Global Irradiation in Children Treated for Hydrocephalus and Its Change over Time—A Single Institutional Analysis
Abstract
:1. Introduction
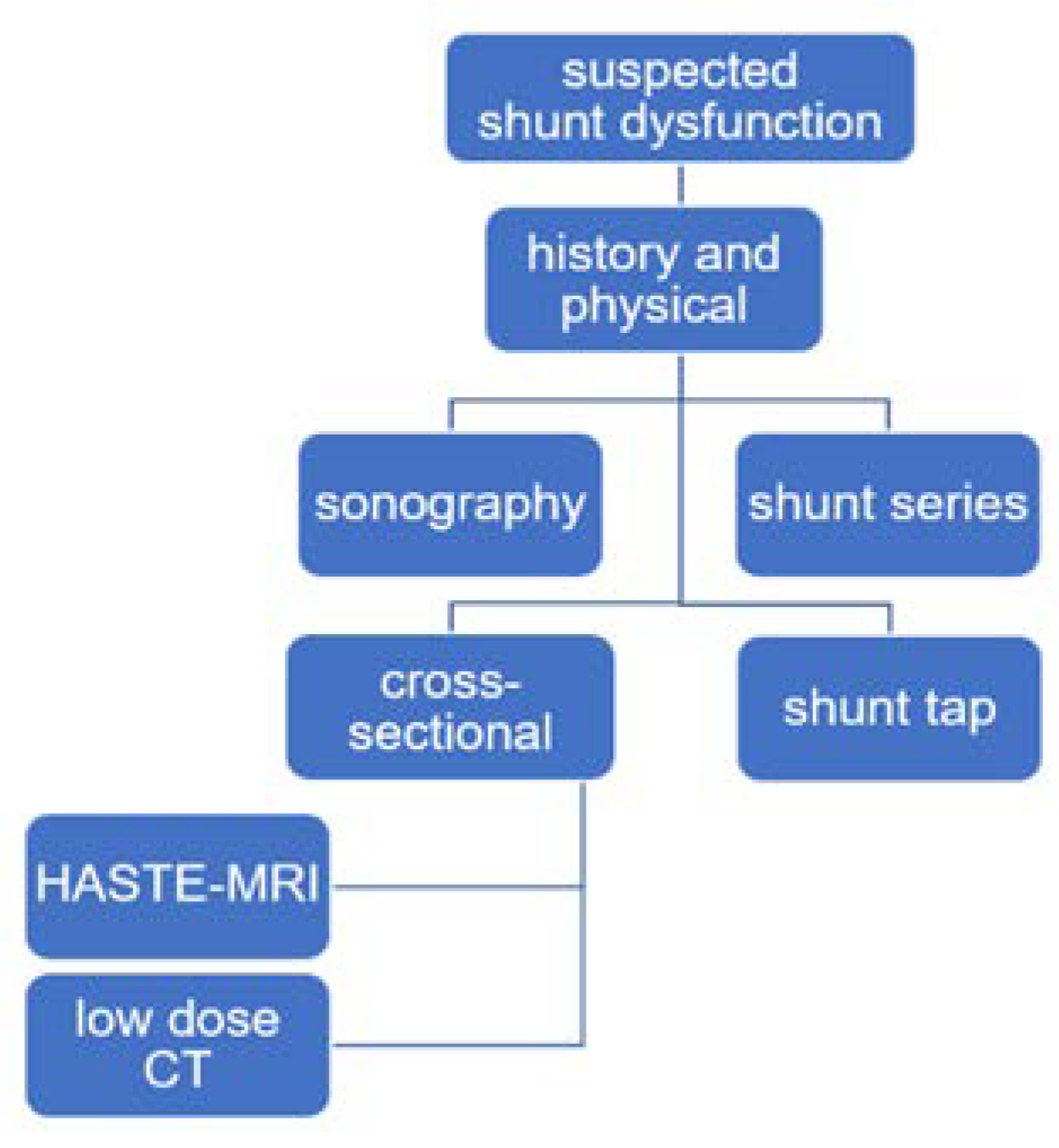
2. Materials and Methods
3. Results
- Overall, 41 patients were exposed to a total of 78 cCTs, of which 18 (23%) were performed during the first year of life, 38 (48%) between the ages of one and six and the remaining 14 (18%) after the age of six. Patients had a mean cCT rate of 1.9 per patient (0–12) and a mean cCT rate of 0.23 per shunt year. There was a mean shunt series rate of 0.92 (range: 0–4) and, for the lateral skull X-rays used to verify shunt settings, the mean rate was 2.37 (range: 0–13). Adding together the cCTs, shunt series X-rays and lateral skull X-rays, patients were exposed to a mean radiation exposure due to hydrocephalus of 3.93 mSv (range: 0–24.38), accounting for 0.49 mSv per shunt year. During the same study period, 272 MRIs were performed on these 41 patients due to hydrocephalus, leading to a mean MRI rate of 6.63 (range: 0–17). Table 1 summarizes these results.
- For patients operated on before 2017—and therefore with a CODMAN shunt system—radiation exposure due to hydrocephalus during the first year of life was calculated as having a mean of 1.68 mSv (range: 0–18.67), taking into consideration 18 cCTs, 8.7 shunt series X-rays and 3 lateral skull X-rays. Patients operated on after 2017—and therefore with a proGAV system—were not exposed to a single cCT, shunt series X-ray or lateral skull X-ray during the first year of life, meaning no additional radiation exposure due to shunt therapy.
- Radiation exposure, considering CT scans for all locations and for indications other than hydrocephalus during the same study period (seven pelvis CT scans (5 mSv), three abdomen CT scans (5 mSv), three PET-CT scans (21.6 mSv), two foot CT scans (1.5 mSv), four chest CT scans (8 mSv), one lower limb CT scan (3.5 mSv), four spine CT scans (6 mSv) and one petrosal bone CT scan (1.2 mSv)), added up to 178.5 mSv, which was 1.85 mSv per shunt year or 4.3 msV per patient (range: 0–80.8 mSv) [11,13,14,15].
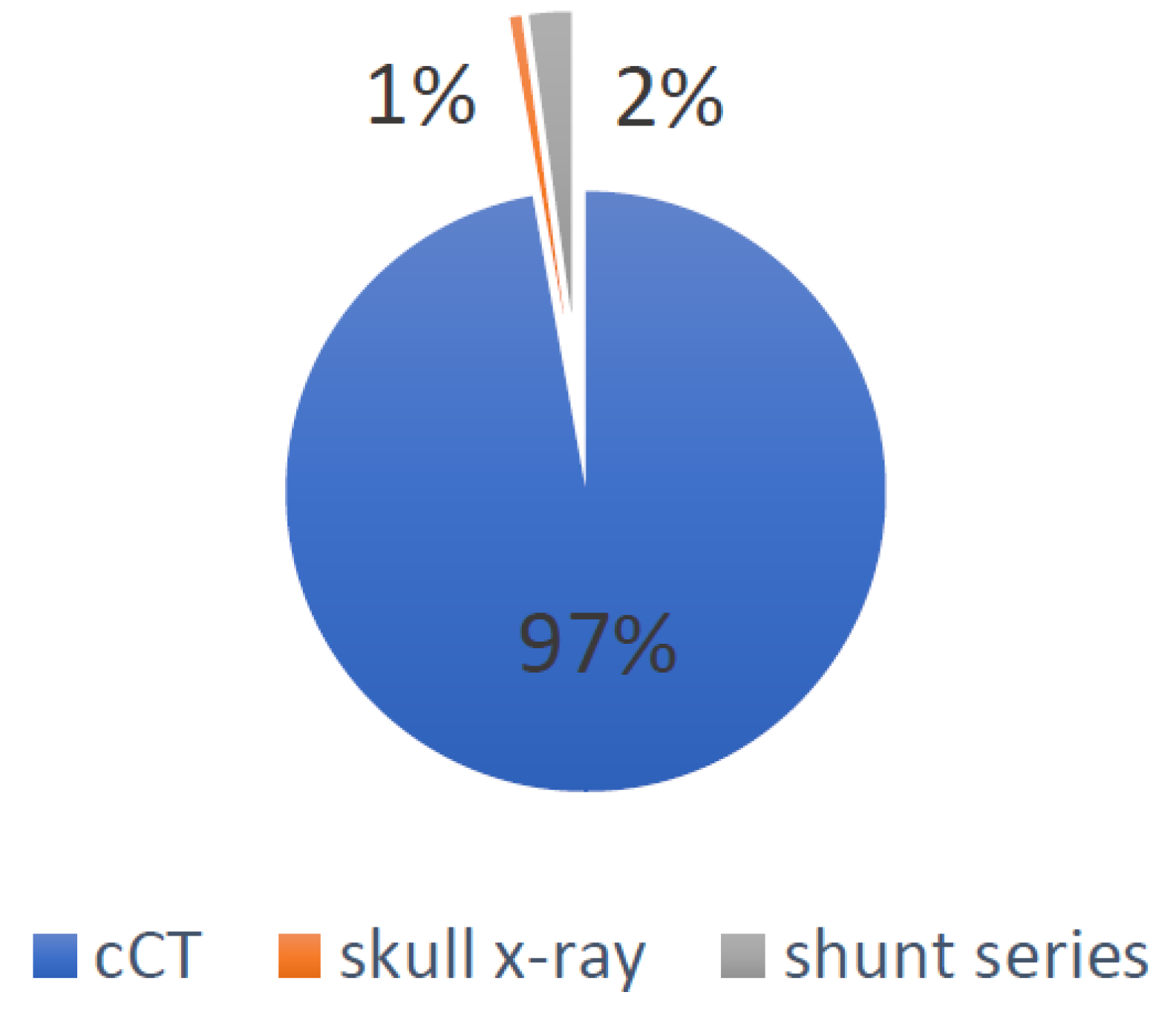
- Global irradiation was 162.64 mSv, of which 158 mSv were caused by cCTs, 3.67 mSv by shunt series X-rays and 0.97 mSv by lateral skull X-rays. The causes for the global irradiation are visualized in Figure 2.
- For all patients, the dates of the MRIs and head CTs were listed in order to evaluate the possible change during the observation period. Figure 3 visualizes this change. Between 2000 and 2010, 4.57 CTs and 4.28 MRIs per patient were performed, in comparison to 1.84 CTs and 8.75 MRIs per patient between 2011 and 2021.
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hung, K.-L.; Liao, H.-T.; Huang, J.-S. Rational management of simple depressed skull fractures in infants. J. Neurosurg. Pediatr. 2005, 103, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Little, M.P.; Wakeford, R.; Borrego, D.; French, B.; Zablotska, L.B.; Adams, M.J.; Allodji, R.; de Vathaire, F.; Lee, C.; Brenner, A.V.; et al. Leukaemia and myeloid malignancy among people exposed to low doses (<100 mSv) of ionising radiation during childhood: A pooled analysis of nine historical cohort studies. Lancet Haematol. 2018, 5, e346–e358. [Google Scholar] [CrossRef]
- Mathews, J.D.; Forsythe, A.V.; Brady, Z.; Butler, M.W.; Goergen, S.K.; Byrnes, G.B.; Giles, G.G.; Wallace, A.B.; Anderson, P.R.; Guiver, T.A.; et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: Data linkage study of 11 million Australians. BMJ 2013, 346, f2360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearce, M.S.; Salotti, A.J.; Little, M.; McHugh, K.; Lee, C.; Kim, K.P.; Howe, N.L.; Ronckers, C.M.; Rajaraman, P.; Craft, A.W.; et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: A retrospective cohort study. Lancet 2012, 380, 499–505. [Google Scholar] [CrossRef] [Green Version]
- Dobson, G.M.; Dalton, A.K.; Nicholson, C.L.; Jenkins, A.J.; Mitchell, P.B.; Cowie, C.J.A. CT scan exposure in children with ventriculo-peritoneal shunts: Single centre experience and review of the literature. Child Nerv. Syst. 2020, 36, 591–599. [Google Scholar] [CrossRef] [Green Version]
- White, I.K.; Shaikh, K.A.; Moore, R.J.; Bullis, C.L.; Sami, M.T.; Gianaris, T.J.; Fulkerson, D.H. Risk of radiation-induced malignancies from CT scanning in children who underwent shunt treatment before 6 years of age: A retrospective cohort study with a minimum 10-year follow-up. J. Neurosurg. Pediatr. 2014, 13, 514–519. [Google Scholar] [CrossRef] [Green Version]
- Antonucci, M.C.; Zuckerbraun, N.S.; Tyler-Kabara, E.C.; Furtado, A.D.; Murphy, M.E.; Marin, J.R. The Burden of Ionizing Radiation Studies in Children with Ventricular Shunts. J. Pediatr. 2017, 182, 210–216.e1. [Google Scholar] [CrossRef]
- Koral, K.; Blackburn, T.; Bailey, A.; Anderson, J. Strengthening the Argument for Rapid Brain MR Imaging: Estimation of Reduction in Lifetime Attributable Risk of Developing Fatal Cancer in Children with Shunted Hydrocephalus by Instituting a Rapid Brain MR Imaging Protocol in Lieu of Head CT. Am. J. Neuroradiol. 2012, 33, 1851–1854. [Google Scholar] [CrossRef] [Green Version]
- Patel, D.M.; Tubbs, R.S.; Pate, G.; Johnston, J.M.; Blount, J.P. Fast-sequence MRI studies for surveillance imaging in pediatric hydrocephalus. J. Neurosurg. Pediatr. 2014, 13, 440–447. [Google Scholar] [CrossRef] [Green Version]
- Afat, S.; Pjontek, R.; Hamou, H.A.; Herz, K.; Nikoubashman, O.; Bamberg, F.; Brockmann, M.A.; Nikolaou, K.; Clusmann, H.; Wiesmann, M.; et al. Imaging of Ventriculoperitoneal Shunt Complications: Comparison of Whole Body Low-Dose Computed Tomography and Radiographic Shunt Series. J. Comput. Assist. Tomogr. 2016, 40, 991–996. [Google Scholar] [CrossRef]
- Smith-Bindman, R.; Lipson, J.; Marcus, R.; Kim, K.-P.; Mahesh, M.; Gould, R.; de González, A.B.; Miglioretti, D.L. Radiation Dose Associated with Common Computed Tomography Examinations and the Associated Lifetime Attributable Risk of Cancer. Arch. Intern. Med. 2009, 169, 2078–2086. [Google Scholar] [CrossRef]
- Biswas, D.; Bible, E.J.; Bohan, M.; Simpson, A.K.; Whang, P.G.; Grauer, J.N. Radiation Exposure from Musculoskeletal Computerized Tomographic Scans. J. Bone Jt. Surg. 2009, 91, 1882–1889. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.G.; Mills, C.N.; Mogensen, M.A.; Lee, C. Radiation Dose from Medical Imaging: A Primer for Emergency Physicians. West. J. Emerg. Med. 2012, 13, 202–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, Y.; Wang, Z.; Liu, Y.; Liu, Z.; Yao, V. Radiation Dose to the Lens Using Different Temporal Bone CT Scanning Protocols. Am. J. Neuroradiol. 2010, 31, 226–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, K.H.; Slovis, B.H.; Runde, D.; Godbout, B.; Newman, D.H.; Lee, J. Radiation exposure among patients with the highest CT scan utilization in the emergency department. Emerg. Radiol. 2013, 20, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.S.; Jamal, N.; Dawes, C.; Chamberlain, J.M.; Atabaki, S.M. Cranial Computed Tomography Utilization for Suspected Ventriculoperitoneal Shunt Malfunction in a Pediatric Emergency Department. J. Emerg. Med. 2014, 46, 449–455. [Google Scholar] [CrossRef]
- Berry, J.G.; Hall, M.A.; Sharma, V.; Goumnerova, L.; Slonim, A.D.; Shah, S.S. A Multi-institutional, 5-year analysis of initial and multiple ventricular shunt revisions in children. Neurosurgery 2008, 62, 445–454. [Google Scholar] [CrossRef]
- Miglioretti, D.L.; Johnson, A.E.; Williams, E.A.; Greenlee, R.T.; Weinmann, S.; Solberg, L.I.; Feigelson, H.S.; Roblin, D.; Flynn, M.J.; Vanneman, N.; et al. The Use of Computed Tomography in Pediatrics and the Associated Radiation Exposure and Estimated Cancer Risk. JAMA Pediatr. 2013, 167, 700–707. [Google Scholar] [CrossRef]
- Mandiwanza, T.; Saidlear, C.; Caird, J.; Crimmins, D. The open fontanelle: A window to less radiation. Child Nerv. Syst. 2013, 29, 1177–1181. [Google Scholar] [CrossRef]
- Udayasankar, U.; Braithwaite, K.; Arvaniti, M.; Tudorascu, D.; Small, W.; Little, S.; Palasis, S. Low-Dose Nonenhanced Head CT Protocol for Follow-Up Evaluation of Children with Ventriculoperitoneal Shunt: Reduction of Radiation and Effect on Image Quality. Am. J. Neuroradiol. 2008, 29, 802–806. [Google Scholar] [CrossRef] [Green Version]
- Marchese, R.F.; Schwartz, E.S.; Heuer, G.G.; Lavelle, J.; Huh, J.W.; Bell, L.M.; Luan, X.; Zorc, J.J. Reduced Radiation in Children Presenting to the ED with Suspected Ventricular Shunt Complication. Pediatrics 2017, 139, e20162431. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Rate per Patient (Range) | Rate per Shunt Year | |
|---|---|---|
| cCT | 1.9 (0–12) | 0.23 |
| Lateral skull X-ray | 2.37 (0–13) | 0.29 |
| Shunt series | 0.92 (0–4) | 0.11 |
| MRI head scan | 6.63 (0–17) | 0.82 |
| mSv per patient (range) | mSv per shunt year | |
| Hydrocephalus-associated radiation | 3.93 (0–24.38) | 0.49 |
| Comorbidity-associated radiation | 4.3 (0–80.8) | 1.85 |
| Study | Patients | Shunt Years | cCTs per Shunt Year | Shunt Series per Shunt Year | Skull X-rays per Shunt Year | Head MRIs per Shunt Year |
|---|---|---|---|---|---|---|
| Present study | 41 | 330 | 0.23 | 0.12 | 0.37 | 0.68 |
| Antonucci et al. | 130 | 1300 | 0.9 | 0.3 | 0.1 | 0.1 |
| White et al. | 62 | 989 | 0.97 | |||
| Dobson et al. | 152 | 778 | 0.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schabl, L.; Küppers, J.; Jhala, T.; Winicker, H.; Esslinger, P.; Lehner, M. Global Irradiation in Children Treated for Hydrocephalus and Its Change over Time—A Single Institutional Analysis. Children 2022, 9, 1062. https://doi.org/10.3390/children9071062
Schabl L, Küppers J, Jhala T, Winicker H, Esslinger P, Lehner M. Global Irradiation in Children Treated for Hydrocephalus and Its Change over Time—A Single Institutional Analysis. Children. 2022; 9(7):1062. https://doi.org/10.3390/children9071062
Chicago/Turabian StyleSchabl, Lukas, Julia Küppers, Tobias Jhala, Hermann Winicker, Peter Esslinger, and Markus Lehner. 2022. "Global Irradiation in Children Treated for Hydrocephalus and Its Change over Time—A Single Institutional Analysis" Children 9, no. 7: 1062. https://doi.org/10.3390/children9071062






