Mitral Atresia with Normal Aortic Root
Abstract
:1. Introduction
2. Pathologic Anatomy
3. Pathophysiology
4. Clinical Presentation
4.1. Inter-Atrial Obstruction
4.2. Severe Pulmonary Stenosis or Pulmonary Atresia
4.3. No Pulmonary Stenosis
5. Physical Examination
6. Chest X-ray
7. Electrocardiogram (ECG)
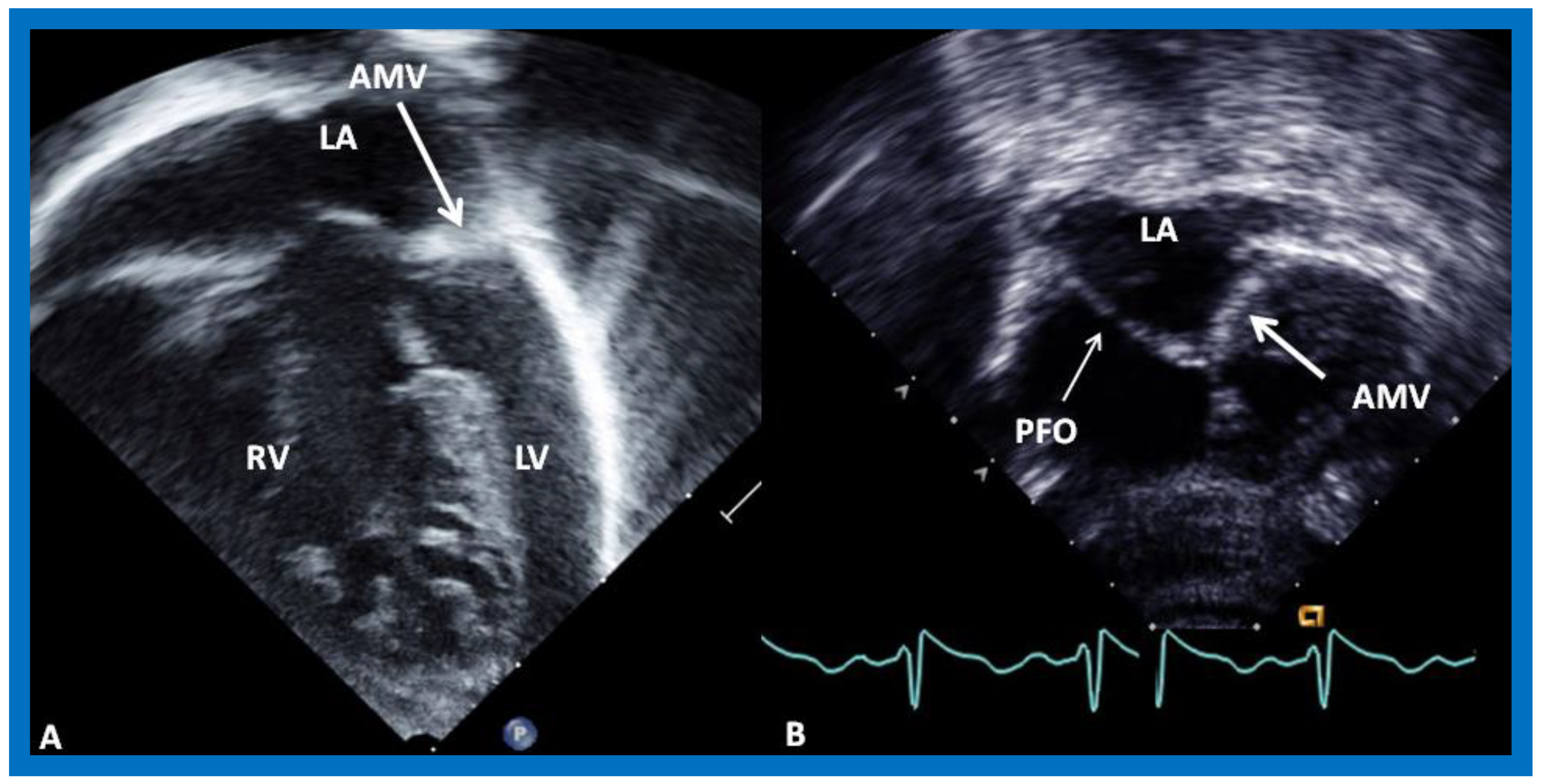
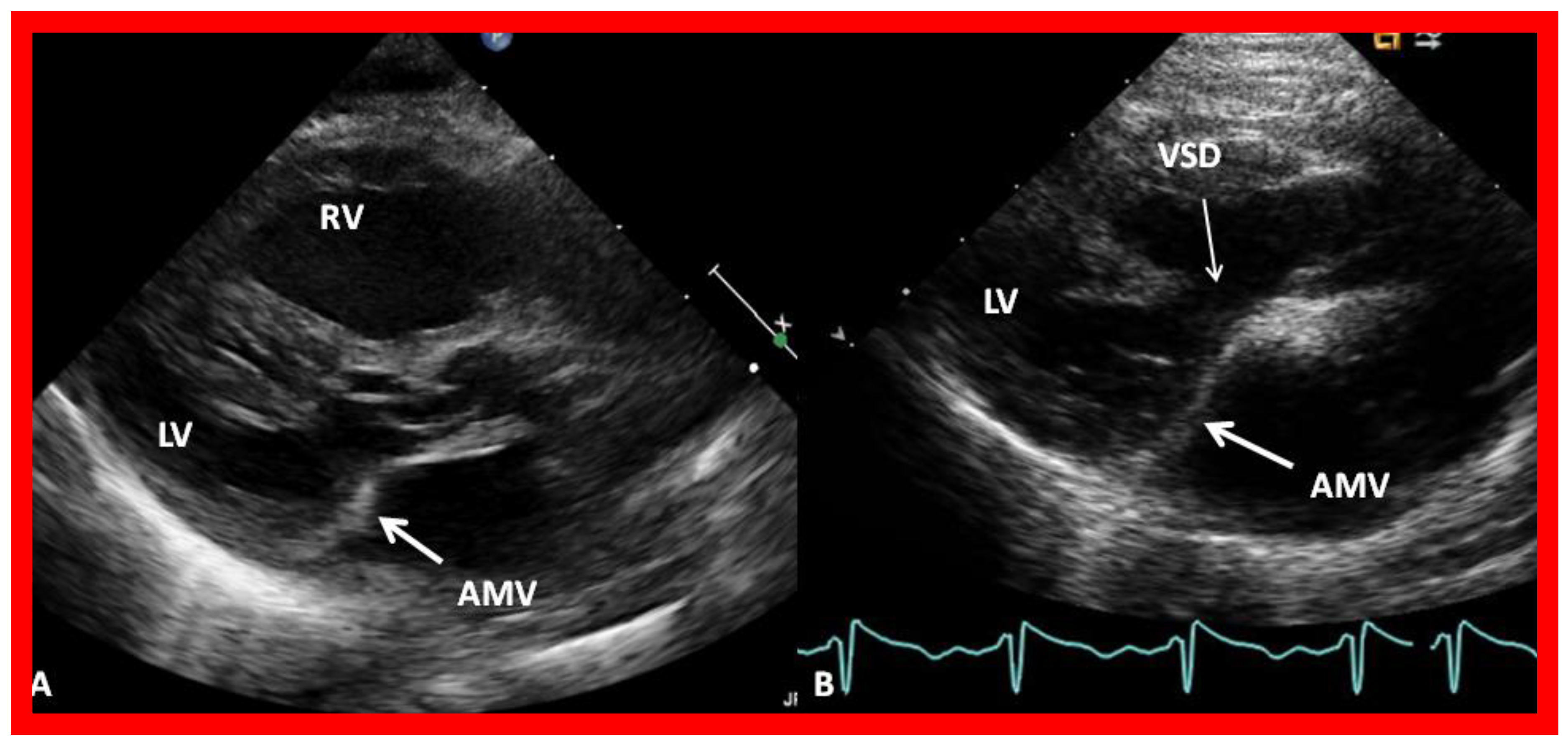
8. Echocardiogram
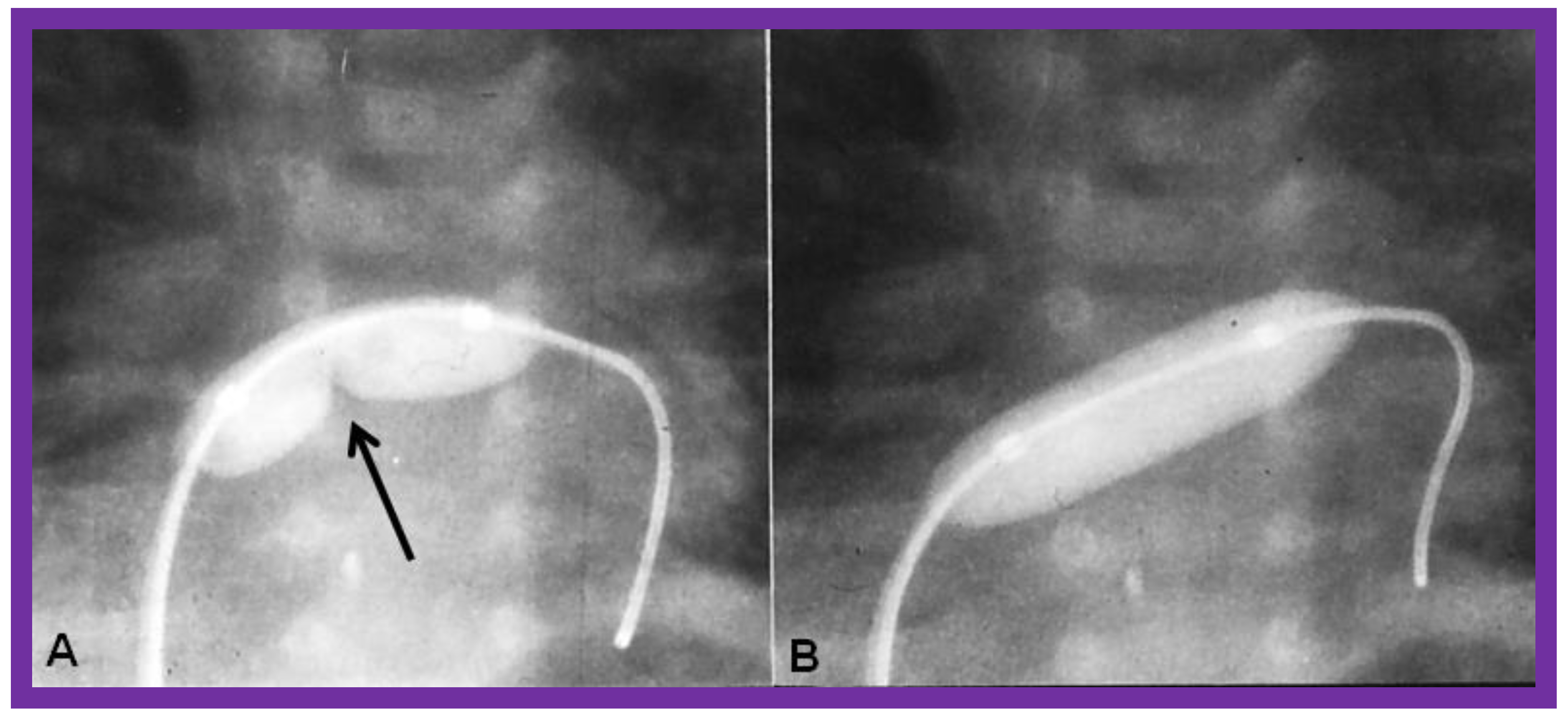
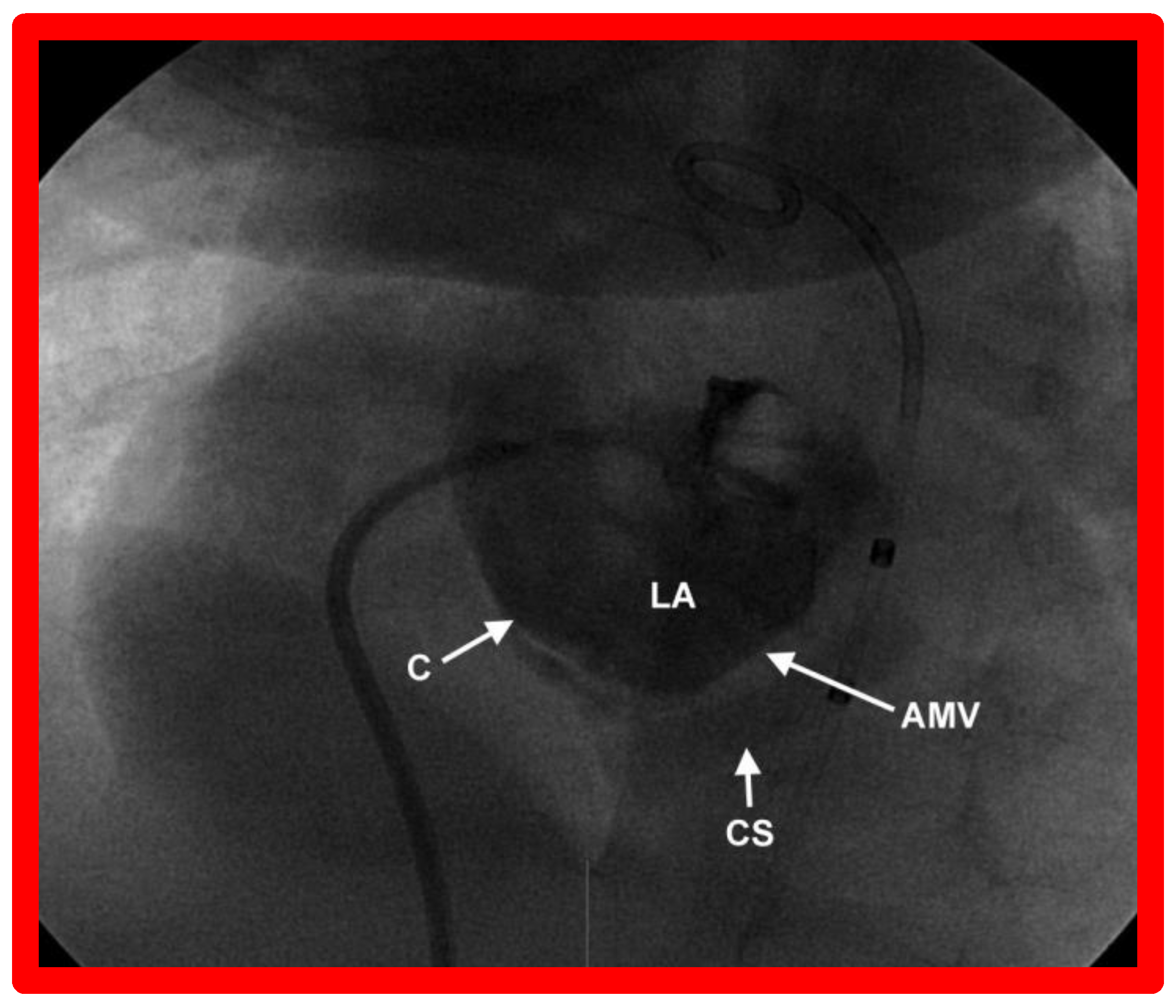
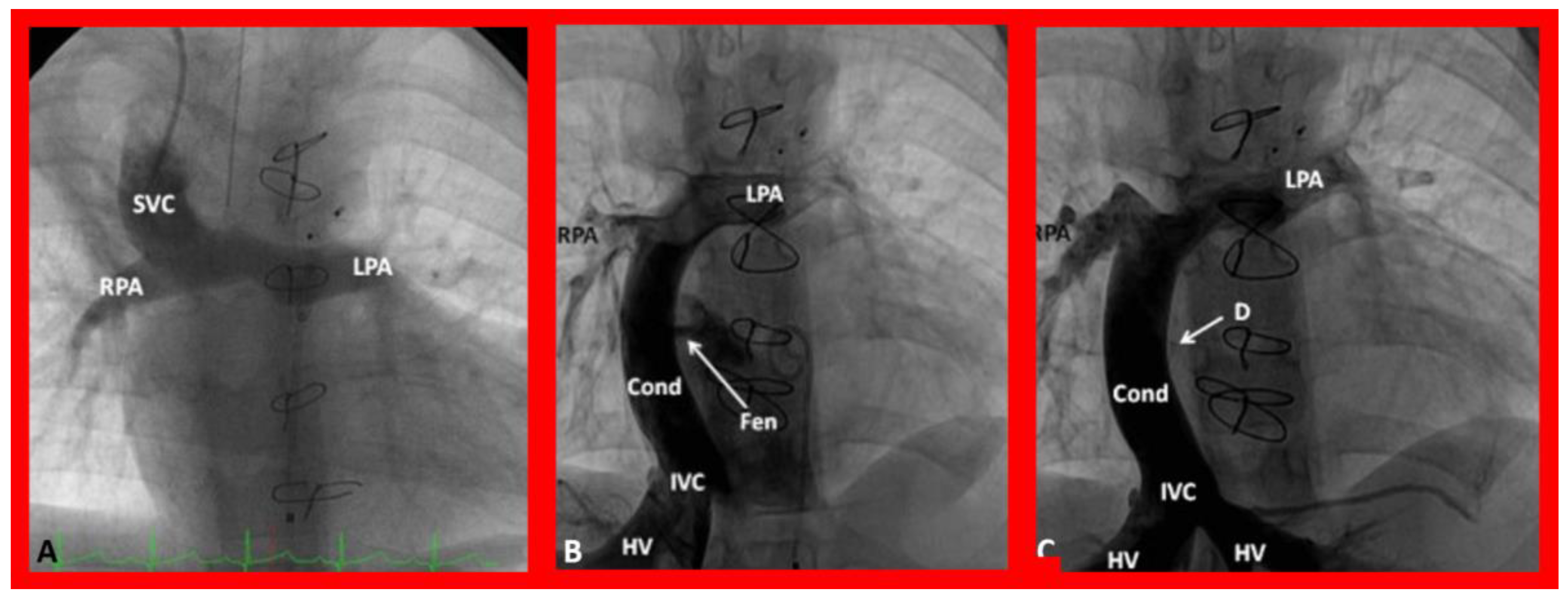
9. Cardiac Catheterization with Angiography, Magnetic Resonance Imaging (MRI) and Computed Tomography (CT)
10. Therapy
10.1. Therapy at Initial Presentation
10.1.1. Inter-Atrial Obstruction
10.1.2. Decreased Pulmonary Blood Flow
10.1.3. Increased Pulmonary Blood Flow with Heart Failure
10.1.4. Increased Pulmonary Blood Flow without Heart Failure
10.1.5. Increased Pulmonary Blood Flow along with Inter-Atrial Obstruction
10.2. Follow-Up after Initial Palliation
10.3. Additional Surgery
10.4. Follow-Up after Palliative and Corrective Surgery
11. Summary and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rao, P.S.; Striepe, V.; Kambam, J. Hypoplastic left heart syndrome. In Cardiac Anesthesia for Infants and Children; Kambam, J., Ed.; Mosby-Year Book: St. Louis, MO, USA, 1994; pp. 296–309. [Google Scholar]
- Rao, P.S.; Alapati, S. Hypoplastic left heart syndrome. In A Comprehensive Approach to Management of Congenital Heart Diseases; Vijayalakshmi, I.B., Rao, P.S., Chugh, R., Eds.; Jaypee Publications: New Delhi, India, 2013; pp. 662–678. [Google Scholar]
- Alapati, S.; Rao, P.S. Hypoplastic left heart syndrome in the neonate. Neonatol. Today 2011, 6, 1–9. [Google Scholar]
- Balaguru, D.; Rao, P.S. Mitral Atresia (with Normal Aortic Root). In A Comprehensive Approach to Management of Congenital Heart Diseases; Vijayalakshmi, I.B., Rao, P.S., Chugh, R., Eds.; Jaypee Publications: New Delhi, India, 2013; pp. 458–467. [Google Scholar]
- Norwood, W.I.; Kirklin, J.K.; Sanders, S.P. Hypoplastic left heart syndrome: Experience with palliative surgery. Am. J. Cardiol. 1980, 45, 87–91. [Google Scholar] [CrossRef]
- Norwood, W.I.; Lang, P.; Hansen, D.D. Physiologic repair of aortic atresia-hypoplastic left heart syndrome. N. Engl. J. Med. 1983, 308, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.S. Other cyanotic heart defects in the neonate. In A Multidisciplinary Approach to Perinatal Cardiology; Rao, P.S., Vidyasagar, D., Eds.; Cambridge Scholars Publishing: New Castle upon Tyne, UK, 2021; Volume 2, pp. 474–509. [Google Scholar]
- Anderson, R.H.; Thiene, G. The clinical morphology of mitral atresia. Atresia of the left atrioventricular valve. G. Ital. Cardio. 1981, 11, 1860–1870. [Google Scholar]
- Thiene, G.; Salient, L.; Frescoer, C.; De Tommasi, M.; Macartney, F.J.; Anderson, R.H. Atresia of left atrioventricular valve. Anatomic investigation in 62 cases. Br. Heart J. 1981, 45, 393–401. [Google Scholar] [CrossRef] [Green Version]
- Rao, P.S. A unified classification of tricuspid atresia. Am. Heart J. 1980, 99, 799–804. [Google Scholar] [CrossRef]
- Keith, J.D. Congenital mitral atresia. In Heart Disease in Infancy and Childhood, 3rd ed.; Keith, J.D., Rowe, R.D., Lad, P., Eds.; Macmillan: New York, NY, USA, 1977; pp. 455–549. [Google Scholar]
- Lucas, R.V., Jr.; Lester, R.G.; Lillehei, C.W.; Edwards, J.E. Mitral atresia with levoatriocardinal vein. A form of congenital pulmonary venous obstruction. Am. J. Cardiol. 1962, 9, 607–613. [Google Scholar] [CrossRef]
- Eliot, R.S.; Shone, J.D.; Kanjuh, V.I.; Ruttenberg, H.D.; Carey, L.S.; Edwards, J.E. Mitral atresia. A study of 32 cases. Am. Heart J. 1965, 70, 6–22. [Google Scholar] [CrossRef]
- Moreno, F.; Quero, M.; Diaz, L.P. Mitral atresia with normal aortic valve: A study of eighteen cases and a review of the literature. Circulation 1976, 53, 1004–1010. [Google Scholar] [CrossRef] [Green Version]
- Watson, D.G.; Rowe, R.D.; Conan, P.E.; Duckworth, J.W. Mitral atresia with normal aortic valve. Report of 11 cases and review of literature. Pediatrics 1960, 25, 450–467. [Google Scholar]
- Rao, P.S. An Approach to the diagnosis of cyanotic neonate for the primary care provider. Neonatol. Today 2007, 2, 1–7. [Google Scholar]
- Rao, P.S. Principles of management of the neonate with congenital heart disease. Neonatol. Today 2007, 2, 1–10. [Google Scholar]
- Rao, P.S. Principles of management of the neonate with congenital heart disease. In A Multidisciplinary Approach to Perinatal Cardiology; Rao, P.S., Vidyasagar, D., Eds.; Cambridge Scholars Publishing: New Castle upon Tyne, UK, 2021; Volume 1, pp. 474–509. [Google Scholar]
- Rashkind, W.J.; Miller, W.W. Creation of an atrial septal defect without thoracotomy: Palliative approach to complete transposition of the great arteries. J. Am. Med. Assoc. 1966, 196, 991–992. [Google Scholar] [CrossRef]
- Rao, P.S. Role of interventional cardiology in neonates: Part I. Non-surgical atrial septostomy. Neonatol. Today 2007, 2, 9–14. [Google Scholar]
- Rao, P.S. Neonatal Catheter Interventions. In Cardiac Catheterization and Imaging (from Pediatrics to Geriatrics); Vijayalakshmi, I.B., Ed.; Jaypee Publications: New Delhi, India, 2015; pp. 388–432. [Google Scholar]
- Rao, P.S.; Kulangara, R.J.; Moore, H.V.; Strong, W.B. Syndrome of single ventricle without pulmonary stenosis but with left atrioventricular vale atresia and interatrial obstruction. J. Thorac. Cardiovasc. Surg. 1981, 81, 127–132. [Google Scholar] [CrossRef]
- Shrivastava, S.; Radhakrishnan, S.; Dev, V.; Singh, L.S.; Rajani, M. Balloon dilatation of atrial septum in complete transposition of great arteries—A new technique. Indian Heart J. 1987, 39, 298–300. [Google Scholar]
- Rao, P.S. Static balloon dilation of atrial septum (Editorial). Am. Heart J. 1993, 125, 1824–1987. [Google Scholar] [PubMed]
- Ross, J., Jr.; Braunwald, E.; Morrow, A.G. Transseptal left atrial puncture: New technique for the measurement of left atrial pressure in man. Am. J. Cardiol. 1959, 3, 653–655. [Google Scholar] [CrossRef]
- Atz, A.M.; Feinstein, J.A.; Jonas, R.A.; Perry, S.B.; Wessel, D.L. Preoperative management of pulmonary venous hypertension in hypoplastic left heart syndrome with restrictive atrial septal defect. Am. J. Cardiol. 1999, 83, 1224–1228. [Google Scholar] [CrossRef]
- Justino, H.; Benson, L.N.; Nykanen, D.G. Transcatheter creation of an atrial septal defect using radiofrequency perforation. Catheter. Cardiovasc. Intervent. 2001, 54, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Gewillig, M.; Boshoff, L.; Mertens, L. Creation with a stent of an unrestrictive lasting atrial communication. Cardiol. Young. 2002, 12, 404–407. [Google Scholar] [CrossRef]
- Blalock, A.; Taussig, H.B. The surgical treatment of malformations of the heart in which there is pulmonary stenosis or atresia. J. Am. Med. Assoc. 1945, 128, 189–194. [Google Scholar] [CrossRef]
- de Leval, M.R.; McKay, R.; Jones, M.; Stark, J.; Macartney, F.J. Modified Blalock-Taussig shunt: Use of subclavian orifice as a flow regulator in prosthetic systemic-pulmonary artery shunts. J. Thorac. Cardiovasc. Surg. 1981, 18, 112–119. [Google Scholar] [CrossRef]
- Siblini, G.; Rao, P.S.; Singh, G.K.; Tinker, K.; Balfour, I.C. Transcatheter management of neonates with pulmonary atresia and intact ventricular septum. Catheter. Cardiovasc. Diagn. 1997, 42, 395–402. [Google Scholar] [CrossRef]
- Gibbs, J.L.; Uzun, O.; Blackburn, M.E.; Wren, C.; Hamilton, J.L.; Watterson, K.G. Fate of stented arterial duct. Circulation 1999, 99, 2621–2625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alwi, M.; Choo, K.K.; Latiff, H.A.; Kandavello, G.; Samion, H.; Mulyadi, M.D. Initial results and medium-term follow-up of stent implantation of patent ductus arteriosus in duct-dependent pulmonary circulation. J. Am. Coll. Cardiol. 2004, 44, 438–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, P.S.; Brais, M. Balloon pulmonary valvuloplasty for congenital cyanotic heart defects. Am. Heart J. 1988, 115, 1105–1110. [Google Scholar] [CrossRef]
- Rao, P.S.; Wilson, A.D.; Thapar, M.K.; Brais, M. Balloon pulmonary valvuloplasty in the management of cyanotic congenital heart defects. Catheter. Cardiovasc. Diagn. 1992, 25, 16–24. [Google Scholar] [CrossRef]
- Rao, P.S. Pulmonary valve disease: Pulmonary valve in cyanotic heart defects with pulmonary oligemia. In Interventions in Structural, Valvular and Congenital Heart Disease; Sievert, H., Qureshi, S.A., Wilson, N., Hijazi, Z., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 297–308. [Google Scholar]
- Muller, W.H., Jr.; Danimann, J.F., Jr. The treatment of certain congenital malformations of the heart by the creation of pulmonic stenosis to reduce pulmonary hypertension and excessive pulmonary blood flow; a preliminary report. Surg. Gynecol. Obstet. 1952, 95, 213–219. [Google Scholar] [PubMed]
- Rao, P.S. Echocardiography in the diagnosis and management of tricuspid atresia. Appl. Sci. 2021, 11, 9472. [Google Scholar] [CrossRef]
- Rao, P.S. Congenital heart disease. In Conn’s Current Therapy; Rakel, R.E., Ed.; WB Saunders Co.: Philadelphia, PA, USA, 1989; pp. 201–213. [Google Scholar]
- Fontan, F.; Baudet, E. Surgical repair of tricuspid atresia. Thorax 1971, 26, 240–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreutzer, G.; Bono, H.; Galindez, E. Una operacion para la correccion de la atresia tricuspidea. In Proceedings of the Ninth Argentinean Congress of Cardiology, Buenos Aires, Argentina, 31 October–6 November 1971. [Google Scholar]
- Chopra, P.S.; Rao, P.S. Corrective surgery for tricuspid atresia: Which modifications of Fontan-Kreutzer procedure should be used? A review. Am. Heart J. 1992, 123, 758–767. [Google Scholar] [CrossRef]
- Rao, P.S.; Chopra, P.S. Modification of Fontan-Kreutzer procedure for tricuspid atresia: Can a choice be made? In Tricuspid Atresia, 2nd ed.; Futura Publishing Co.: Mount Kisco, NY, USA, 1992; pp. 361–375. [Google Scholar]
- Rao, P.S. Fontan operation: Indications, short- and long-term outcomes. Indian J. Pediatr. 2015, 82, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.S. Pediatric Tricuspid Atresia. Medscape Drugs & Diseases. Updated 17 January 2017. Available online: http://emedicine.medscape.com/article/900832-overview (accessed on 29 July 2022).
- Rao, P.S. Fontan operation: A comprehensive review. In Fontan Surgery; Khan, I., Ed.; InTechOpen: Rijeka, Croatia, 2020. [Google Scholar] [CrossRef]
- Rao, P.S. Single Ventricle—A Comprehensive Review. Children 2021, 8, 441. [Google Scholar] [CrossRef]
- de Leval, M.R.; Kilner, P.; Gewillig, M.; Bull, C.; McGoon, D.C. Total cavopulmonary connection: A logical alternative to atriopulmonary connection for complex Fontan operation. J. Thorac. Cardiovasc. Surg. 1988, 96, 682–695. [Google Scholar] [CrossRef]
- Marcelletti, C.; Corno, A.; Giannico, S.; Marino, B. Inferior vena cava-pulmonary artery extra-cardiac conduit. A new form of right heart bypass. J. Thorac. Cardiovasc. Surg. 1990, 100, 228–232. [Google Scholar] [CrossRef]
- Marcelletti, C.; Iorio, F.S.; Abella, R.F. Late results of extra-cardiac Fontan repair. In Seminars in Thoracic and Cardiovascular Surgery: Pediatric Cardiac Surgery Annual; WB Saunders: Philadelphia, PA, USA, 1999; Volume 2, pp. 131–141. [Google Scholar]
- Bridges, N.D.; Lock, J.E.; Castaneda, A.R. Baffle fenestration with subsequent transcatheter closure. Modification of the Fontan operation for patients at increased risk. Circulation 1990, 82, 1681–1689. [Google Scholar] [CrossRef] [Green Version]
- Laks, H.; Pearl, J.M.; Haas, G.S.; Drinkwater, D.C.; Milgalter, E.; Jarmakani, J.M.; Isabel-Jones, J.; George, B.L.; Williams, R.G. Partial Fontan: Advantages of an adjustable interatrial communication. Ann. Thorac. Surg. 1991, 52, 1084–1094. [Google Scholar] [CrossRef]
- Rao, P.S.; Chandar, J.S.; Sideris, E.B. Role of inverted buttoned device in transcatheter occlusion of atrial septal defects or patent foramen ovale with right-to-left shunting associated with previously operated complex congenital cardiac anomalies. Am. J. Cardiol. 1997, 80, 914–921. [Google Scholar] [CrossRef]
- Goff, D.A.; Blume, E.D.; Gauvreau, K.; Mayer, J.E.; Lock, J.E.; Jenkins, K.J. Clinical advances in complex valvular disease: Outcome of fenestrated Fontan patients after closure: The first 10 years. Circulation 2000, 102, 2094–2099. [Google Scholar] [CrossRef] [Green Version]
- Boudjemline, Y.; Bonnet, D.; Sidi, D.; Agnoletti, G. Closure of extracardiac Fontan fenestration by using the Amplatzer duct occluder. Arch. Mal. Coeur Vaiss. 2005, 98, 449–454. [Google Scholar] [PubMed]
- Tweddell, J.S.; Hoffman, G.M.; Mussatto, K.A.; Fedderly, R.T.; Berger, S.; Jaquiss, R.D.; Ghanayem, N.S.; Frisbee, S.J.; Litwin, S.B. Improved survival of patients undergoing palliation of hypoplastic left heart syndrome: Lessons learned from 115 consecutive patients. Circulation 2002, 106, 82–89. [Google Scholar] [CrossRef]
- Yates, M.C.; Rao, P.S. Pediatric cardiac emergencies. Emerg. Med. 2013, 3, 164. [Google Scholar] [CrossRef] [Green Version]
- Bartram, U.; Grunenfelder, J.; Van Praagh, R. Causes of death after the modified Norwood procedure: A study of 122 postmortem cases. Ann. Thorac. Surg. 1997, 64, 1795–1802. [Google Scholar] [CrossRef]
- Cashen, K.; Gupta, P.; Lieh-Lai, M.; Mastropietro, C. Infants with single ventricle physiology in the emergency department: Are physicians prepared? J. Pediatr. 2011, 159, 273–277.e1. [Google Scholar] [CrossRef]
- Rao, P.S. Protein-losing enteropathy following the Fontan operation (editorial). J. Invasive Cardiol. 2007, 19, 447–448. [Google Scholar]
- Rao, P.S. What an adult cardiologist should know about cyanotic congenital heart disease? J. Cardiovasc. Dis. Diagn. 2013, 1, 104. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, P.S. Mitral Atresia with Normal Aortic Root. Children 2022, 9, 1148. https://doi.org/10.3390/children9081148
Rao PS. Mitral Atresia with Normal Aortic Root. Children. 2022; 9(8):1148. https://doi.org/10.3390/children9081148
Chicago/Turabian StyleRao, P. Syamasundar. 2022. "Mitral Atresia with Normal Aortic Root" Children 9, no. 8: 1148. https://doi.org/10.3390/children9081148
APA StyleRao, P. S. (2022). Mitral Atresia with Normal Aortic Root. Children, 9(8), 1148. https://doi.org/10.3390/children9081148





