Defining Equinus Foot in Cerebral Palsy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Eligibility Criteria
2.2. Study Participants and Measurements
2.3. Statistical Analysis
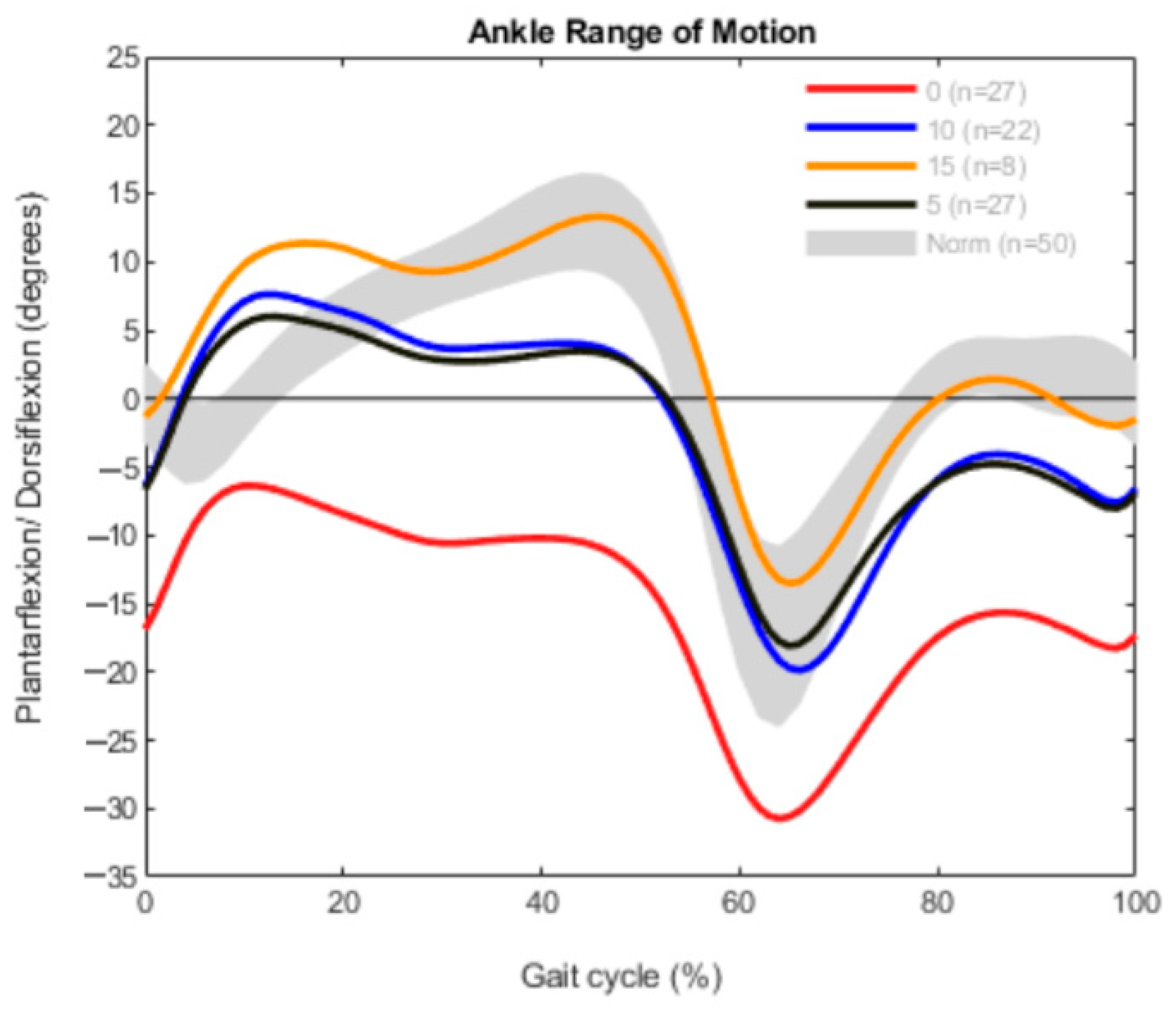
3. Results
3.1. Baseline Characteristics of Included Participants
3.2. Measured Outcomes in the Bilateral CP Group
3.3. Measured Outcomes in the Unilateral Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DeHeer, P.A. Equinus and Lengthening Techniques. Clin. Podiatr. Med. Surg. 2017, 34, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, X.; Pu, F.; Yang, Y.; Wang, L.; Liu, H.; Fan, Y. Conservative treatment for equinus deformity in children with cerebral palsy using an adjustable splint-assisted ankle-foot orthosis. Medicine 2017, 96, e8186. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, M.; Harper, D.C. Management of cerebral palsy: Equinus gait. Dev. Med. Child Neurol. 2001, 43, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Rutz, E.; McCarthy, J.; Shore, B.J.; Shrader, M.W.; Veerkamp, M.; Chambers, H.; Davids, J.R.; Kay, R.M.; Narayanan, U.; Novacheck, T.F.; et al. Indications for gastrocsoleus lengthening in ambulatory children with cerebral palsy: A Delphi consensus study. J. Child. Orthop. 2020, 14, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Shore, B.J.; White, N.; Kerr Graham, H. Surgical correction of equinus deformity in children with cerebral palsy: A systematic review. J. Child. Orthop. 2010, 4, 277–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gendy, S.; ElGebeily, M.; El-Sobky, T.A.; Khoshhal, K.I.; Jawadi, A.H. Current practice and preferences to management of equinus in children with ambulatory cerebral palsy: A survey of orthopedic surgeons. Sicot-J. 2019, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Lovell, W.W.; Winter, R.B. Lovell and Winter’s Pediatric Orthopaedics; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1990; Volume 1. [Google Scholar]
- Horsch, A.; Götze, M.; Geisbüsch, A.; Beckmann, N.; Tsitlakidis, S.; Berrsche, G.; Klotz, M. Prevalence and classification of equinus foot in bilateral spastic cerebral palsy. World J. Pediatrics 2019, 15, 276–280. [Google Scholar] [CrossRef]
- Dietz, F.R.; Albright, J.C.; Dolan, L. Medium-term follow-up of Achilles tendon lengthening in the treatment of ankle equinus in cerebral palsy. Iowa Orthop. J. 2006, 26, 27–32. [Google Scholar] [PubMed]
- Ferreira, L.A.; Cimolin, V.; Costici, P.F.; Albertini, G.; Oliveira, C.S.; Galli, M. Effects of gastrocnemius fascia lengthening on gait pattern in children with cerebral palsy using the gait profile score. Res. Dev. Disabil. 2014, 35, 1137–1143. [Google Scholar] [CrossRef]
- Firth, G.B.; Passmore, E.; Sangeux, M.; Thomason, P.; Rodda, J.; Donath, S.; Selber, P.; Graham, H.K. Multilevel surgery for equinus gait in children with spastic diplegic cerebral palsy: Medium-term follow-up with gait analysis. J. Bone Jt. Surg. Am. Vol. 2013, 95, 931–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franki, I.; De Cat, J.; Deschepper, E.; Molenaers, G.; Desloovere, K.; Himpens, E.; Vanderstraeten, G.; Van den Broeck, C. A clinical decision framework for the identification of main problems and treatment goals for ambulant children with bilateral spastic cerebral palsy. Res. Dev. Disabil. 2014, 35, 1160–1176. [Google Scholar] [CrossRef] [PubMed]
- Steinwender, G.; Saraph, V.; Zwick, E.B.; Uitz, C.; Linhart, W. Fixed and dynamic equinus in cerebral palsy: Evaluation of ankle function after multilevel surgery. J. Pediatric Orthop. 2001, 21, 102–107. [Google Scholar] [CrossRef]
- Horsch, A.; Klotz, M.C.M.; Platzer, H.; Seide, S.; Zeaiter, N.; Ghandour, M. Is the Prevalence of Equinus Foot in Cerebral Palsy Overestimated? Results from a Meta-Analysis of 4814 Feet. J. Clin. Med. 2021, 10, 4128. [Google Scholar] [CrossRef] [PubMed]
- Galen, S.; Wiggins, L.; McWilliam, R.; Granat, M. A combination of Botulinum Toxin A therapy and Functional Electrical Stimulation in children with cerebral palsy–a pilot study. Technol. Health Care Off. J. Eur. Soc. Eng. Med. 2012, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hastings-Ison, T.; Sangeux, M.; Thomason, P.; Rawicki, B.; Fahey, M.; Graham, H.K. Onabotulinum toxin-A (Botox) for spastic equinus in cerebral palsy: A prospective kinematic study. J. Child. Orthop. 2018, 12, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Maas, J.; Dallmeijer, A.; Huijing, P.; Brunstrom-Hernandez, J.; van Kampen, P.; Bolster, E.; Dunn, C.; Herndon, K.; Jaspers, R.; Becher, J. A randomized controlled trial studying efficacy and tolerance of a knee-ankle-foot orthosis used to prevent equinus in children with spastic cerebral palsy. Clin. Rehabil. 2014, 28, 1025–1038. [Google Scholar] [CrossRef]
- Nakagawa, S.; Mutsuzaki, H.; Mataki, Y.; Endo, Y.; Matsuda, M.; Yoshikawa, K.; Kamada, H.; Iwasaki, N.; Yamazaki, M. Safety and immediate effects of Hybrid Assistive Limb in children with cerebral palsy: A pilot study. Brain Dev. 2020, 42, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Putz, C.; Mertens, E.M.; Wolf, S.I.; Geisbüsch, A.; Niklasch, M.; Gantz, S.; Döderlein, L.; Dreher, T.; Klotz, M.C. Equinus Correction During Multilevel Surgery in Adults With Cerebral Palsy. Foot Ankle Int. 2018, 39, 812–820. [Google Scholar] [CrossRef]
- Palisano, R.; Rosenbaum, P.; Walter, S.; Russell, D.; Wood, E.; Galuppi, B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev. Med. Child Neurol. 1997, 39, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, H.K.; Kadaba, M.P. On the estimation of joint kinematics during gait. J. Biomech. 1991, 24, 969–977. [Google Scholar] [CrossRef]
- Perry, J.; Burnfield, J.M. Gait Analysis Normal and Pathological Function; Slack Incorporated: Thorofare, NJ, USA, 2010; Volume 2. [Google Scholar]
- Lamm, B.M.; Paley, D.; Herzenberg, J.E. Gastrocnemius soleus recession: A simpler, more limited approach. J. Am. Podiatr. Med. Assoc. 2005, 95, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Southerland, J.T.; Boberg, J.S.; Downey, M.S.; Nakra, A.; Rabjohn, L.V. McGlamry’s Comprehensive Textbook of Foot and Ankle Surgery; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Herzenberg, J.E.; Lamm, B.M.; Corwin, C.; Sekel, J. Isolated recession of the gastrocnemius muscle: The Baumann procedure. Foot Ankle Int. 2007, 28, 1154–1159. [Google Scholar] [CrossRef] [PubMed]
- Digiovanni, C.W.; Holt, S.; Czerniecki, J.M.; Ledoux, W.R.; Sangeorzan, B.J. Can the presence of equinus contracture be established by physical exam alone? J. Rehabil. Res. Dev. 2001, 38, 335–340. [Google Scholar] [PubMed]
- Sobel, E.; Caselli, M.A.; Velez, Z. Effect of persistent toe walking on ankle equinus. Analysis of 60 idiopathic toe walkers. J. Am. Podiatr. Med. Assoc. 1997, 87, 17–22. [Google Scholar] [CrossRef]
- Orendurff, M.S.; Rohr, E.S.; Sangeorzan, B.J.; Weaver, K.; Czerniecki, J.M. An equinus deformity of the ankle accounts for only a small amount of the increased forefoot plantar pressure in patients with diabetes. J. Bone Jt. Surg. Br. Vol. 2006, 88, 65–68. [Google Scholar] [CrossRef] [Green Version]
- DiGiovanni, C.W.; Kuo, R.; Tejwani, N.; Price, R.; Hansen, S.T., Jr.; Cziernecki, J.; Sangeorzan, B.J. Isolated gastrocnemius tightness. J. Bone Jt. Surg. Am. Vol. 2002, 84, 962–970. [Google Scholar] [CrossRef]
- Perry, J.; Burnfield, J.M.; Gronley, J.K.; Mulroy, S.J. Toe walking: Muscular demands at the ankle and knee. Arch. Phys. Med. Rehabil. 2003, 84, 7–16. [Google Scholar] [CrossRef]
- Meyer, D.C.; Werner, C.M.; Wyss, T.; Vienne, P. A mechanical equinometer to measure the range of motion of the ankle joint: Interobserver and intraobserver reliability. Foot Ankle Int. 2006, 27, 202–205. [Google Scholar] [CrossRef]
- Ma, N.; Sclavos, N.; Passmore, E.; Thomason, P.; Graham, K.; Rutz, E. Three-Dimensional Gait Analysis in Children Undergoing Gastrocsoleus Lengthening for Equinus Secondary to Cerebral Palsy. Medicina 2021, 57, 98. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horsch, A.; Petzinger, L.; Ghandour, M.; Putz, C.; Renkawitz, T.; Götze, M. Defining Equinus Foot in Cerebral Palsy. Children 2022, 9, 956. https://doi.org/10.3390/children9070956
Horsch A, Petzinger L, Ghandour M, Putz C, Renkawitz T, Götze M. Defining Equinus Foot in Cerebral Palsy. Children. 2022; 9(7):956. https://doi.org/10.3390/children9070956
Chicago/Turabian StyleHorsch, Axel, Lara Petzinger, Maher Ghandour, Cornelia Putz, Tobias Renkawitz, and Marco Götze. 2022. "Defining Equinus Foot in Cerebral Palsy" Children 9, no. 7: 956. https://doi.org/10.3390/children9070956
APA StyleHorsch, A., Petzinger, L., Ghandour, M., Putz, C., Renkawitz, T., & Götze, M. (2022). Defining Equinus Foot in Cerebral Palsy. Children, 9(7), 956. https://doi.org/10.3390/children9070956





