Standalone Axial Malrotation after Pediatric Supracondylar Fracture Does Not Seem to Be an Indication for Immediate Postoperative Revision Surgery
Abstract
:1. Introduction
2. Materials and Methods
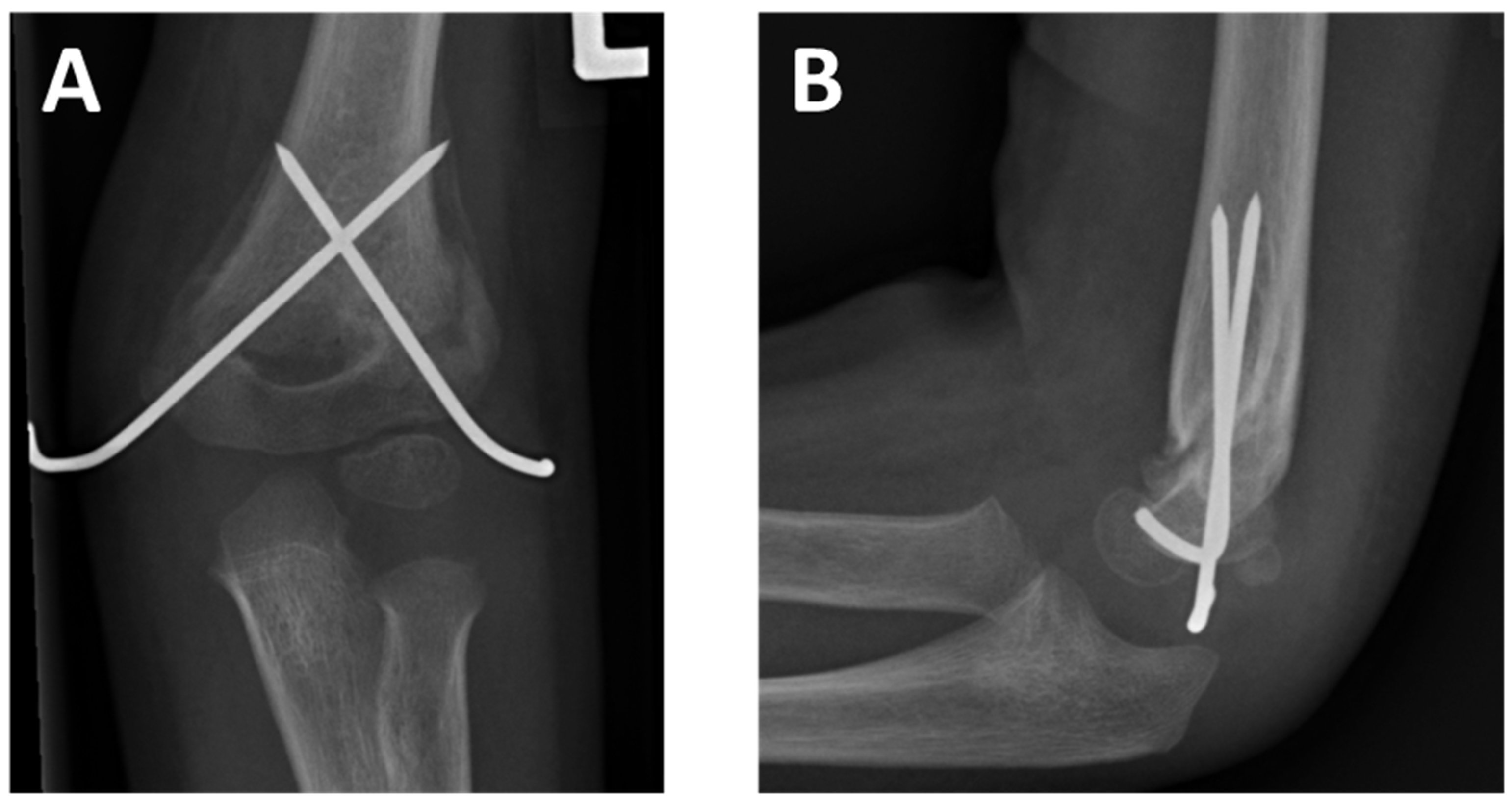
2.1. Patient Collective
2.2. Retrospective Analysis
2.3. Follow-Up Examination
2.3.1. Clinical Examination
2.3.2. Radiological Examination
Humeral Ulnar Angle
Baumann’s Angle
Antecurvation Angle
Humeral Trochlear Angle
2.3.3. Scores
2.4. Statistics
2.5. Ethics
3. Results
3.1. Retrospective Analysis
3.2. Follow-Up Examination
3.2.1. Range of Motion
3.2.2. Radiological Analysis
3.2.3. Scores
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khoshbin, A.; Leroux, T.; Wasserstein, D.; Wolfstadt, J.; Law, P.W.; Mahomed, N.; Wright, J.G. The epidemiology of paediatric supracondylar fracture fixation: A population-based study. Injury 2014, 45, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Omid, R.; Choi, P.D.; Skaggs, D.L. Supracondylar humeral fractures in children. J. Bone Jt. Surg. Am. 2008, 90, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Farnsworth, C.L.; Silva, P.D.; Mubarak, S.J. Etiology of supracondylar humerus fractures. J. Pediatr. Orthop. 1998, 18, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Kropelnicki, A.; Ali, A.M.; Popat, R.; Sarraf, K.M. Paediatric supracondylar humerus fractures. Br. J. Hosp. Med. 2019, 80, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Acton, J.D.; McNally, M.A. Baumann’s confusing legacy. Injury 2001, 32, 41–43. [Google Scholar] [CrossRef]
- Shenoy, P.M.; Islam, A.; Puri, R. Current Management of Paediatric Supracondylar Fractures of the Humerus. Cureus 2020, 12, e8137. [Google Scholar] [CrossRef]
- Linhart, W.E.; Kraus, T. Reconstruction of humeroradial joint. Oper. Orthop. Traumatol. 2008, 20, 396–408. [Google Scholar] [CrossRef]
- Smith, L. Deformity following supracondylar fractures of the humerus. J. Bone Jt. Surg. Am. 1965, 47, 1668. [Google Scholar] [CrossRef]
- Gaddy, B.C.; Manske, P.R.; Pruitt, D.L.; Schoenecker, P.L.; Rouse, A.M. Distal humeral osteotomy for correction of posttraumatic cubitus varus. J. Pediatr. Orthop. 1994, 14, 214–219. [Google Scholar] [CrossRef]
- Handelsman, J.E.; Weinberg, J.; Hersch, J.C. Corrective supracondylar humeral osteotomies using the small AO external fixator. J. Pediatr. Orthop. B 2006, 15, 194–197. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, M.A., 3rd; Roach, J.W. Corrective osteotomy for cubitus varus deformity. J. Pediatr. Orthop. 1994, 14, 487–491. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, S.W.; Spinner, R.J.; McKee, M.D.; Kibler, W.B.; Hastings, H., 2nd; Morrey, B.F.; Kato, H.; Takayama, S.; Imatani, J.; Toh, S.; et al. Tardy posterolateral rotatory instability of the elbow due to cubitus varus. J. Bone Jt. Surg. Am. 2001, 83, 1358–1369. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, K.E. Residuals of elbow trauma in children. Orthop. Clin. N. Am. 1990, 21, 291–314. [Google Scholar] [CrossRef]
- von Laer, L. The supracondylar fracture of the humerus in children (author’s transl). Arch. Orthop. Trauma. Surg. 1979, 95, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Takahara, M.; Sasaki, I.; Kimura, T.; Kato, H.; Minami, A.; Ogino, T. Second fracture of the distal humerus after varus malunion of a supracondylar fracture in children. J. Bone Jt. Surg. Br. 1998, 80, 791–797. [Google Scholar] [CrossRef]
- Yamamoto, I.; Ishii, S.; Usui, M.; Ogino, T.; Kaneda, K. Cubitus varus deformity following supracondylar fracture of the humerus. A method for measuring rotational deformity. Clin. Orthop. Relat. Res. 1985, 179–185. [Google Scholar]
- Beaton, D.E.; Wright, J.G.; Katz, J.N.; Upper Extremity Collaborative, G. Development of the QuickDASH: Comparison of three item-reduction approaches. J. Bone Jt. Surg. Am. 2005, 87, 1038–1046. [Google Scholar] [CrossRef]
- Flynn, J.M.; Hresko, T.; Reynolds, R.A.; Blasier, R.D.; Davidson, R.; Kasser, J. Titanium elastic nails for pediatric femur fractures: A multicenter study of early results with analysis of complications. J. Pediatr. Orthop. 2001, 21, 4–8. [Google Scholar] [CrossRef]
- Dodds, S.D.; Grey, M.A.; Bohl, D.D.; Mahoney, E.M.; DeLuca, P.A. Clinical and radiographic outcomes of supracondylar humerus fractures treated surgically by pediatric and non-pediatric orthopedic surgeons. J. Child. Orthop. 2015, 9, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Suganuma, S.; Tada, K.; Takagawa, S.; Yasutake, H.; Takata, M.; Shimanuki, K.; Fujita, K.; Tsuchiya, H. Independent predictors affecting the reduction of pediatric supracondylar humerus fractures: A retrospective cohort study. Eur. J. Orthop. Surg. Traumatol. 2021, 31, 399–406. [Google Scholar] [CrossRef]
- Hubbard, E.W.; Riccio, A.I. Pediatric Orthopedic Trauma: An Evidence-Based Approach. Orthop. Clin. N. Am. 2018, 49, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, A.M.; Marzi, I.; Gunter, S.M.; Wessel, L.; Riedel, J.; von Laer, L. Supracondylar humerus fracture in childhood—An efficacy study. Results of a multicenter study by the Pediatric Traumatology Section of the German Society of Trauma Surgery—I: Epidemiology, effectiveness evaluation and classification. Unfallchirurg 2002, 105, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Mulpuri, K.; Wilkins, K. The treatment of displaced supracondylar humerus fractures: Evidence-based guideline. J. Pediatr. Orthop. 2012, 32 (Suppl. S2), S143–S152. [Google Scholar] [CrossRef] [PubMed]
- Novais, E.N.; Andrade, M.A.; Gomes, D.C. The use of a joystick technique facilitates closed reduction and percutaneous fixation of multidirectionally unstable supracondylar humeral fractures in children. J. Pediatr. Orthop. 2013, 33, 14–19. [Google Scholar] [CrossRef]
- Prabhakar, P.; Pierce, W.A.; Standefer, K.D.; Ho, C.A. Can We Estimate the Amount of Malrotation in Supracondylar Humerus Fractures after CRPP? J. Orthop. Trauma 2020, 34, e245–e249. [Google Scholar] [CrossRef]
- Wessely, J.; Decker, S. Radiograph of the shoulder girdle in identification of rotation defects of the humerus (author’s transl). Unfallheilkunde 1978, 81, 123–128. [Google Scholar]
- Arino, V.L.; Lluch, E.E.; Ramirez, A.M.; Ferrer, J.; Rodriguez, L.; Baixauli, F. Percutaneous fixation of supracondylar fractures of the humerus in children. J. Bone Jt. Surg. Am. 1977, 59, 914–916. [Google Scholar] [CrossRef]
- Arnold, J.A.; Nasca, R.J.; Nelson, C.L. Supracondylar fractures of the humerus: The role of dynamic factors in prevention of deformity. J. Bone Jt. Surg. Am. 1977, 59, 589–595. [Google Scholar] [CrossRef]
- Norman, O. Roentgenological studies on dislocations in supracondylar fractures of the humerus. Ann. Radiol. 1975, 18, 395–399. [Google Scholar]
- Henderson, E.R.; Egol, K.A.; van Bosse, H.J.; Schweitzer, M.E.; Pettrone, S.K.; Feldman, D.S. Calculation of rotational deformity in pediatric supracondylar humerus fractures. Skelet. Radiol. 2007, 36, 229–235. [Google Scholar] [CrossRef]
- Mahaisavariya, B.; Laupattarakasem, W. Supracondylar fracture of the humerus: Malrotation versus cubitus varus deformity. Injury 1993, 24, 416–418. [Google Scholar] [CrossRef]
- Nigst, H. Spezielle Fracturen und Luxationslehre, Band II/1; Georg Thieme Verlag: Stuttgart, Germany, 1965. [Google Scholar]
- Ham, J.; Flipsen, M.; Koolen, M.; van der Zwan, A.; Mader, K. Multiple osteochondromas (MO) in the forearm: A 12-year single-centre experience. Strateg. Trauma Limb Reconstr. 2016, 11, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Solfelt, D.A.; Hill, B.W.; Anderson, C.P.; Cole, P.A. Supracondylar osteotomy for the treatment of cubitus varus in children: A systematic review. Bone Jt. J. 2014, 96-B, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.A. Cubitus Varus-It’s More Than Just a Crooked Arm! J. Pediatr. Orthop. 2017, 37 (Suppl. S2), S37–S41. [Google Scholar] [CrossRef]
- Bauer, A.S.; Pham, B.; Lattanza, L.L. Surgical Correction of Cubitus Varus. J. Hand Surg. Am. 2016, 41, 447–452. [Google Scholar] [CrossRef]
- Sommerfeldt, D.W. Corrective osteotomies around the elbow in childhood and adolescence: Indications and techniques for posttraumatic deformities. Unfallchirurg 2019, 122, 353–363. [Google Scholar] [CrossRef]



| Sex | 19 (49%) male | 20 (51%) female |
| Affected side | 11 (28%) right side | 28 (72%) left side |
| Fracture geometry | 19 (54%) transverse | 16 (46%) oblique |
| Gartland classification | 6 (17%) type 2 | 28 (83%) type 3 |
| Surgery | 14 (36%) PCP | 25 (64%) AN |
| Sex | 7 (58%) male | 5 (42%) female |
| Affected side | 3 (25%) right side | 9 (75%) left side |
| Fracture geometry | 7 (58%) transverse | 5 (42%) oblique |
| Gartland classification | 4 (33%) type 2 | 8 (67%) type 3 |
| Surgery | 3 (25%) PCP | 9 (75%) AN |
| Injured Side Mean/Median (Range) | Contralateral Side Mean/Median (Range) | Difference Mean/Median (Range) | p-Value | |
|---|---|---|---|---|
| Flexion | 143°/143° (130°–150°) | 145°/145° (135°–150°) | 1°/0° (−12°–5°) | 0.268 # |
| Extension | 18°/15° (10°–25°) | 15°/13° (10°–20°) | 3°/0° (−5°–20°) | 0.266 § |
| Pronation | 84°/85° (60°–100°) | 88°/90° (60°–100°) | 4°/3° (−20°–10°) | 0.176 § |
| Supination | 103°/98 (70°–115°) | 103°/100° (80°–115°) | 0°/0° (−15°–20°) | >0.999 # |
| Yamamoto | −2° (–30°–40°) | −4° (−30°–20°) | 2°/0° (−20°–20°) | 0.516 # |
| Injured Mean/Median (Range) | Unaffected Mean/Median (Range) | Difference Mean/Median (Range) | p-Value | |
|---|---|---|---|---|
| Humeral ulnar angle | 7°/8° (2°–11°) | 11°/10 (8°–18°) | 3°/3° (−1°–9°) | 0.023 * § |
| Antecurvation angle | 50°/45° (37°–76°) | 50°/51° (31°–73°) | 0.7°/1° (−27°–16°) | 0.847 # |
| Baumann’s angle | 70°/70° (60°–83°) | 67°/74° (46°–79°) | 3°/5° (−14°–9°) | 0.688 § |
| Humerus trochlear angle | 2°/2° (−5°–7°) | 5°/4° (2°–10°) | 3°/1.5° (−3–8°) | 0.313 § |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greve, F.; Müller, M.; Wurm, M.; Biberthaler, P.; Singer, G.; Till, H.; Wegmann, H. Standalone Axial Malrotation after Pediatric Supracondylar Fracture Does Not Seem to Be an Indication for Immediate Postoperative Revision Surgery. Children 2022, 9, 1013. https://doi.org/10.3390/children9071013
Greve F, Müller M, Wurm M, Biberthaler P, Singer G, Till H, Wegmann H. Standalone Axial Malrotation after Pediatric Supracondylar Fracture Does Not Seem to Be an Indication for Immediate Postoperative Revision Surgery. Children. 2022; 9(7):1013. https://doi.org/10.3390/children9071013
Chicago/Turabian StyleGreve, Frederik, Michael Müller, Markus Wurm, Peter Biberthaler, Georg Singer, Holger Till, and Helmut Wegmann. 2022. "Standalone Axial Malrotation after Pediatric Supracondylar Fracture Does Not Seem to Be an Indication for Immediate Postoperative Revision Surgery" Children 9, no. 7: 1013. https://doi.org/10.3390/children9071013





