Children with Esophageal Atresia. Indian J. Pediatr. 2015, 82, 586–590. [Google Scholar] [CrossRef]
- Schneider, D.J.; Moore, J.W. Patent ductus arteriosus. Circulation 2006, 114, 1873–1882. [Google Scholar] [CrossRef]
| Characteristic | n (%) | |
|---|---|---|
| Sex | Female | 17 (46.0%) |
| Male | 20 (54.0%) | |
| Gestational age (weeks), median (IQR) | Total | 36.5 (34.5–40) |
| <37 | 18 (48.7%) | |
| ≥37 | 18 (48.7%) | |
| Missing | 1 (2.7%) | |
| Age at surgery (days), total median (IQR) | 6.5 (3–10.5) | |
| Birth weight (g), mean ± SD | Total | 2327 ± 626 |
| <2500 | 24 (64.9%) | |
| ≥2500 | 12 (32.4%) | |
| Missing | 1 (2.7%) | |
| Weight at surgery (g), median (IQR) | 2366 (1575–3105) | |
| Proportions of weight groups | <2500 | 23 (62.2%) |
| ≥2500 | 13 (35.1%) | |
| Missing | 1 (2.7%) | |
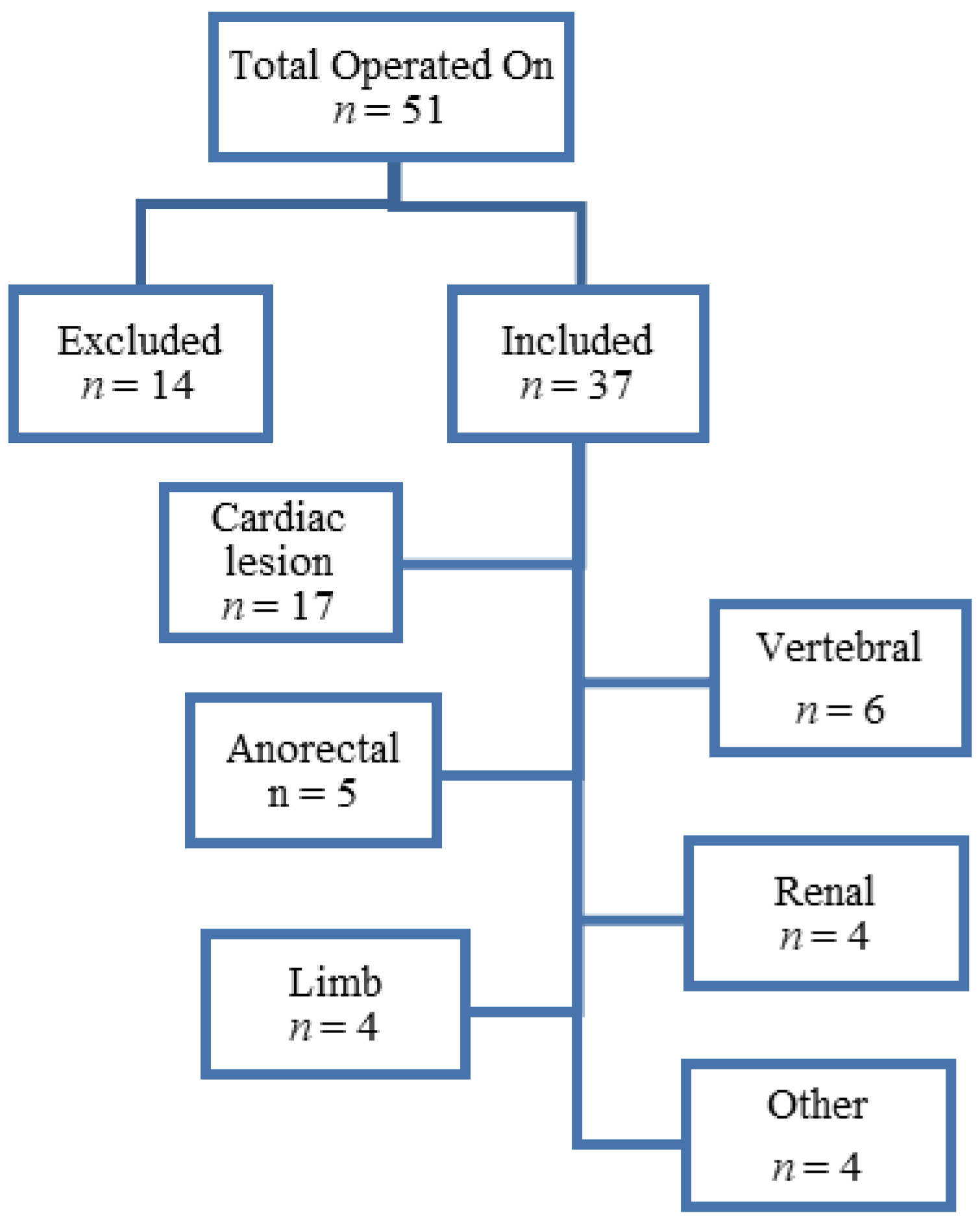
| Type of Abnormality | n (%) | |
|---|---|---|
| Vertebral | 6 (16.2%) | |
| Anorectal | 5 (13.5%) | |
| Cardiac | 17 (45.9%) | |
| Renal | 4 (10.8%) | |
| Limb | 4 (10.8%) | |
| Other | Genitalia | 2 (5.3%) |
| Choanal atresia | 1 (2.6%) | |
| Pulmonary artery malformation | 1 (2.6%) | |
| Echocardiography | n (%) | |
| Number of neonates who had echocardiography | 30 (81.1%) | |
| Presence of cardiac lesions | 17 (45.9%) | |
| Cardiac lesion types | Tetralogy of Fallot | 1 (5.2%) |
| Atrial septal defect | 10 (52.6%) | |
| Ventricular septal defect | 6 (31.6%) | |
| Patent ductus arteriosus | 10 (52.6%) | |
| Pulmonary hypertension | 2 (10.5%) | |
| Bidirectional shunt | 3 (15.8%) | |
| Tricuspid regurgitation | 3 (15.8%) | |
| Dextroposition | 2 (10.5%) | |
| Complications and Outcomes | Overall | No Cardiac Abnormalities | Had Cardiac Abnormalities | p Value |
|---|---|---|---|---|
| Desaturation | 13 (35.1%) | 6 (30.0%) | 7 (41.2%) | 0.478 |
| Mechanical ventilation | 11 (29.7%) | 6 (30.0%) | 5 (29.4%) | 0.969 |
| Inotropes used or increased | 3 (8.1%) | 1 (5.0%) | 2 (11.8%) | 0.452 |
| Invasive monitors | 11 (29.7%) | 6 (30.0%) | 5 (29.4%) | 0.545 |
| Difficult intubation | 4 (10.8%) | 0 (0.0%) | 4 (23.5%) | 0.022 |
| Difficult ventilation | 15 (40.5%) | 7 (35.0%) | 8 (47.1%) | 0.457 |
| Desaturation > 10% from baseline | 17 (45.9%) | 7 (35.0%) | 10 (58.8%) | 0.147 |
| Hypotension | 9 (24.3%) | 2 (10.0%) | 7 (41.2%) | 0.028 |
| Inotropes used or increased | 4 (10.8%) | 1 (5.0%) | 3 (17.7%) | 0.315 |
| Arrythmias | 2 (5.4%) | 1 (5.0%) | 1 (5.9%) | 0.906 |
| Blood transfusions used | 10 (27.0%) | 5 (25.0%) | 5 (29.4%) | 0.763 |
| Mechanical ventilation (days) Median (IQR) | 13.5 (7–30) | 14.5 (6–30) | 12 (7–30) | 0.959 |
| Nosocomial infection | 22 (59.5%) | 14 (70.0%) | 8 (47.1%) | 0.157 |
| Strictures | 6 (16.2%) | 4 (20.0%) | 2 (11.8%) | 0.498 |
| Septic shock | 4 (10.8%) | 2 (10.0%) | 2 (11.8%) | 0.863 |
| Anaemia | 5 (13.5%) | 3 (15.0%) | 2 (11.8%) | 0.774 |
| Chronic lung disease | 3 (8.1%) | 0 (0.0%) | 3 (17.7%) | 0.050 |
| Pneumonia | 4 (10.8%) | 4 (20.0%) | 0 (0.0%) | 0.050 |
| Sepsis | 3 (8.1%) | 1 (5.0%) | 2 (11.8%) | 0.452 |
| Length of stay ICU | 22.5 (11–36) | 16.5 (12–35) | 30 (9.5–50) | 0.378 |
| Length of stay hospital | 37 (18–64) | 39.5 (30–60) | 36 (14–86) | 0.729 |
| Mortality | 9 (25.0%) | 2 (10.5%) | 7 (41.2%) | 0.034 |
| Variable Median (IQR)/n% | Overall (n = 36) | Term Birth (≥37 weeks), (n = 18) | Prematurity (<37 weeks), (n = 18) | p Value |
|---|---|---|---|---|
| Preoperative critical events | ||||
| Desaturation | 13 (36.1%) | 5 (27.8%) | 8 (44.4%) | 0.489 |
| Mechanical ventilation | 11 (30.6%) | 3 (16.7%) | 8 (44.4%) | 0.146 |
| Inotropes used or increased | 4 (11.1%) | 2 (11.1%) | 2 (11.1%) | 1.000 |
| Invasive/non-invasive | 11 (30.6%) | 3 (16.7%) | 8 (44.4%) | 0.146 |
| Intraoperative critical events | ||||
| Difficult intubation | 4 (11.1%) | 3 (16.7%) | 1 (5.6%) | 0.603 |
| Difficult ventilation | 14 (38.9%) | 10 (55.6%) | 4 (22.2%) | 0.086 |
| Desaturation > 10% from baseline | 16 (44.4%) | 12 (66.7%) | 4 (22.2%) | 0.018 |
| Hypotension | 8 (22.2%) | 7 (38.9%) | 1 (5.6%) | 0.041 |
| Arrythmias | 2 (5.6%) | 0 (0.0%) | 2 (11.1%) | 0.486 |
| Blood transfusions used | 10 (27.8%) | 5 (27.8%) | 5 (27.8%) | 1.000 |
| Postoperative critical events and Complications | ||||
| Mechanical ventilation (days) | 13 (7–30) | 12.5 (6–30) | 14 (7–25) | 0.814 |
| Nosocomial infection | 22 (61.1%) | 11 (61.1%) | 11 (61.1%) | 1.000 |
| Strictures | 5 (13.9%) | 3 (16.7%) | 2 (11.1%) | 1.000 |
| Septic shock | 4 (11.0%) | 0 (0.0%) | 4 (22.2%) | 0.104 |
| Anaemia | 5 (13.9%) | 0 (0.0%) | 5 (27.8%) | 0.045 |
| Chronic lung disease | 2 (5.6%) | 1 (5.6%) | 1 (5.6%) | 1.000 |
| Pneumonia | 4 (11.0%) | 2 (11.1%) | 2 (11.1%) | 1.000 |
| Sepsis | 3 (8.3%) | 1 (5.6%) | 2 (11.1%) | 1.000 |
| Mortality | 9 (25.7%) | 5 (29.4%) | 4 (22.2%) | 0.711 |
| Variable Median (IQR)/n (%) | Overall n = 17 | Single Cardiac Lesion (n = 6) | Multiple Cardiac Lesions (n = 11) | p Value |
|---|---|---|---|---|
| Preoperative and critical events | ||||
| Desaturation | 7 (41.2%) | 1 (16.7%) | 6 (54.6%) | 0.304 |
| Mechanical ventilation | 5 (29.4%) | 1 (16.7%) | 4 (36.4%) | 0.600 |
| Inotropes used or increased | 3 (8.1%) | 1 (16.7%) | 2 (18.2%) | 1.000 |
| Invasive/non-invasive | 5 (29.4%) | 1 (16.7%) | 4 (36.4%) | 0.600 |
| Intraoperative and critical events | ||||
| Difficult intubation | 4 (23.5%) | 1 (16.7%) | 3 (27.3%) | 1.000 |
| Difficult ventilation | 8 (47.1%) | 2 (33.3%) | 6 (54.6%) | 0.620 |
| Desaturation > 10% from baseline | 10 (58.8%) | 2 (33.3%) | 8 (72.7%) | 0.162 |
| Hypotension | 7 (41.2%) | 2 (33.3%) | 5 (45.5%) | 1.000 |
| Arrythmias | 1 (5.9%) | 1 (16.7%) | 0 (0.0%) | 0.353 |
| Blood transfusions used | 5 (29.4%) | 1 (16.7%) | 4 (36.4%) | 0.600 |
| Postoperative critical events and complications | ||||
| Mechanical ventilation (days) | 12 (7–30) | 25 (12–60) | 9 (5–30) | 0.099 |
| Nosocomial infection | 8 (47.1%) | 2 (33.3%) | 6 (54.6%) | 0.620 |
| Strictures | 2 (11.8%) | 1 (16.7%) | 1 (9.1%) | 1.000 |
| Septic shock | 2 (11.8%) | 1 (16.7%) | 1 (9.1%) | 1.000 |
| Anaemia | 2 (11.8%) | 1 (16.7%) | 1 (9.1%) | 1.000 |
| Chronic lung disease | 3 (17.7%) | 2 (33.3%) | 1 (9.1%) | 0.515 |
| Pneumonia | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | - |
| Sepsis | 2 (11.8%) | 0 (0.0%) | 2 (18.2%) | 0.515 |
| Mortality | 7 (41.2%) | 2 (33.3%) | 5 (45.5%) | 0.627 |
| Parameter | uOR (95%CI) | p Value |
|---|---|---|
| Cardiac Lesions | 6.758 (1.089–41.938) | 0.040 |
| Prematurity | 1.087 (0.199–5.935) | 0.923 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoyi, N.; Mogane, P.; Madima, N.; Motshabi, P. The Phenotypical Profile and Outcomes of Neonates with Congenital Tracheoesophageal Fistula Associated with Congenital Cardiac Anomalies Presenting for Surgery. Children 2022, 9, 887. https://doi.org/10.3390/children9060887
Hoyi N, Mogane P, Madima N, Motshabi P. The Phenotypical Profile and Outcomes of Neonates with Congenital Tracheoesophageal Fistula Associated with Congenital Cardiac Anomalies Presenting for Surgery. Children. 2022; 9(6):887. https://doi.org/10.3390/children9060887
Chicago/Turabian StyleHoyi, Nomvuyo, Palesa Mogane, Nthatheni Madima, and Palesa Motshabi. 2022. "The Phenotypical Profile and Outcomes of Neonates with Congenital Tracheoesophageal Fistula Associated with Congenital Cardiac Anomalies Presenting for Surgery" Children 9, no. 6: 887. https://doi.org/10.3390/children9060887
APA StyleHoyi, N., Mogane, P., Madima, N., & Motshabi, P. (2022). The Phenotypical Profile and Outcomes of Neonates with Congenital Tracheoesophageal Fistula Associated with Congenital Cardiac Anomalies Presenting for Surgery. Children, 9(6), 887. https://doi.org/10.3390/children9060887





