Helicobacter pylori Eradication Efficacy of Therapy Based on the Antimicrobial Susceptibility in Children with Gastritis and Peptic Ulcer in Mekong Delta, Vietnam
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting and Study Design
2.2. Helicobacter pylori Culture and Antimicrobial Susceptibility Testing
2.3. Tailored Therapy
2.4. Follow up to Assess the Efficacy of H. pylori Eradication Therapy
2.5. Statistical Analysis
2.6. Ethical Issues
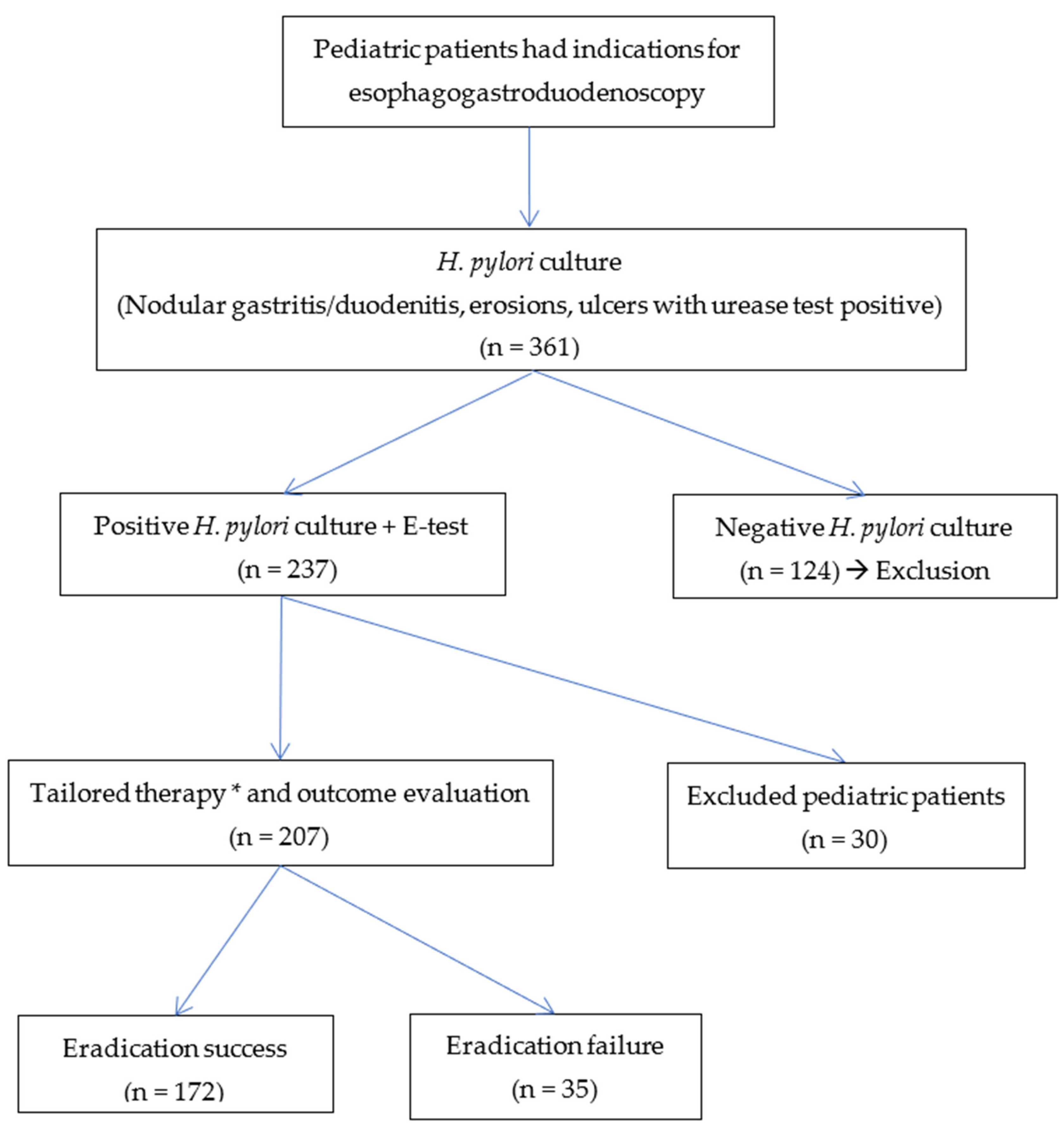
3. Results
- Susceptibility-guided triple therapy: esomeprazole + amoxicillin (AMX) + clarithromycin (CLR)/metronidazole (MTZ) or levofloxacin (LEV)/adolescent.
- If double resistance:
- +
- Can only choose one sensitive antibiotic agent: (AMX or MTZ or TET)≤8 years old: bismuth + esomeprazole + high-dose AMX + MTZ.Eight years old: Bismuth + Esomeprazole + TET + MTZ.
- +
- Can choose two sensitive antibiotic agents:Esomeprazole + two sensitive antibiotic agents.
3.1. Characteristics of Patients
3.2. Results of H. pylori Eradication Treatment
3.3. Adverse Effects of Therapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amieva, M.; Peek, R.M., Jr. Pathobiology of Helicobacter pylori-induced Gastric Cancer. Gastroenterology 2016, 150, 64–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Gastroenterology Organisation. World Gastroenterology Organisation Global Guidelines. Helicobacter pylori; World Gastroenterology Organisation: Milwaukee, WI, USA, 2021. [Google Scholar]
- Quach, D.T.; Vilaichone, R.K.; Vu, K.V. Helicobacter pylori Infection and Related Gastrointestinal Diseases in Southeast Asian Countries: An Expert Opinion Survey. Asian. Pac. J. Cancer Prev. 2018, 19, 3565–3569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, N.L. Joint ESPGHAN/NASPGHAN Guidelines for the Management of Helicobacter pylori in Children and Adolescents (Update 2016). J. Pediatr. Gastroenterol. Nutr. 2017, 64, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, M. How and when investigating and treating Helicobacter pylori infection in children. Acta Biomed. 2018, 89, 65–71. [Google Scholar] [PubMed]
- Ikuse, T. Antibiotic Resistance of Helicobacter pylori and Eradication Rate in Japanese Pediatric Patients. Adv. Microbiol. 2017, 07, 241–252. [Google Scholar] [CrossRef] [Green Version]
- Butenko, T.; Jeverica, S.; Orel, R.; Homan, M. Antibacterial resistance and the success of tailored triple therapy in Helicobacter pylori strains isolated from Slovenian children. Helicobacter 2017, 22, e12400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.D.; Dong, Q.W.; Zhang, S.H.; Gu, F.; Zhang, Y.; Song, H.B.; Zuo, N.Y.; Zhang, S.S.; Ma, L.; Ding, Z.L. Effectiveness of eradication regimen based on the bacterial susceptibility and CYP2C19 genotype in children with refractory Helicobacter pylori infection. Zhonghua Er Ke Za Zhi 2020, 58, 41–45. [Google Scholar] [PubMed]
- European Committee on Antimicrobial Susceptibility. Breakpoint Tables for Interpretation of MICs and Zone Diameters European Committee on Antimicrobial Susceptibility Testing; European Committee on Antimicrobial Susceptibility, EU: Växjö, Sweden, 2019. [Google Scholar]
- Kato, S.; Shimizu, T.; Toyoda, S. The updated JSPGHAN guidelines for the management of Helicobacter pylori infection in childhood. Pediatrics Int. 2020, 62, 1315–1331. [Google Scholar] [CrossRef] [PubMed]
- Poblet, G.G.; Cavero, T.A.; Pérez, N.A.; Martínez, B.B.; Arcos, G.B.; Pascual, M.L.C.; Martín, L.M.G.; Hernández, A.H.; Escribano, B.M.; Castells, X.O.; et al. Management of Helicobacter pylori infection in the pediatric age. An. Pediatr. 2021, 95, 383.e1–383.e9. [Google Scholar]
- Kotilea, K.; Mekhael, J.; Salame, A.; Mahler, T.; Miendje-Deyi, V.Y.; Cadranel, S.; Bontems, P. Eradication rate of Helicobacter Pylori infection is directly influenced by adherence to therapy in children. Helicobacter 2017, 22, e123832. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.M. Helicobacter pylori antimicrobial resistance in a pediatric population. Helicobacter 2018, 23, e12528. [Google Scholar] [CrossRef] [PubMed]
- Marcus, E.A.; Sachs, G.; Scott, D.R. Eradication of Helicobacter pylori infection. Kaohsiung J. Med. Sci. 2014, 30, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Uotani, T.; Miftahussurur, M.; Yamaoka, Y. Effect of bacterial and host factors on Helicobacter pylori eradication therapy. Expert Opin. Targets 2015, 19, 1637–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, S.C.; Iyer, P.G.; Moss, S.F. AGA Clinical Practice Update on the Management of Refractory Helicobacter pylori Infection: Expert Review. Gastroenterology 2021, 160, 1831–1841. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.J.; Thomas, C.D.; Barbarino, J.; Desta, Z.; Van Driest, S.L.; El Rouby, N.; Johnson, J.A.; Cavallari, L.H.; Shakhnovich, V.; Thacker, D.L.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2C19 and Proton Pump Inhibitor Dosing. Clin. Pharmacol. 2021, 109, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.D. Research on the Situation, Clinical and Subclinical Characteristics and Evaluation of Results of Treatment for Eradication of Helicobacter pylori in Children with Gastritis and Peptic Ulcers at Can Tho Children’s Hospital in 2017–2019. Master’s Thesis, Can Tho University of Medicine and Pharmacy, Can Tho, Vietnam, 2019. [Google Scholar]
- Nguyen, U.T. Epidemiological, Clinical Characteristics and Results of Some Regimens for the Treatment of Peptic Ulcer and Peptic Ulcer Caused by Antibiotic-Resistant Helicobacter pylori in Children at the National Children’s Hospital. Ph.D. Thesis, Central Institute of Hygiene and Epidemiology, Hanoi, Vietnam, 2016. [Google Scholar]
- Le, T.; Ngoc, C. Characteristics of Antibiotic Resistance and Response to Treatment in Children with Gastritis Caused by Helicobacter pylori at Children’s Hospital 2. Specialized Thesis II, Ho Chi Minh City University of Medicine and Pharmacy, Ho Chi Minh City, Vietnam, 2018. [Google Scholar]
- Van Thieu, H. Antimicrobial Resistance and the Successful Eradication of Helicobacter pylori-induced Gastroduodenal Ulcers in Vietnamese Children. Med. Arch. 2021, 75, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Thanh, T.T.; Phuc, N.T.T.; Hang, D.T.; Hau, D.T.; Yen, H.H. Antibiotic resistance of Helicobacter pylori strain in children’s gastritis. J. Pediatric Res. Pract. 2020, 4, 1–8. [Google Scholar]
- Nayoung, K. Helicobacter Pylori; Springer: Singapore, 2016. [Google Scholar]
- Zullo, A.; de Francesco, V.; Hassan, C. Predicting Helicobacter pylori eradication: How to teach an old dog new tricks! J. Clin. Gastroenterol. 2012, 46, 259–261. [Google Scholar] [CrossRef] [PubMed]
| Demographic Characteristics | Frequency | Rate (%) |
|---|---|---|
| Sex | ||
| Male | 116 | 48.9 |
| Female | 121 | 51.1 |
| Age | ||
| Mean age | 10.03 ± 2.53 | |
| 5–8 years | 60 | 25.3 |
| 9–12 years | 122 | 51.5 |
| 13–16 years | 55 | 23.2 |
| Geographic area | ||
| Can Tho city | 150 | 63.3 |
| Nearby regions | 87 | 36.7 |
| Endoscopy findings | ||
| Nodular Gastritis/duodenitis | 168 | 69.2 |
| Gastric ulcer | 05 | 2.1 |
| Duodenal ulcer | 68 | 28.7 |
| History eradication | ||
| Without previous therapy | 183 | 77.2 |
| Prior to H. pylori treatment | 54 | 22.8 |
| Symptom | Frequency | Rate (%) |
|---|---|---|
| Epigastric pain | 213 | 89.9 |
| Periumbilical pain | 14 | 6.1 |
| Pain when hungry | 57 | 24.1 |
| Nausea and vomiting | 155 | 65.4 |
| Heartburn | 78 | 32.9 |
| Constipation | 10 | 4.2 |
| GI bleeding | 07 | 3.0 |
| Anemia | 33 | 13.9 |
| Antibiotic Agents | Frequency | Rate (%) |
|---|---|---|
| Overall resistance to agents | ||
| CLR | 191 | 80.6 |
| AMX | 170 | 71.7 |
| MTZ | 114 | 49.4 |
| LEV | 107 | 45.1 |
| TET | 27 | 11.4 |
| Mono resistance to agents | 24 | 10.1 |
| Double resistance to agents | 78 | 32.9 |
| Triple resistance to agents | 111 | 46.8 |
| Quadruple resistance to agents | 21 | 8.9 |
| All resistance to agents | 03 | 1.3 |
| Regimen | Overall | Eradication Result | |||
|---|---|---|---|---|---|
| Success | Failure | ||||
| n | % | n | % | ||
| EAC | 23 | 12 | 52.2 | 11 | 47.8 |
| EAM | 32 | 25 | 78.1 | 7 | 21.9 |
| EAL | 3 | 2 | 66.6 | 1 | 33.4 |
| EB(T/A)M | 43 | 38 | 88.4 | 5 | 11.6 |
| Other triple regimens based on AS | 106 | 95 | 89.6 | 11 | 10.4 |
| Overall eradication rate | 207 | 172 | 83.1 | 35 | 16.9 |
| Factor | n | Eradication | p | OR | |
|---|---|---|---|---|---|
| Success | Failure | (95% Confidence Interval) | |||
| n (%) | n (%) | ||||
| Age (years) | |||||
| 5–8 | 44 | 24 (54.5) | 20 (45.5) | 0.00 | 8.2 (3.71–18.23) |
| 9–16 | 163 | 148 (90.8) | 15 (9.2) | ||
| Sex | |||||
| Boy | 101 | 84 (83.2) | 17 (16.8) | 0.98 | 0.99 (0.4–2.09) |
| Girl | 106 | 88 (83.0) | 18 (17.0) | ||
| Prior treatment | |||||
| No | 162 | 135 (83.3) | 27 (16.7) | 0.86 | 0.93 (0.39–2.21) |
| Yes | 45 | 37 (82.2) | 8 (17.8) | ||
| Peptic diseases | |||||
| Gastritis | 144 | 114 (79.2) | 30 (20.8) | 0.03 | 3.05 (1.13–8.28) |
| Peptic ulcer | 63 | 58 (92.1) | 5 (7.9) | ||
| Using bismuth | |||||
| Yes | 43 | 38 (88.4) | 5 (11.6) | 0.3 | 0.59 (0.21–1.62) |
| No | 164 | 134 (81.7) | 30 (18.3) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, L.T.T.; Nguyen, T.A.; Nguyen, N.A.; Nguyen, Y.T.H.; Nguyen, H.T.B.; Nguyen, L.T.; Vi, M.T.; Nguyen, T. Helicobacter pylori Eradication Efficacy of Therapy Based on the Antimicrobial Susceptibility in Children with Gastritis and Peptic Ulcer in Mekong Delta, Vietnam. Children 2022, 9, 1019. https://doi.org/10.3390/children9071019
Le LTT, Nguyen TA, Nguyen NA, Nguyen YTH, Nguyen HTB, Nguyen LT, Vi MT, Nguyen T. Helicobacter pylori Eradication Efficacy of Therapy Based on the Antimicrobial Susceptibility in Children with Gastritis and Peptic Ulcer in Mekong Delta, Vietnam. Children. 2022; 9(7):1019. https://doi.org/10.3390/children9071019
Chicago/Turabian StyleLe, Loan T. T., Tuan A. Nguyen, Nghia A. Nguyen, Yen T. H. Nguyen, Hai T. B. Nguyen, Liem T. Nguyen, Mai T. Vi, and Thang Nguyen. 2022. "Helicobacter pylori Eradication Efficacy of Therapy Based on the Antimicrobial Susceptibility in Children with Gastritis and Peptic Ulcer in Mekong Delta, Vietnam" Children 9, no. 7: 1019. https://doi.org/10.3390/children9071019







