Combination of Hemoglobin-for-Age Z-Score and Plasma Hepcidin Identified as a Novel Predictor for Kawasaki Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Quantification of Plasma Hepcidin by Enzyme-Linked Immunosorbent Assay
2.3. Support Vector Machine
2.4. Statistical Analysis
3. Results
3.1. Significantly Higher Plasma Hepcidin and Lower HbZ in KD Compared to FC
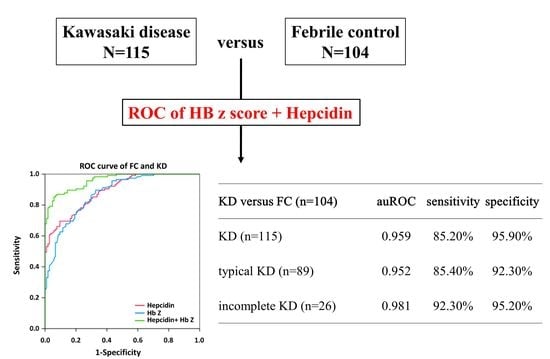
3.2. Significantly Better Preditor of the Combination of Plasma Hepcidin and HbZ in KD
3.3. SVM Classification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, Y.H.; Lin, K.M.; Ho, S.C.; Yan, J.H.; Lo, M.H.; Kuo, H.C. Increased Incidence of Kawasaki Disease in Taiwan in Recent Years: A 15 Years Nationwide Population-Based Cohort Study. Front. Pediatr. 2019, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Wu, Y.T.; Liu, C.A.; Kuo, H.C.; Yang, K.D. Kawasaki disease: Infection, immunity and genetics. Pediatr. Infect. Dis. J. 2005, 24, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.C.P.; Beaton, A.; Acquatella, H.; Bern, C.; Bolger, A.F.; Echeverria, L.E.; Dutra, W.O.; Gascon, J.; Morillo, C.A.; Oliveira-Filho, J.; et al. Chagas Cardiomyopathy: An Update of Current Clinical Knowledge and Management: A Scientific Statement from the American Heart Association. Circulation 2018, 138, e169–e209. [Google Scholar] [CrossRef]
- Fukazawa, R.; Kobayashi, J.; Ayusawa, M.; Hamada, H.; Miura, M.; Mitani, Y.; Tsuda, E.; Nakajima, H.; Matsuura, H.; Ikeda, K.; et al. JCS/JSCS 2020 Guideline on Diagnosis and Management of Cardiovascular Sequelae in Kawasaki Disease. Circ. J. 2020, 84, 1348–1407. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Chen, K.D.; Kuo, K.C.; Guo, M.M.; Chang, L.S.; Yang, Y.L.; Kuo, H.C. Human Transcriptome Array Analysis Identifies CDR2 as a Novel Suppressed Gene for Kawasaki Disease. Diagnostics 2022, 12, 240. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, X.; Cao, L.; Chen, Y.; Yan, W.; Xu, Q.; Lv, H. Characteristics and Indications of Kawasaki Disease Among Infants Under 6 Months. Front. Pediatr. 2020, 8, 470. [Google Scholar] [CrossRef] [PubMed]
- Newburger, J.W.; Takahashi, M.; Gerber, M.A.; Gewitz, M.H.; Tani, L.Y.; Burns, J.C.; Shulman, S.T.; Bolger, A.F.; Ferrieri, P.; Baltimore, R.S.; et al. Diagnosis, treatment, and long-term management of Kawasaki disease: A statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation 2004, 110, 2747–2771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, H.C.; Ho, J.C.; Guo, M.M.; Lo, M.H.; Hsieh, K.S.; Tsai, W.C.; Kuo, H.C.; Lee, C.H. Bull’s eye dermatoscopy pattern at bacillus Calmette-Guerin inoculation site correlates with systemic involvements in patients with Kawasaki disease. J. Dermatol. 2016, 43, 1044–1050. [Google Scholar] [CrossRef]
- Huang, Y.H.; Kuo, H.C.; Huang, F.C.; Yu, H.R.; Hsieh, K.S.; Yang, Y.L.; Sheen, J.M.; Li, S.C.; Kuo, H.C. Hepcidin-Induced Iron Deficiency Is Related to Transient Anemia and Hypoferremia in Kawasaki Disease Patients. Int. J. Mol. Sci. 2016, 17, 715. [Google Scholar] [CrossRef] [Green Version]
- Kuo, H.C.; Hsu, Y.W.; Wu, M.S.; Chien, S.C.; Liu, S.F.; Chang, W.C. Intravenous immunoglobulin, pharmacogenomics, and Kawasaki disease. J. Microbiol. Immunol. Infect. 2016, 49, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Alves, N.R.; Magalhaes, C.M.; Almeida Rde, F.; Santos, R.C.; Gandolfi, L.; Pratesi, R. Prospective study of Kawasaki disease complications: Review of 115 cases. Rev. Assoc. Med. Bras. 2011, 57, 295–300. [Google Scholar] [CrossRef]
- Ishikawa, T.; Wada, Y.; Namba, H.; Kawai, T. Hepcidin in Kawasaki disease: Upregulation by acute inflammation in patients having resistance to intravenous immunoglobulin therapy. Clin. Rheumatol. 2021, 40, 5019–5024. [Google Scholar] [CrossRef]
- Atsumi, Y.; Sakakibara, H.; Morikawa, Y.; Miyata, K.; Yamagishi, H.; Misawa, M.; Miura, M. Decreased hemoglobin after initial treatment is associated with treatment resistance in Kawasaki disease in Kobayashi risk stratification. World J. Pediatr. 2020, 16, 623–628. [Google Scholar] [CrossRef]
- Iio, K.; Morikawa, Y.; Miyata, K.; Kaneko, T.; Misawa, M.; Yamagishi, H.; Miura, M. Risk Factors of Coronary Artery Aneurysms in Kawasaki Disease with a Low Risk of Intravenous Immunoglobulin Resistance: An Analysis of Post RAISE. J. Pediatr. 2022, 240, 158–163. [Google Scholar] [CrossRef]
- Li, W.; He, X.; Zhang, L.; Wang, Z.; Wang, Y.; Lin, H.; Yuan, J.; Xie, X.; Qin, Y.; Huang, P. A Retrospective Cohort Study of Intravenous Immunoglobulin Therapy in the Acute Phase of Kawasaki Disease: The Earlier, the Better? Cardiovasc. Ther. 2021, 2021, 6660407. [Google Scholar] [CrossRef]
- Ling, X.B.; Lau, K.; Kanegaye, J.T.; Pan, Z.; Peng, S.; Ji, J.; Liu, G.; Sato, Y.; Yu, T.T.; Whitin, J.C.; et al. A diagnostic algorithm combining clinical and molecular data distinguishes Kawasaki disease from other febrile illnesses. BMC Med. 2011, 9, 130. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.J.; Cheng, M.C.; Lo, M.H.; Chien, S.J. Early Differentiation of Kawasaki Disease Shock Syndrome and Toxic Shock Syndrome in a Pediatric Intensive Care Unit. Pediatr. Infect. Dis. J. 2015, 34, 1163–1167. [Google Scholar] [CrossRef]
- Liu, X.P.; Huang, Y.S.; Kuo, H.C.; Xia, H.B.; Yi, S.; Huang, W.D.; Lang, X.L.; Liu, C.Y.; Liu, X. A novel nomogram model for differentiating Kawasaki disease from sepsis. Sci. Rep. 2020, 10, 13745. [Google Scholar] [CrossRef]
- Tsai, C.M.; Chu, C.H.; Liu, X.; Weng, K.P.; Liu, S.F.; Huang, Y.H.; Kuo, H.C. A novel score system of blood tests for differentiating Kawasaki disease from febrile children. PLoS ONE 2021, 16, e0244721. [Google Scholar] [CrossRef]
- Kuo, K.C.; Yeh, Y.C.; Chiu, I.M.; Tang, K.S.; Su, C.M.; Huang, Y.H. The clinical features and therapy of community-acquired gram negative bacteremia in children less than three years old. Pediatr. Neonatol. 2020, 61, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Kuo, H.C.; Yang, Y.L.; Chuang, J.H.; Tiao, M.M.; Yu, H.R.; Huang, L.T.; Yang, K.D.; Chang, W.C.; Lee, C.P.; Huang, Y.H. Inflammation-induced hepcidin is associated with the development of anemia and coronary artery lesions in Kawasaki disease. J. Clin. Immunol. 2012, 32, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Kossiva, L.; Gourgiotis, D.I.; Tsentidis, C.; Anastasiou, T.; Marmarinos, A.; Vasilenko, H.; Sdogou, T.; Georgouli, H. Serum hepcidin and ferritin to iron ratio in evaluation of bacterial versus viral infections in children: A single-center study. Pediatr. Infect. Dis. J. 2012, 31, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Lanser, L.; Fuchs, D.; Kurz, K.; Weiss, G. Physiology and Inflammation Driven Pathophysiology of Iron Homeostasis-Mechanistic Insights into Anemia of Inflammation and Its Treatment. Nutrients 2021, 13, 3732. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, C.; Tsuchiya, K.; Maeda, K. Reticulocyte hemoglobin content. Clin. Chim. Acta 2020, 504, 138–145. [Google Scholar] [CrossRef]
- Wang, F.S.; Lian, W.S.; Lee, M.S.; Weng, W.T.; Huang, Y.H.; Chen, Y.S.; Sun, Y.C.; Wu, S.L.; Chuang, P.C.; Ko, J.Y. Histone demethylase UTX counteracts glucocorticoid deregulation of osteogenesis by modulating histone-dependent and -independent pathways. J. Mol. Med. 2017, 95, 499–512. [Google Scholar] [CrossRef]
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Baker, A.L.; Jackson, M.A.; Takahashi, M.; Shah, P.B.; et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation 2017, 135, e927–e999. [Google Scholar] [CrossRef]
- Huang, Y.H.; Kuo, H.C.; Li, S.C.; Cai, X.Y.; Liu, S.F.; Kuo, H.C. HAMP promoter hypomethylation and increased hepcidin levels as biomarkers for Kawasaki disease. J. Mol. Cell Cardiol. 2018, 117, 82–87. [Google Scholar] [CrossRef] [Green Version]
- Kain, P.; Boyle, S.M.; Tharadra, S.K.; Guda, T.; Pham, C.; Dahanukar, A.; Ray, A. Odour receptors and neurons for DEET and new insect repellents. Nature 2013, 502, 507–512. [Google Scholar] [CrossRef] [Green Version]
- Kvon, E.Z.; Kazmar, T.; Stampfel, G.; Yanez-Cuna, J.O.; Pagani, M.; Schernhuber, K.; Dickson, B.J.; Stark, A. Genome-scale functional characterization of Drosophila developmental enhancers in vivo. Nature 2014, 512, 91–95. [Google Scholar] [CrossRef]
- Kuo, H.C.; Hsieh, K.S.; Guo, M.M.-H.; Weng, K.P.; Ger, L.P.; Chan, W.C.; Li, S.C. Next-generation sequencing identifies micro-RNA-based biomarker panel for Kawasaki disease. J. Allergy Clin. Immunol. 2016, 138, 1227–1230. [Google Scholar] [CrossRef] [Green Version]
- Addo, O.Y.; Yu, E.X.; Williams, A.M.; Young, M.F.; Sharma, A.J.; Mei, Z.; Kassebaum, N.J.; Jefferds, M.E.D.; Suchdev, P.S. Evaluation of Hemoglobin Cutoff Levels to Define Anemia Among Healthy Individuals. JAMA Netw. Open 2021, 4, e2119123. [Google Scholar] [CrossRef]
- Yang, C.P.; Lin, L.U. Normal blood cell values for Chinese children of various ages. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi 1988, 29, 167–173. [Google Scholar]
- Chen, Y.J.; Guo, M.M.; Chang, L.S.; Kuo, H.C. The Impact of Onset Age on Eosinophils in Kawasaki Disease. Biomedicines 2022, 10, 835. [Google Scholar] [CrossRef]
- Grasa, C.D.; Fernandez-Cooke, E.; Sanchez-Manubens, J.; Carazo-Gallego, B.; Aracil-Santos, J.; Anton, J.; Lirola, M.J.; Mercader, B.; Villalobos, E.; Bustillo, M.; et al. Kawasaki disease in children younger than 6 months of age: Characteristics of a Spanish cohort. Eur. J. Pediatr. 2022, 181, 589–598. [Google Scholar] [CrossRef]
- Nomura, Y.; Yashiro, M.; Masuda, K.; Nakamura, Y. Treatment change and coronary artery abnormality in incomplete Kawasaki disease. Pediatr. Int. 2020, 62, 779–784. [Google Scholar] [CrossRef]
- Fukushige, J.; Takahashi, N.; Ueda, Y.; Ueda, K. Incidence and clinical features of incomplete Kawasaki disease. Acta Paediatr. 1994, 83, 1057–1060. [Google Scholar] [CrossRef]
- Kuo, H.C.; Wang, C.L.; Liang, C.D.; Yu, H.R.; Chen, H.H.; Wang, L.; Yang, K.D. Persistent monocytosis after intravenous immunoglobulin therapy correlated with the development of coronary artery lesions in patients with Kawasaki disease. J. Microbiol. Immunol. Infect. 2007, 40, 395–400. [Google Scholar]
- Kuo, H.C.; Yang, K.D.; Liang, C.D.; Bong, C.N.; Yu, H.R.; Wang, L.; Wang, C.L. The relationship of eosinophilia to intravenous immunoglobulin treatment failure in Kawasaki disease. Pediatr. Allergy. Immunol. 2007, 18, 354–359. [Google Scholar] [CrossRef]
- Huang, Y.H.; Kuo, H.C. Anemia in Kawasaki Disease: Hepcidin as a Potential Biomarker. Int. J. Mol. Sci. 2017, 18, 820. [Google Scholar] [CrossRef]
- Huang, Y.H.; Yang, K.D.; Hsu, Y.W.; Lu, H.F.; Wong, H.S.; Yu, H.R.; Kuo, H.C.; Huang, F.C.; Lo, M.H.; Hsieh, K.S.; et al. Correlation of HAMP gene polymorphisms and expression with the susceptibility and length of hospital stays in Taiwanese children with Kawasaki disease. Oncotarget 2017, 8, 51859–51868. [Google Scholar] [CrossRef] [Green Version]
- Kali, A.; Charles, M.V.; Seetharam, R.S. Hepcidin-A novel biomarker with changing trends. Pharmacogn. Rev. 2015, 9, 35–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tacke, F.; Nuraldeen, R.; Koch, A.; Strathmann, K.; Hutschenreuter, G.; Trautwein, C.; Strnad, P. Iron Parameters Determine the Prognosis of Critically Ill Patients. Crit. Care Med. 2016, 44, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.W.; Tabangin, M.; Kusano, R.; Ma, Y.; Ridsdale, R.; Akinbi, H. The utility of serum hepcidin as a biomarker for late-onset neonatal sepsis. J. Pediatr. 2013, 162, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Prentice, S.; Jallow, A.T.; Sinjanka, E.; Jallow, M.W.; Sise, E.A.; Kessler, N.J.; Wegmuller, R.; Cerami, C.; Prentice, A.M. Hepcidin mediates hypoferremia and reduces the growth potential of bacteria in the immediate post-natal period in human neonates. Sci. Rep. 2019, 9, 16596. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.H.; Cai, X.Y.; Huang, Y.H. The clinical value of plasma hepcidin levels in predicting bacterial infections in febrile children. Pediatr. Neonatol. 2019, 60, 377–381. [Google Scholar] [CrossRef]
- Olinder, J.; Ehinger, D.; Liljenborg, E.; Herwald, H.; Ryden, C. Plasma Levels of Hepcidin and Reticulocyte Haemoglobin during Septic Shock. J. Innate Immun. 2020, 12, 448–460. [Google Scholar] [CrossRef]
- Jiang, Y.; Jiang, F.Q.; Kong, F.; An, M.M.; Jin, B.B.; Cao, D.; Gong, P. Inflammatory anemia-associated parameters are related to 28-day mortality in patients with sepsis admitted to the ICU: A preliminary observational study. Ann. Intensive Care 2019, 9, 67. [Google Scholar] [CrossRef] [Green Version]
- Wakakuri, H.; Hyodo, H.; Ohara, T.; Yasutake, M. Serum Hepcidin-25 Levels Reflect the Presence of Bacteremia in Patients with Systemic Inflammatory Response Syndrome. J. Nippon. Med. Sch. 2019, 86, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Peng, D.; Gao, Y.; Zhang, L.; Liu, Z.; Wang, H.; Liu, Y. The Relationship Between Hepcidin-Mediated Iron Dysmetabolism and COVID-19 Severity: A Meta-Analysis. Front. Public Health 2022, 10, 881412. [Google Scholar] [CrossRef]
- Delaye, J.B.; Alarcan, H.; Vallet, N.; Veyrat-Durebex, C.; Bernard, L.; Herault, O.; Ropert, M.; Marlet, J.; Gyan, E.; Andres, C.; et al. Specific changes of erythroid regulators and hepcidin in patients infected by SARS-COV-2. J. Investig. Med. 2022, 70, 934–938. [Google Scholar] [CrossRef]
- Bautista-Rodriguez, C.; Sanchez-de-Toledo, J.; Clark, B.C.; Herberg, J.; Bajolle, F.; Randanne, P.C.; Salas-Mera, D.; Foldvari, S.; Chowdhury, D.; Munoz, R.; et al. Multisystem Inflammatory Syndrome in Children: An International Survey. Pediatrics 2021, 147, e2020024554. [Google Scholar] [CrossRef]
- Ghosh, P.; Katkar, G.D.; Shimizu, C.; Kim, J.; Khandelwal, S.; Tremoulet, A.H.; Kanegaye, J.T.; Pediatric Emergency Medicine Kawasaki Disease Research Group. An Artificial Intelligence-guided signature reveals the shared host immune response in MIS-C and Kawasaki disease. Nat. Commun. 2022, 13, 2687. [Google Scholar] [CrossRef]
- Pelkonen, T.; Roine, I.; Kallio, M.; Jahnukainen, K.; Peltola, H. Prevalence and significance of anaemia in childhood bacterial meningitis: A secondary analysis of prospectively collected data from clinical trials in Finland, Latin America and Angola. BMJ Open 2022, 12, e057285. [Google Scholar] [CrossRef]
- Shi, T.; Chen, C.; Huang, L.; Fan, H.; Lu, G.; Yang, D.; Zhao, C.; Zhang, D. Risk factors for mortality from severe community-acquired pneumonia in hospitalized children transferred to the pediatric intensive care unit. Pediatr. Neonatol. 2020, 61, 577–583. [Google Scholar] [CrossRef]
- Lonberg-Holm, K.; Reed, D.L.; Roberts, R.C.; Hebert, R.R.; Hillman, M.C.; Kutney, R.M. Three high molecular weight protease inhibitors of rat plasma. Isolation, characterization, and acute phase changes. J. Biol. Chem. 1987, 262, 438–445. [Google Scholar] [CrossRef]
- Weyand, A.C.; McGann, P.T. Eliminating race-based reference ranges in haematology: A call to action. Lancet Haematol. 2021, 8, e462–e466. [Google Scholar] [CrossRef]
- Kuo, H.C. Preventing coronary artery lesions in Kawasaki disease. Biomed. J. 2017, 40, 141–146. [Google Scholar] [CrossRef]
- Scherler, L.; Haas, N.A.; Tengler, A.; Pattathu, J.; Mandilaras, G.; Jakob, A. Acute phase of Kawasaki disease: A review of national guideline recommendations. Eur. J. Pediatr. 2022, 181, 2563–2573. [Google Scholar] [CrossRef]
- Huang, Z.; Tan, X.H.; Wang, H.; Pan, B.; Lv, T.W.; Tian, J. A New Diagnostic Model to Distinguish Kawasaki Disease From Other Febrile Illnesses in Chongqing: A Retrospective Study on 10,367 Patients. Front. Pediatr. 2020, 8, 533759. [Google Scholar] [CrossRef]
- Xiu-Yu, S.; Jia-Yu, H.; Qiang, H.; Shu-Hui, D. Platelet count and erythrocyte sedimentation rate are good predictors of Kawasaki disease: ROC analysis. J. Clin. Lab. Anal. 2010, 24, 385–388. [Google Scholar] [CrossRef]
- Liu, X.P.; Huang, Y.S.; Xia, H.B.; Sun, Y.; Lang, X.L.; Li, Q.Z.; Liu, C.Y.; Kuo, H.C.; Huang, W.D.; Liu, X. A Nomogram Model Identifies Eosinophilic Frequencies to Powerfully Discriminate Kawasaki Disease From Febrile Infections. Front. Pediatr. 2020, 8, 559389. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C.; Wang, C.L.; Liang, C.D.; Yu, H.R.; Huang, C.F.; Wang, L.; Hwang, K.P.; Yang, K.D. Association of lower eosinophil-related T helper 2 (Th2) cytokines with coronary artery lesions in Kawasaki disease. Pediatr. Allergy Immunol. 2009, 20, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.P.; Huang, Y.H.; Tsai, Y.C.; Liu, S.F.; Kuo, H.C. Comparison of Laboratory Data between Children with Kawasaki Disease and COVID-19. Children 2022, 9, 638. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Fan, Q. The Role of Procalcitonin for Predicting Kawasaki Disease. Fetal. Pediatr. Pathol. 2022, 1–8. [Google Scholar] [CrossRef]
- Rodriguez-Gonzalez, M.; Perez-Reviriego, A.A.; Castellano-Martinez, A.; Cascales-Poyatos, H.M. N-terminal probrain natriuretic peptide as biomarker for diagnosis of Kawasaki disease. Biomark. Med. 2019, 13, 307–323. [Google Scholar] [CrossRef]
- Ye, Q.; Shao, W.X.; Shang, S.Q.; Zhang, T.; Hu, J.; Zhang, C.C. A Comprehensive Assessment of the Value of Laboratory Indices in Diagnosing Kawasaki Disease. Arthritis Rheumatol. 2015, 67, 1943–1950. [Google Scholar] [CrossRef] [Green Version]




| Characteristic | Febrile Controls (n = 104) | KD (n = 115) | p-Value |
|---|---|---|---|
| Male gender, n (%) | 53 (51.0) | 75 (65.2) | 0.033 |
| Age (year) | 2.3 ± 0.2 | 1.8 ± 0.1 | 0.023 |
| Fever duration (day) | 4.5 ± 0.3 | 7.1 ± 0.2 | <0.001 |
| White blood cells (1000/uL) | 9.8 ± 0.5 | 13.8 ± 0.5 | <0.001 |
| Hemoglobin (g/dL) | 12.1 ± 0.1 | 11.1 ± 0.1 | <0.001 |
| C-reactive protein | 20.0 ± 2.4 | 92.2 ± 6.6 | <0.001 |
| Characteristic | Typical KD (n = 89) | Incomplete KD (n = 26) | p-Value |
|---|---|---|---|
| Male gender, n (%) | 55 (61.8) | 20 (76.9) | 0.170 |
| Age (year) | 1.7 ± 0.1 | 1.8 ± 0.4 | 0.815 |
| Fever duration (day) | 6.9 ± 0.2 | 8.1 ± 0.7 | 0.031 |
| White blood cell (1000/uL) | 13.6 ± 0.5 | 14.5 ± 1.3 | 0.529 |
| Hemoglobin (g/dL) | 11.1 ± 0.1 | 10.8 ± 0.2 | 0.200 |
| C-reactive protein (mg/L) | 96.2 ± 7.9 | 78.2 ± 11.6 | 0.263 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.-L.; Kuo, H.-C.; Chen, K.-D.; Chu, C.-H.; Kuo, K.-C.; Guo, M.M.-H.; Chang, L.-S.; Huang, Y.-H. Combination of Hemoglobin-for-Age Z-Score and Plasma Hepcidin Identified as a Novel Predictor for Kawasaki Disease. Children 2022, 9, 913. https://doi.org/10.3390/children9060913
Yang Y-L, Kuo H-C, Chen K-D, Chu C-H, Kuo K-C, Guo MM-H, Chang L-S, Huang Y-H. Combination of Hemoglobin-for-Age Z-Score and Plasma Hepcidin Identified as a Novel Predictor for Kawasaki Disease. Children. 2022; 9(6):913. https://doi.org/10.3390/children9060913
Chicago/Turabian StyleYang, Ya-Ling, Ho-Chang Kuo, Kuang-Den Chen, Chi-Hsiang Chu, Kuang-Che Kuo, Mindy Ming-Huey Guo, Ling-Sai Chang, and Ying-Hsien Huang. 2022. "Combination of Hemoglobin-for-Age Z-Score and Plasma Hepcidin Identified as a Novel Predictor for Kawasaki Disease" Children 9, no. 6: 913. https://doi.org/10.3390/children9060913
APA StyleYang, Y. -L., Kuo, H. -C., Chen, K. -D., Chu, C. -H., Kuo, K. -C., Guo, M. M. -H., Chang, L. -S., & Huang, Y. -H. (2022). Combination of Hemoglobin-for-Age Z-Score and Plasma Hepcidin Identified as a Novel Predictor for Kawasaki Disease. Children, 9(6), 913. https://doi.org/10.3390/children9060913