Diagnostic Performance of Height-Estimated Baseline Creatinine in Diagnosing Acute Kidney Injury in Children with Type 1 Diabetes Mellitus Onset
Abstract
:1. Introduction
2. Methods
2.1. AKI Definition
2.2. Post-Hoc Power Calculation
2.3. Statistical Analysis
3. Results
3.1. AKI Definition on the Basis of mbSCr
3.2. AKI Definition on the Basis of ebSCr
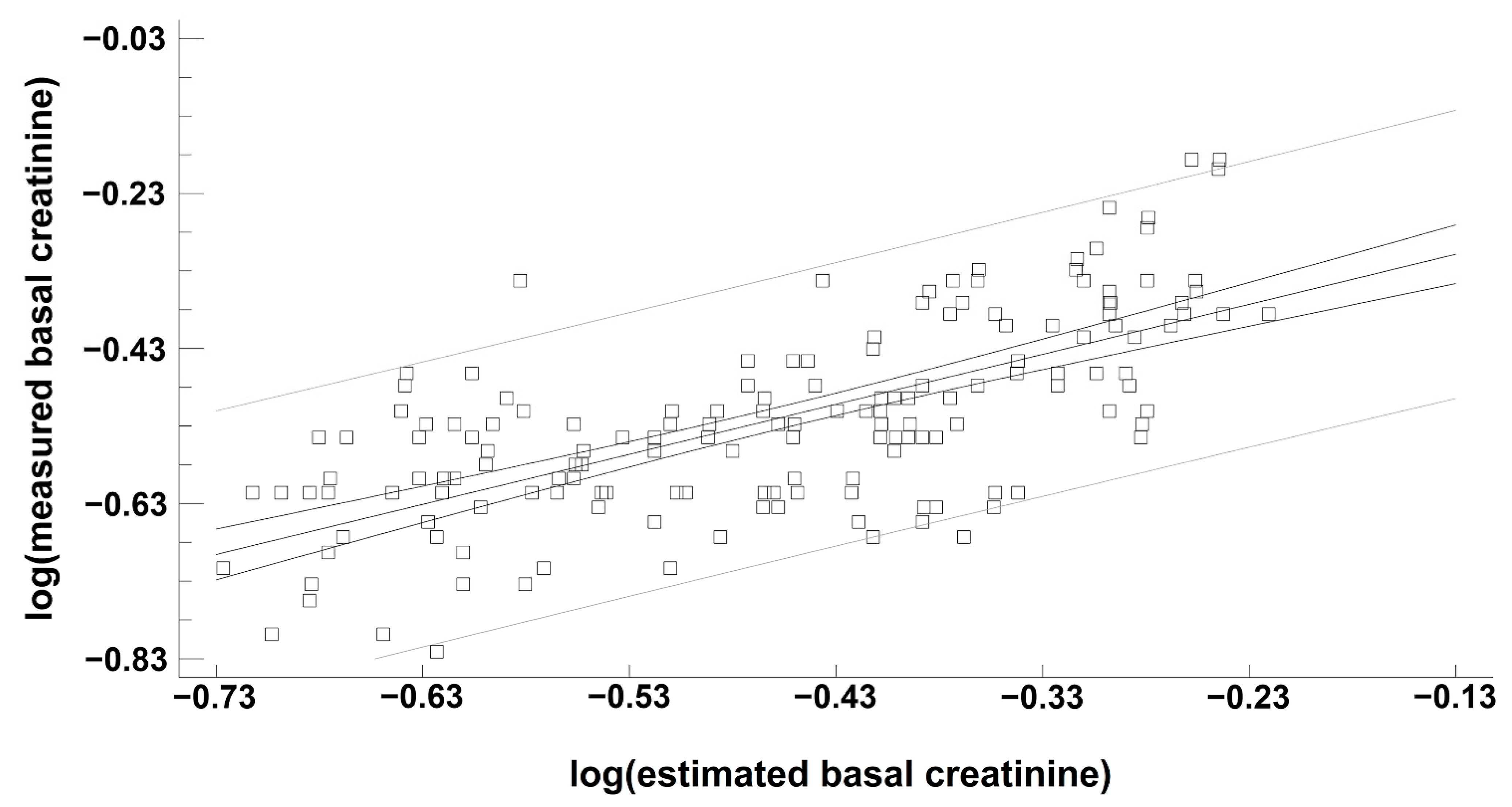
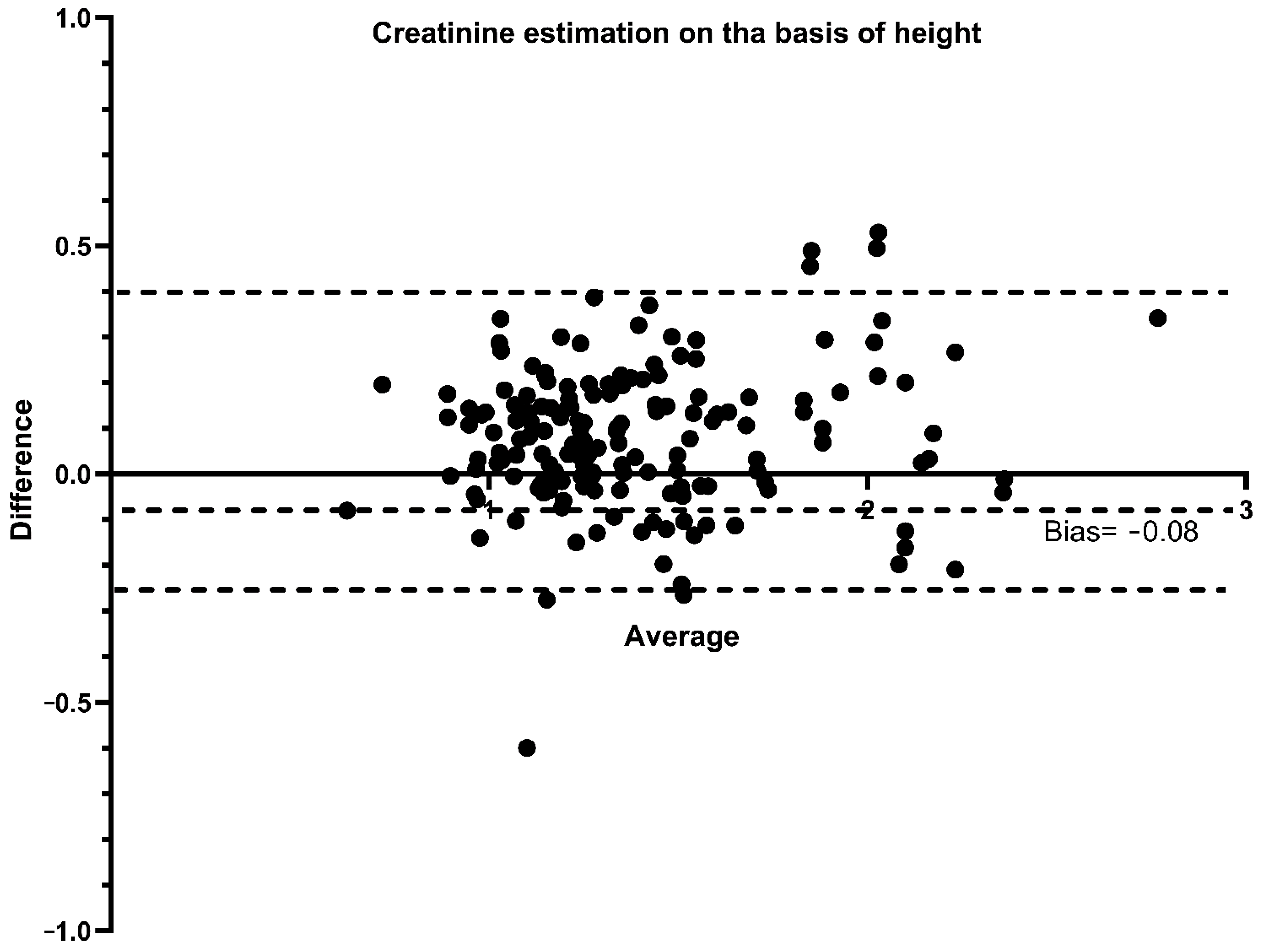
3.3. Performance of ebSCr Compared with mbSCr in Diagnosing AKI
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marzuillo, P.; Pezzella, V.; Guarino, S.; di Sessa, A.; Baldascino, M.; Polito, C.; Miraglia Del Giudice, E.; Nunziata, F. Acute Kidney Injury in children hospitalized for community acquired pneumonia. Pediatr. Nephrol. 2021, 36, 2883–2890. [Google Scholar] [CrossRef]
- Marzuillo, P.; Baldascino, M.; Guarino, S.; Perrotta, S.; Miraglia del Giudice, E.; Nunziata, F. Acute Kidney Injury in children hospitalized for acute gastroenteritis: Prevalence and risk factors. Pediatr. Nephrol. 2021, 36, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Marzuillo, P.; Coppola, C.; Caiazzo, R.; Macchini, G.; Di Sessa, A.; Guarino, S.; Esposito, F.; del Giudice, E.M.; Tipo, V. Acute Kidney Injury in Children with Acute Appendicitis. Children 2022, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.; Neu, A.; Fadrowski, J. AKI in Hospitalized Children: Poorly Documented (and Underrecognized). Front. Pediatr. 2022, 9, 790509. [Google Scholar] [CrossRef] [PubMed]
- Carmody, J.B.; Swanson, J.R.; Rhone, E.T.; Charlton, J.R. Recognition and reporting of AKI in very low birth weight infants. Clin. J. Am. Soc. Nephrol. 2014, 9, 2036–2043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenberg, J.H.; Zappitelli, M.; Devarajan, P.; Thiessen-Philbrook, H.R.; Krawczeski, C.; Li, S.; Garg, A.X.; Coca, S.; Parikh, C.R. Kidney Outcomes 5 Years After Pediatric Cardiac Surgery: The TRIBE-AKI Study. JAMA Pediatr. 2016, 170, 1071–1078. [Google Scholar] [CrossRef]
- Coca, S.G.; Singanamala, S.; Parikh, C.R. Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int. 2012, 81, 442–448. [Google Scholar] [CrossRef] [Green Version]
- Harel, Z.; Wald, R.; Bargman, J.M.; Mamdani, M.; Etchells, E.; Garg, A.X.; Ray, J.G.; Luo, J.; Li, P.; Quinn, R.R.; et al. Nephrologist follow-up improves all-cause mortality of severe acute kidney injury survivors. Kidney Int. 2013, 83, 901–908. [Google Scholar] [CrossRef] [Green Version]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; et al. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar] [CrossRef] [Green Version]
- Hessey, E.; Ali, R.; Dorais, M.; Morissette, G.; Pizzi, M.; Rink, N.; Jouvet, P.; Lacroix, J.; Phan, V.; Zappitelli, M. Evaluation of height-dependent and height-independent methods of estimating baseline serum creatinine in critically ill children. Pediatr. Nephrol. 2017, 32, 1953–1962. [Google Scholar] [CrossRef]
- Hursh, B.E.; Ronsley, R.; Islam, N.; Mammen, C.; Panagiotopoulos, C. Acute Kidney Injury in Children With Type 1 Diabetes Hospitalized for Diabetic Ketoacidosis. JAMA Pediatr. 2017, 171, e170020. [Google Scholar] [CrossRef] [PubMed]
- Marzuillo, P.; Iafusco, D.; Zanfardino, A.; Guarino, S.; Piscopo, A.; Casaburo, F.; Capalbo, D.; Ventre, M.; Arienzo, M.R.; Cirillo, G.; et al. Acute kidney injury and renal tubular damage in children with type 1 diabetes mellitus onset. J. Clin. Endocrinol. Metab. 2021, 106, e2720–e2737. [Google Scholar] [CrossRef] [PubMed]
- Weissbach, A.; Zur, N.; Kaplan, E.; Kadmon, G.; Gendler, Y.; Nahum, E. Acute Kidney Injury in Critically Ill Children Admitted to the PICU for Diabetic Ketoacidosis. A Retrospective Study. Pediatr. Crit. Care Med. 2019, 20, e10–e14. [Google Scholar] [CrossRef] [PubMed]
- Martinez Herrada, A.; Shein, S.L.; Rotta, A.T. Methodologic Challenges in the Diagnosis of Acute Kidney Injury in Children With Diabetic Ketoacidosis. Pediatr. Crit. Care Med. 2019, 20, 589. [Google Scholar] [CrossRef] [PubMed]
- Baalaaji, M.; Jayashree, M.; Nallasamy, K.; Singhi, S.; Bansal, A. Predictors and Outcome of Acute Kidney Injury in Children with Diabetic Ketoacidosis. Indian Pediatrics 2018, 55, 311–314. [Google Scholar] [CrossRef]
- Schwartz, G.J.; Muñoz, A.; Schneider, M.F.; Mak, R.H.; Kaskel, F.; Warady, B.A.; Furth, S.L. New equations to estimate GFR in children with CKD. J. Am. Soc. Nephrol. 2009, 20, 629–637. [Google Scholar] [CrossRef] [Green Version]
- Ibiebele, I.; Algert, C.S.; Bowen, J.R.; Roberts, C.L. Pediatric admissions that include intensive care: A population-based study. BMC Health Serv. Res. 2018, 18, 264. [Google Scholar] [CrossRef]
- Schwartz, G.J.; Furth, S.L. Glomerular filtration rate measurement and estimation in chronic kidney disease. Pediatr. Nephrol. 2007, 22, 1839–1848. [Google Scholar] [CrossRef] [Green Version]
- Marzuillo, P.; Guarino, S.; Grandone, A.; Di Somma, A.; Diplomatico, M.; Rambaldi, P.F.; Decimo, F.; Miraglia del Giudice, E.; La Manna, A.; Polito, C. Congenital solitary kidney size at birth could predict reduced eGFR levels later in life. J. Perinatol. 2019, 39, 129–134. [Google Scholar] [CrossRef]
- Marzuillo, P.; Grandone, A.; Di Sessa, A.; Guarino, S.; Diplomatico, M.; Umano, G.R.; Polito, C.; La Manna, A.; Perrone, L.; Miraglia del Giudice, E. Anthropometric and Biochemical Determinants of Estimated Glomerular Filtration Rate in a Large Cohort of Obese Children. J. Ren. Nutr. 2018, 28, 359–362. [Google Scholar] [CrossRef]
- Schwartz, G.J.; Brion, L.P.; Spitzer, A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr. Clin. N. Am. 1987, 34, 571–590. [Google Scholar] [CrossRef]
- Landis, J.; Koch, G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giavarina, D. Understanding Bland Altman analysis. Biochem. Med. 2015, 25, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanley, J.A.; McNeil, B.J. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983, 148, 839–843. [Google Scholar] [CrossRef] [Green Version]
- Bogdanović, R. Diabetic nephropathy in children and adolescents. Pediatr. Nephrol. 2008, 23, 507–525. [Google Scholar] [CrossRef]
- Huang, J.X.; Casper, T.C.; Pitts, C.; Myers, S.; Loomba, L.; Ramesh, J.; Kuppermann, N.; Glaser, N. Association of Acute Kidney Injury during Diabetic Ketoacidosis with Risk of Microalbuminuria in Children with Type 1 Diabetes. JAMA Pediatr. 2022, 176, 169–175. [Google Scholar] [CrossRef]
- Devarajan, P. Acute Kidney Injury in Children: Clinical Features, Etiology, Evaluation, and Diagnosis; UpToDate: Waltham, MA, USA, 2019. [Google Scholar]
- Devarajan, P. Acute kidney injury: Still misunderstood and misdiagnosed. Nat. Rev. Nephrol. 2017, 13, 137–138. [Google Scholar] [CrossRef]
- Laskin, B.L.; Goebel, J. Acute kidney injury in children admitted with diabetic ketoacidosis: Finding the sweet spot of fluid management. JAMA Pediatr. 2017, 171, 12–13. [Google Scholar] [CrossRef]
- Kuppermann, N.; Ghetti, S.; Schunk, J.; Stoner, M.; Rewers, A.; McManemy, J.; Myers, S.R.; Nigrovic, L.E.; Garro, A.; Brown, K.M.; et al. Clinical Trial of Fluid Infusion Rates for Pediatric Diabetic Ketoacidosis. N. Engl. J. Med. 2018, 378, E793–E794. [Google Scholar] [CrossRef]
- Pottel, H.; Hoste, L.; Martens, F. A simple height-independent equation for estimating glomerular filtration rate in children. Pediatr. Nephrol. 2012, 27, 973–979. [Google Scholar] [CrossRef]
- Kemperman, F.A.; Weber, J.A.; Gorgels, J.; van Zanten, A.P.; Krediet, R.T.; Arisz, L. The influence of ketoacids on plasma creatinine assays in diabetic ketoacidosis. J. Intern. Med. 2000, 248, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, P. Biomarkers for the Early Detection of Acute Kidney Injury. Curr. Opin. Pediatr. 2011, 23, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonneijck, L.; Muskiet, M.H.A.; Smits, M.M.; Van Bommel, E.J.; Heerspink, H.J.L.; Van Raalte, D.H.; Joles, J.A. Glomerular hyperfiltration in diabetes: Mechanisms, clinical significance, and treatment. J. Am. Soc. Nephrol. 2017, 28, 1023–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, M.T.; Maynard, S.E.; Kimmel, P.L. Misapplications of Commonly Used Kidney Equations: Renal Physiology in Practice. Clin. J. Am. Soc. Nephrol. 2009, 4, 528. [Google Scholar] [CrossRef] [Green Version]
- CDC Surveillance System: Laboratory Reporting Using IDMS-Traceable Creatinine Calibration. Available online: https://nccd.cdc.gov/ckd/detail.aspx?Qnum=Q223 (accessed on 4 April 2022).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guarino, S.; Rivetti, G.; Di Sessa, A.; De Lucia, M.; Palma, P.L.; Miraglia del Giudice, E.; Polito, C.; Marzuillo, P. Diagnostic Performance of Height-Estimated Baseline Creatinine in Diagnosing Acute Kidney Injury in Children with Type 1 Diabetes Mellitus Onset. Children 2022, 9, 899. https://doi.org/10.3390/children9060899
Guarino S, Rivetti G, Di Sessa A, De Lucia M, Palma PL, Miraglia del Giudice E, Polito C, Marzuillo P. Diagnostic Performance of Height-Estimated Baseline Creatinine in Diagnosing Acute Kidney Injury in Children with Type 1 Diabetes Mellitus Onset. Children. 2022; 9(6):899. https://doi.org/10.3390/children9060899
Chicago/Turabian StyleGuarino, Stefano, Giulio Rivetti, Anna Di Sessa, Maeva De Lucia, Pier Luigi Palma, Emanuele Miraglia del Giudice, Cesare Polito, and Pierluigi Marzuillo. 2022. "Diagnostic Performance of Height-Estimated Baseline Creatinine in Diagnosing Acute Kidney Injury in Children with Type 1 Diabetes Mellitus Onset" Children 9, no. 6: 899. https://doi.org/10.3390/children9060899
APA StyleGuarino, S., Rivetti, G., Di Sessa, A., De Lucia, M., Palma, P. L., Miraglia del Giudice, E., Polito, C., & Marzuillo, P. (2022). Diagnostic Performance of Height-Estimated Baseline Creatinine in Diagnosing Acute Kidney Injury in Children with Type 1 Diabetes Mellitus Onset. Children, 9(6), 899. https://doi.org/10.3390/children9060899







