Benefits of Physical Exercise as Approach to Prevention and Reversion of Non-Alcoholic Fatty Liver Disease in Children and Adolescents with Obesity
Abstract
:1. Introduction
2. Methods
3. Childhood Obesity and Non-Alcoholic Fatty Liver Disease
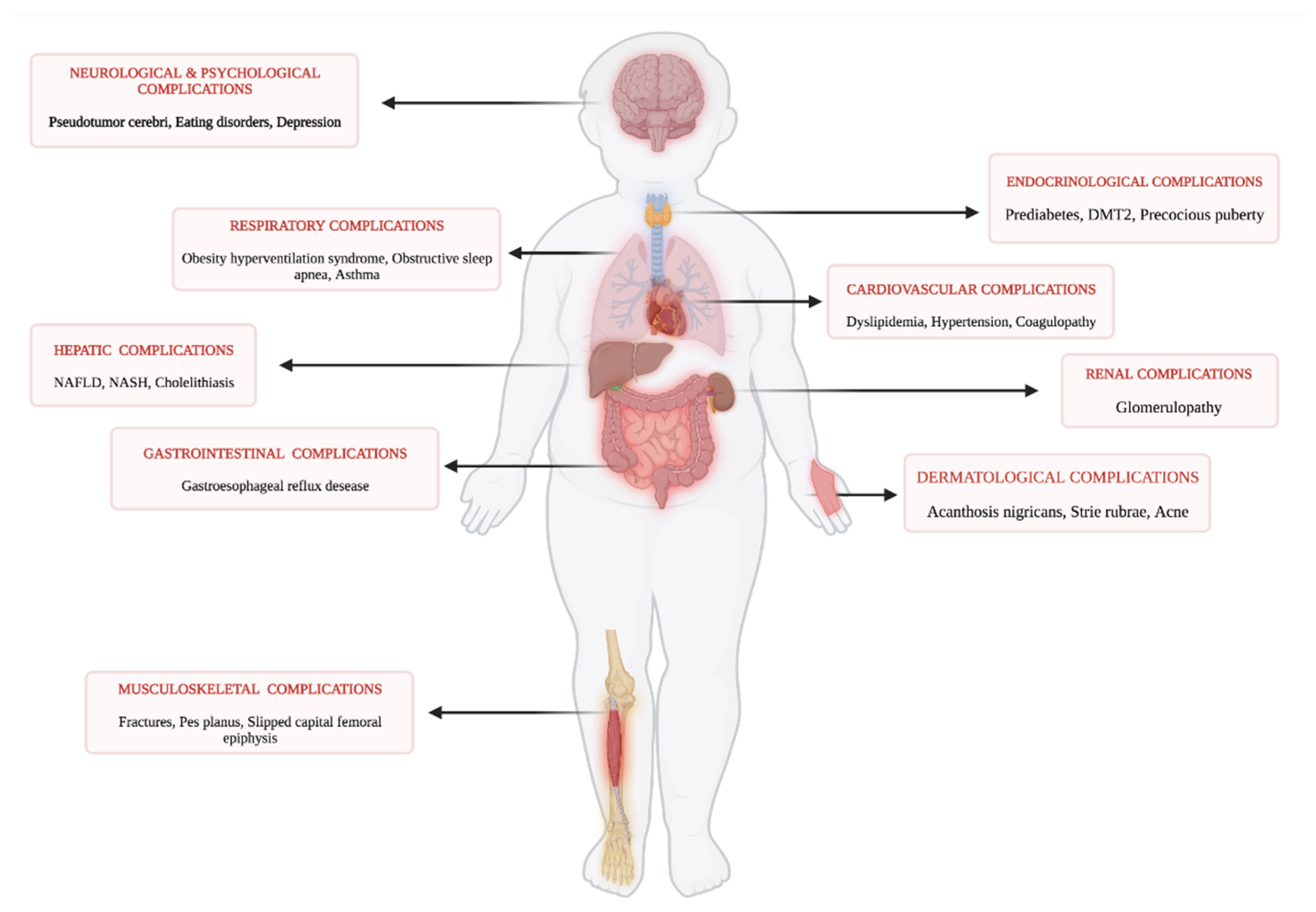
3.1. Childhood Obesity
3.2. Epidemiology of NAFLD in General Pediatric Population and in Children with Obesity
3.3. Pathogenesis and Risk Factors of NAFLD
3.4. Diagnosis of NAFLD
3.5. Treatments of NAFLD
4. Physical Activity Levels in Children and Adolescents with Obesity
5. The Effects of Physical Exercise on NAFLD
5.1. Exercise Intervention in NAFLD Patients
5.2. Exercise in NAFLD: Mechanisms of Action
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nobili, V.; Alisi, A.; Valenti, L.; Miele, L.; Feldstein, A.E.; Alkhouri, N. NAFLD in Children: New Genes, New Diagnostic Modalities and New Drugs. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Shaunak, M.; Byrne, C.D.; Davis, N.; Afolabi, P.; Faust, S.N.; Davies, J.H. Non-Alcoholic Fatty Liver Disease and Childhood Obesity. Arch. Dis. Child. 2021, 106, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Africa, J.A.; Newton, K.P.; Schwimmer, J.B. Lifestyle Interventions Including Nutrition, Exercise, and Supplements for Nonalcoholic Fatty Liver Disease in Children. Dig. Dis. Sci. 2016, 61, 1375–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nobili, V.; Svegliati-Baroni, G.; Alisi, A.; Miele, L.; Valenti, L.; Vajro, P. A 360-Degree Overview of Paediatric NAFLD: Recent Insights. J. Hepatol. 2013, 58, 1218–1229. [Google Scholar] [CrossRef] [Green Version]
- Cananzi, M.; Vajro, P.; Rela, M.; Dhawan, A. NAFLD and Liver Transplantation in Children—Working Group Report From the ILTS Single Topic Conference on NAFLD. Transplantation 2019, 103, 68–70. [Google Scholar] [CrossRef]
- Mann, J.P.; Tang, G.Y.; Nobili, V.; Armstrong, M.J. Evaluations of Lifestyle, Dietary, and Pharmacologic Treatments for Pediatric Nonalcoholic Fatty Liver Disease: A Systematic Review. Clin. Gastroenterol. Hepatol. 2019, 17, 1457–1476. [Google Scholar] [CrossRef] [Green Version]
- Kwak, M.-S.; Kim, D. Non-Alcoholic Fatty Liver Disease and Lifestyle Modifications, Focusing on Physical Activity. Korean J. Intern. Med. 2018, 33, 64–74. [Google Scholar] [CrossRef] [Green Version]
- Lin, G.; Xinhe, Z.; Haoyu, T.; Xing, J.; Dan, L.; Ningning, W.; Jing, S.; Xue, W.; Zilu, Z.; Yiling, L. Epidemiology and Lifestyle Survey of Non-Alcoholic Fatty Liver Disease in School-Age Children and Adolescents in Shenyang, Liaoning. BMC Pediatr. 2022, 22, 286. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Gregory, A.T.; Denniss, A.R. An Introduction to Writing Narrative and Systematic Reviews—Tasks, Tips and Traps for Aspiring Authors. Heart Lung Circ. 2018, 27, 893–898. [Google Scholar] [CrossRef] [Green Version]
- Jebeile, H.; Kelly, A.S.; O’Malley, G.; Baur, L.A. Obesity in Children and Adolescents: Epidemiology, Causes, Assessment, and Management. Lancet Diabetes Endocrinol. 2022, 10, 351–365. [Google Scholar] [CrossRef]
- Childhood Obesity Facts|Overweight & Obesity|CDC. Available online: https://www.cdc.gov/obesity/data/childhood.html (accessed on 29 June 2022).
- Lange, S.J.; Kompaniyets, L.; Freedman, D.S.; Kraus, E.M.; Porter, R.; Blanck, H.M.; Goodman, A.B. Longitudinal Trends in Body Mass Index Before and During the COVID-19 Pandemic Among Persons Aged 2–19 Years—United States, 2018–2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Dyer, O. Obesity in US Children Increased at an Unprecedented Rate during the Pandemic. BMJ 2021, 374, n2332. [Google Scholar] [CrossRef]
- Sahoo, K.; Sahoo, B.; Choudhury, A.; Sofi, N.; Kumar, R.; Bhadoria, A. Childhood Obesity: Causes and Consequences. J. Fam. Med. Prim. Care 2015, 4, 187. [Google Scholar] [CrossRef]
- Vandoni, M.; Calcaterra, V.; Pellino, V.C.; De Silvestri, A.; Marin, L.; Zuccotti, G.V.; Tranfaglia, V.; Giuriato, M.; Codella, R.; Lovecchio, N. “Fitness and Fatness” in Children and Adolescents: An Italian Cross-Sectional Study. Children 2021, 8, 762. [Google Scholar] [CrossRef]
- Calcaterra, V.; Zuccotti, G. Prevention and Treatment of Cardiometabolic Diseases in Children with Overweight and Obesity: The Future of Healthcare. Children 2022, 9, 176. [Google Scholar] [CrossRef]
- Calcaterra, V.; Larizza, D.; Codrons, E.; De Silvestri, A.; Brambilla, P.; Abela, S.; Arpesella, M.; Vandoni, M. Improved Metabolic and Cardiorespiratory Fitness during a Recreational Training Program in Obese Children. J. Pediatr. Endocrinol. Metab. 2013, 26, 271–276. [Google Scholar] [CrossRef]
- Calcaterra, V.; Verduci, E.; Ghezzi, M.; Cena, H.; Pascuzzi, M.C.; Regalbuto, C.; Lamberti, R.; Rossi, V.; Manuelli, M.; Bosetti, A.; et al. Pediatric Obesity-Related Asthma: The Role of Nutrition and Nutrients in Prevention and Treatment. Nutrients 2021, 13, 3708. [Google Scholar] [CrossRef]
- Calcaterra, V.; Verduci, E.; Cena, H.; Magenes, V.C.; Todisco, C.F.; Tenuta, E.; Gregorio, C.; De Giuseppe, R.; Bosetti, A.; Di Profio, E.; et al. Polycystic Ovary Syndrome in Insulin-Resistant Adolescents with Obesity: The Role of Nutrition Therapy and Food Supplements as a Strategy to Protect Fertility. Nutrients 2021, 13, 1848. [Google Scholar] [CrossRef]
- Kumar, S.; Kelly, A.S. Review of Childhood Obesity. Mayo Clin. Proc. 2017, 92, 251–265. [Google Scholar] [CrossRef] [Green Version]
- Valerio, G.; Maffeis, C.; Saggese, G.; Ambruzzi, M.A.; Balsamo, A.; Bellone, S.; Bergamini, M.; Bernasconi, S.; Bona, G.; Calcaterra, V.; et al. Diagnosis, Treatment and Prevention of Pediatric Obesity: Consensus Position Statement of the Italian Society for Pediatric Endocrinology and Diabetology and the Italian Society of Pediatrics. Ital. J. Pediatr. 2018, 44, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calcaterra, V.; Vandoni, M.; Rossi, V.; Berardo, C.; Grazi, R.; Cordaro, E.; Tranfaglia, V.; Carnevale Pellino, V.; Cereda, C.; Zuccotti, G. Use of Physical Activity and Exercise to Reduce Inflammation in Children and Adolescents with Obesity. Int. J. Environ. Res. Public. Health 2022, 19, 6908. [Google Scholar] [CrossRef]
- Calcaterra, V.; Cena, H.; Pelizzo, G.; Porri, D.; Regalbuto, C.; Vinci, F.; Destro, F.; Vestri, E.; Verduci, E.; Bosetti, A.; et al. Bariatric Surgery in Adolescents: To Do or Not to Do? Children 2021, 8, 453. [Google Scholar] [CrossRef] [PubMed]
- Bellentani, S.; Marino, M. Epidemiology and Natural History of Non-Alcoholic Fatty Liver Disease (NAFLD). Ann. Hepatol. 2009, 8 (Suppl. S1), S4–S8. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The Diagnosis and Management of Nonalcoholic Fatty Liver Disease: Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global Epidemiology of Nonalcoholic Fatty Liver Disease-Meta-Analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Lu, F.-B.; Hu, E.-D.; Xu, L.-M.; Chen, L.; Wu, J.-L.; Li, H.; Chen, D.-Z.; Chen, Y.-P. The Relationship between Obesity and the Severity of Non-Alcoholic Fatty Liver Disease: Systematic Review and Meta-Analysis. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 491–502. [Google Scholar] [CrossRef]
- Golabi, P.; Paik, J.M.; Arshad, T.; Younossi, Y.; Mishra, A.; Younossi, Z.M. Mortality of NAFLD According to the Body Composition and Presence of Metabolic Abnormalities. Hepatol. Commun. 2020, 4, 1136–1148. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; Celedon, M.A.; Lavine, J.E.; Salem, R.; Campbell, N.; Schork, N.J.; Shiehmorteza, M.; Yokoo, T.; Chavez, A.; Middleton, M.S.; et al. Heritability of Nonalcoholic Fatty Liver Disease. Gastroenterology 2009, 136, 1585–1592. [Google Scholar] [CrossRef] [Green Version]
- Wattacheril, J.; Lavine, J.E.; Chalasani, N.P.; Guo, X.; Kwon, S.; Schwimmer, J.; Molleston, J.P.; Loomba, R.; Brunt, E.M.; Chen, Y.-D.I.; et al. Genome-Wide Associations Related to Hepatic Histology in Nonalcoholic Fatty Liver Disease in Hispanic Boys. J. Pediatr. 2017, 190, 100–107.e2. [Google Scholar] [CrossRef]
- Santoro, N.; Caprio, S.; Pierpont, B.; Van Name, M.; Savoye, M.; Parks, E.J. Hepatic De Novo Lipogenesis in Obese Youth Is Modulated by a Common Variant in the GCKR Gene. J. Clin. Endocrinol. Metab. 2015, 100, E1125–E1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oniki, K.; Saruwatari, J.; Izuka, T.; Kajiwara, A.; Morita, K.; Sakata, M.; Otake, K.; Ogata, Y.; Nakagawa, K. Influence of the PNPLA3 Rs738409 Polymorphism on Non-Alcoholic Fatty Liver Disease and Renal Function among Normal Weight Subjects. PLoS ONE 2015, 10, e0132640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, Z.Y.; Wong, V.W.; Fan, J.G. Epidemiology of Nonalcoholic Fatty Liver Disease in Non-obese Populations: Meta-analytic Assessment of Its Prevalence, Genetic, Metabolic, and Histological Profiles. J. Dig. Dis. 2020, 21, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Schwimmer, J.B.; Deutsch, R.; Kahen, T.; Lavine, J.E.; Stanley, C.; Behling, C. Prevalence of Fatty Liver in Children and Adolescents. Pediatrics 2006, 118, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Wieckowska, A.; Feldstein, A. Diagnosis of Nonalcoholic Fatty Liver Disease: Invasive versus Noninvasive. Semin. Liver Dis. 2008, 28, 386–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, E.L.; Howe, L.D.; Jones, H.E.; Higgins, J.P.T.; Lawlor, D.A.; Fraser, A. The Prevalence of Non-Alcoholic Fatty Liver Disease in Children and Adolescents: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0140908. [Google Scholar] [CrossRef] [Green Version]
- Welsh, J.A.; Karpen, S.; Vos, M.B. Increasing Prevalence of Nonalcoholic Fatty Liver Disease Among United States Adolescents, 1988–1994 to 2007–2010. J. Pediatr. 2013, 162, 496–500.e1. [Google Scholar] [CrossRef] [Green Version]
- Patton, H.M.; Sirlin, C.; Behling, C.; Middleton, M.; Schwimmer, J.B.; Lavine, J.E. Pediatric Nonalcoholic Fatty Liver Disease: A Critical Appraisal of Current Data and Implications for Future Research. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 413–427. [Google Scholar] [CrossRef]
- Guzzaloni, G.; Grugni, G.; Minocci, A.; Moro, D.; Morabito, F. Liver Steatosis in Juvenile Obesity: Correlations with Lipid Profile, Hepatic Biochemical Parameters and Glycemic and Insulinemic Responses to an Oral Glucose Tolerance Test. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2000, 24, 772–776. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, R.Z.; Jalaludin, M.Y.; Anuar Zaini, A. Predictors of Non-Alcoholic Fatty Liver Disease (NAFLD) among Children with Obesity. J. Pediatr. Endocrinol. Metab. JPEM 2020, 33, 247–253. [Google Scholar] [CrossRef]
- Maleki, F.; Hosseinpour, M.; Motlagh, B.M.; Esmaeili Gouvarchin Ghaleh, H.; Gheibi, S. OC49 Non-Alcoholic Fatty Liver Disease in Obese and Overweight Iranian Children: A Cross Sectional Study. Arch. Dis. Child. 2019, 104, A20–A21. [Google Scholar]
- Dyson, J.K.; Anstee, Q.M.; McPherson, S. Non-Alcoholic Fatty Liver Disease: A Practical Approach to Diagnosis and Staging. Frontline Gastroenterol. 2014, 5, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Jennison, E.; Patel, J.; Scorletti, E.; Byrne, C.D. Diagnosis and Management of Non-Alcoholic Fatty Liver Disease. Postgrad. Med. J. 2019, 95, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Selmi, C.; Tang, R.; Gershwin, M.E.; Ma, X. The Microbiome and Autoimmunity: A Paradigm from the Gut–Liver Axis. Cell. Mol. Immunol. 2018, 15, 595–609. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.-L.; Chen, H.; Wang, C.-L.; Liang, L. Pathogenesis of Non-Alcoholic Fatty Liver Disease in Children and Adolescence: From “Two Hit Theory” to “Multiple Hit Model”. World J. Gastroenterol. 2018, 24, 2974–2983. [Google Scholar] [CrossRef]
- Lawlor, D.A.; Callaway, M.; Macdonald-Wallis, C.; Anderson, E.; Fraser, A.; Howe, L.D.; Day, C.; Sattar, N. Nonalcoholic Fatty Liver Disease, Liver Fibrosis, and Cardiometabolic Risk Factors in Adolescence: A Cross-Sectional Study of 1874 General Population Adolescents. J. Clin. Endocrinol. Metab. 2014, 99, E410–E417. [Google Scholar] [CrossRef] [Green Version]
- Jain, A. Pediatric Fatty Liver Disease. Mo. Med. 2019, 116, 123. [Google Scholar]
- Chiesa, C.; Andreoli, G.M.; Pacifico, L. Pediatric Nonalcoholic Fatty Liver Disease. J. Pediatr. Rio J. 2019, 95, 4–6. [Google Scholar] [CrossRef]
- Vos, M.B.; Abrams, S.H.; Barlow, S.E.; Caprio, S.; Daniels, S.R.; Kohli, R.; Mouzaki, M.; Sathya, P.; Schwimmer, J.B.; Sundaram, S.S.; et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J. Pediatr. Gastroenterol. Nutr. 2017, 64, 319–334. [Google Scholar] [CrossRef] [Green Version]
- Di Sessa, A.; Cirillo, G.; Guarino, S.; Marzuillo, P.; Miraglia del Giudice, E. Pediatric Non-Alcoholic Fatty Liver Disease: Current Perspectives on Diagnosis and Management. Pediatr. Health Med. Ther. 2019, 10, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Nassir, F.; Rector, R.S.; Hammoud, G.M.; Ibdah, J.A. Pathogenesis and Prevention of Hepatic Steatosis. Gastroenterol. Hepatol. 2015, 11, 167–175. [Google Scholar]
- Berardo, C.; Di Pasqua, L.G.; Cagna, M.; Richelmi, P.; Vairetti, M.; Ferrigno, A. Nonalcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis: Current Issues and Future Perspectives in Preclinical and Clinical Research. Int. J. Mol. Sci. 2020, 21, 9646. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The Multiple-Hit Pathogenesis of Non-Alcoholic Fatty Liver Disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Wu, C.-C.; Ni, Y.-H. New Perspectives on Genetic Prediction for Pediatric Metabolic Associated Fatty Liver Disease. Front. Pediatr. 2020, 8, 603654. [Google Scholar] [CrossRef] [PubMed]
- Jonas, W.; Schürmann, A. Genetic and Epigenetic Factors Determining NAFLD Risk. Mol. Metab. 2021, 50, 101111. [Google Scholar] [CrossRef] [PubMed]
- Dongiovanni, P.; Crudele, A.; Panera, N.; Romito, I.; Meroni, M.; De Stefanis, C.; Palma, A.; Comparcola, D.; Fracanzani, A.L.; Miele, L.; et al. β-Klotho Gene Variation Is Associated with Liver Damage in Children with NAFLD. J. Hepatol. 2020, 72, 411–419. [Google Scholar] [CrossRef] [Green Version]
- Goyal, N.P.; Schwimmer, J.B. The Genetics of Pediatric Nonalcoholic Fatty Liver Disease. Clin. Liver Dis. 2018, 22, 59–71. [Google Scholar] [CrossRef]
- Valenti, L.; Al-Serri, A.; Daly, A.K.; Galmozzi, E.; Rametta, R.; Dongiovanni, P.; Nobili, V.; Mozzi, E.; Roviaro, G.; Vanni, E.; et al. Homozygosity for the Patatin-like Phospholipase-3/Adiponutrin I148M Polymorphism Influences Liver Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Hepatology 2010, 51, 1209–1217. [Google Scholar] [CrossRef]
- Romeo, S.; Sentinlli, F.; Cambuli, V.M.; Incani, M.; Congiu, T.; Matta, V.; Pilia, S.; Huang-Doran, I.; Cossu, E.; Loche, S.; et al. The 148M Allele of the PNPLA3 Gene Is Associated with Indices of Liver Damage Early in Life. J. Hepatol. 2010, 53, 335–338. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Chang, P.-F.; Hu, F.-C.; Yang, W.-S.; Chang, M.-H.; Ni, Y.-H. A Common Variant in the PNPLA3 Gene Is a Risk Factor for Non-Alcoholic Fatty Liver Disease in Obese Taiwanese Children. J. Pediatr. 2011, 158, 740–744. [Google Scholar] [CrossRef]
- Goran, M.I.; Walker, R.; Le, K.-A.; Mahurkar, S.; Vikman, S.; Davis, J.N.; Spruijt-Metz, D.; Weigensberg, M.J.; Allayee, H. Effects of PNPLA3 on Liver Fat and Metabolic Profile in Hispanic Children and Adolescents. Diabetes 2010, 59, 3127–3130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frasinariu, O.E.; Ceccarelli, S.; Alisi, A.; Moraru, E.; Nobili, V. Gut-Liver Axis and Fibrosis in Nonalcoholic Fatty Liver Disease: An Input for Novel Therapies. Dig. Liver Dis. 2013, 45, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Berardis, S.; Sokal, E. Pediatric Non-Alcoholic Fatty Liver Disease: An Increasing Public Health Issue. Eur. J. Pediatr. 2014, 173, 131–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kneeman, J.M.; Misdraji, J.; Corey, K.E. Secondary Causes of Nonalcoholic Fatty Liver Disease. Ther. Adv. Gastroenterol. 2012, 5, 199–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mann, J.; Valenti, L.; Scorletti, E.; Byrne, C.; Nobili, V. Nonalcoholic Fatty Liver Disease in Children. Semin. Liver Dis. 2018, 38, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Faa, G.; Ekstrom, J.; Castagnola, M.; Gibo, Y.; Ottonello, G.; Fanos, V. A Developmental Approach to Drug-Induced Liver Injury in Newborns and Children. Curr. Med. Chem. 2012, 19, 4581–4594. [Google Scholar] [CrossRef] [Green Version]
- Chornomydz, I.; Boyarchuk, O.; Chornomydz, A. Reye (Ray’s) syndrome: A problem everyone should remember. Georgian Med. News 2017, 272, 110–118. [Google Scholar]
- Di Pasqua, L.G.; Cagna, M.; Berardo, C.; Vairetti, M.; Ferrigno, A. Detailed Molecular Mechanisms Involved in Drug-Induced Non-Alcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis: An Update. Biomedicines 2022, 10, 194. [Google Scholar] [CrossRef]
- Mouzaki, M.; Yodoshi, T.; Arce-Clachar, A.C.; Bramlage, K.; Fei, L.; Ley, S.L.; Xanthakos, S.A. Psychotropic Medications Are Associated with Increased Liver Disease Severity in Pediatric Nonalcoholic Fatty Liver Disease. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 339–343. [Google Scholar] [CrossRef]
- Ahmed Amin, D.; Ahmed Isma, M.; Mohamed Ib, A. Non Alcoholic Fatty Liver Disease, Insulin Resistance, Dyslipidemia and Atherogenic Ratios in Epileptic Children and Adolescents on Long Term Antiepileptic Drug Therapy. Pak. J. Biol. Sci. 2012, 15, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Yıldız, Y.; Sivri, H.S. Inborn Errors of Metabolism in the Differential Diagnosis of Fatty Liver Disease. Turk. J. Gastroenterol. 2020, 31, 3–16. [Google Scholar] [CrossRef]
- Han, S.; Zhu, F.; Huang, X.; Yan, P.; Xu, K.; Shen, F.; Sun, J.; Yang, Z.; Jin, G.; Teng, Y. Maternal Obesity Accelerated Non-alcoholic Fatty Liver Disease in Offspring Mice by Reducing Autophagy. Exp. Ther. Med. 2021, 22, 716. [Google Scholar] [CrossRef] [PubMed]
- Brumbaugh, D.E.; Tearse, P.; Cree-Green, M.; Fenton, L.Z.; Brown, M.; Scherzinger, A.; Reynolds, R.; Alston, M.; Hoffman, C.; Pan, Z.; et al. Intrahepatic Fat Is Increased in the Neonatal Offspring of Obese Women with Gestational Diabetes. J. Pediatr. 2013, 162, 930–936.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bugianesi, E.; Bizzarri, C.; Rosso, C.; Mosca, A.; Panera, N.; Veraldi, S.; Dotta, A.; Giannone, G.; Raponi, M.; Cappa, M.; et al. Low Birthweight Increases the Likelihood of Severe Steatosis in Pediatric Non-Alcoholic Fatty Liver Disease. Am. J. Gastroenterol. 2017, 112, 1277–1286. [Google Scholar] [CrossRef]
- Amadou, C.; Nabi, O.; Serfaty, L.; Lacombe, K.; Boursier, J.; Mathurin, P.; Ribet, C.; de Ledinghen, V.; Zins, M.; Charles, M. Association between Birth Weight, Preterm Birth, and Nonalcoholic Fatty Liver Disease in a Community-based Cohort. Hepatology, 2022; in press. [Google Scholar] [CrossRef]
- Newton, K.P.; Feldman, H.S.; Chambers, C.D.; Wilson, L.; Behling, C.; Clark, J.M.; Molleston, J.P.; Chalasani, N.; Sanyal, A.J.; Fishbein, M.H.; et al. Low and High Birth Weights Are Risk Factors for Nonalcoholic Fatty Liver Disease in Children. J. Pediatr. 2017, 187, 141–146.e1. [Google Scholar] [CrossRef]
- Tock, L.; Dâmaso, A.R.; de Piano, A.; Carnier, J.; Sanches, P.L.; Lederman, H.M.; Ernandes, R.M.Y.; de Mello, M.T.; Tufik, S. Long-Term Effects of Metformin and Lifestyle Modification on Nonalcoholic Fatty Liver Disease Obese Adolescents. J. Obes. 2010, 2010, 831901. [Google Scholar] [CrossRef]
- Vajro, P.; Lenta, S.; Socha, P.; Dhawan, A.; McKiernan, P.; Baumann, U.; Durmaz, O.; Lacaille, F.; McLin, V.; Nobili, V. Diagnosis of Nonalcoholic Fatty Liver Disease in Children and Adolescents: Position Paper of the ESPGHAN Hepatology Committee. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 700–713. [Google Scholar] [CrossRef]
- Brambilla, P.; Bedogni, G.; Heo, M.; Pietrobelli, A. Waist Circumference-to-Height Ratio Predicts Adiposity Better than Body Mass Index in Children and Adolescents. Int. J. Obes. 2013, 37, 943–946. [Google Scholar] [CrossRef] [Green Version]
- Schwimmer, J.B.; Newton, K.P.; Awai, H.I.; Choi, L.J.; Garcia, M.A.; Ellis, L.L.; Vanderwall, K.; Fontanesi, J. Paediatric Gastroenterology Evaluation of Overweight and Obese Children Referred from Primary Care for Suspected Non-Alcoholic Fatty Liver Disease. Aliment. Pharmacol. Ther. 2013, 38, 1267–1277. [Google Scholar] [CrossRef]
- Molleston, J.P.; Schwimmer, J.B.; Yates, K.P.; Murray, K.F.; Cummings, O.W.; Lavine, J.E.; Brunt, E.M.; Scheimann, A.O.; Unalp-Arida, A. Histological Abnormalities in Children with Nonalcoholic Fatty Liver Disease and Normal or Mildly Elevated Alanine Aminotransferase Levels. J. Pediatr. 2014, 164, 707–713.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwok, R.; Tse, Y.-K.; Wong, G.L.-H.; Ha, Y.; Lee, A.U.; Ngu, M.C.; Chan, H.L.-Y.; Wong, V.W.-S. Systematic Review with Meta-Analysis: Non-Invasive Assessment of Non-Alcoholic Fatty Liver Disease—The Role of Transient Elastography and Plasma Cytokeratin-18 Fragments. Aliment. Pharmacol. Ther. 2014, 39, 254–269. [Google Scholar] [CrossRef] [PubMed]
- Schwimmer, J.B.; Dunn, W.; Norman, G.J.; Pardee, P.E.; Middleton, M.S.; Kerkar, N.; Sirlin, C.B. SAFETY Study: Alanine Aminotransferase Cutoff Values Are Set Too High for Reliable Detection of Pediatric Chronic Liver Disease. Gastroenterology 2010, 138, 1357–1364.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Singh, A.K.; Panda, P.K.; Nischal, N.; Soneja, M. Non-Alcoholic Fatty Liver Disease Diagnosis, Grading and Staging; a Simplified Tool for Clinicians. J. Adv. Med. 2017, 6, 15. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; Deutsch, R.; Rauch, J.B.; Behling, C.; Newbury, R.; Lavine, J.E. Obesity, Insulin Resistance, and Other Clinicopathological Correlates of Pediatric Nonalcoholic Fatty Liver Disease. J. Pediatr. 2003, 143, 500–505. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Oliveira, N.; Reis, J.C.; Santos, M.V.; Silva, A.M.; Adan, L. Triglycerides and Alanine Aminotransferase as Screening Markers for Suspected Fatty Liver Disease in Obese Children and Adolescents. Horm. Res. Paediatr. 2009, 71, 83–88. [Google Scholar] [CrossRef]
- Engiz, Ö.; Berberoglu, M.; Siklar, Z.; Öçal, G. Risk Factors for Non-Alcoholic Fatty Liver Disease in Obese Children. Horm. Res. 2009, 72, 63–64. [Google Scholar] [CrossRef]
- Nobili, V.; Alkhouri, N.; Bartuli, A.; Manco, M.; Lopez, R.; Alisi, A.; Feldstein, A.E. Severity of Liver Injury and Atherogenic Lipid Profile in Children with Nonalcoholic Fatty Liver Disease. Pediatr. Res. 2010, 67, 665–670. [Google Scholar] [CrossRef] [Green Version]
- Lonardo, A.; Loria, P.; Leonardi, F.; Borsatti, A.; Neri, P.; Pulvirenti, M.; Verrone, A.M.; Bagni, A.; Bertolotti, M.; Ganazzi, D.; et al. Fasting Insulin and Uric Acid Levels but Not Indices of Iron Metabolism Are Independent Predictors of Non-Alcoholic Fatty Liver Disease. A Case-Control Study. Dig. Liver Dis. 2002, 34, 204–211. [Google Scholar] [CrossRef]
- Sartorio, A.; Del Col, A.; Agosti, F.; Mazzilli, G.; Bellentani, S.; Tiribelli, C.; Bedogni, G. Predictors of Non-Alcoholic Fatty Liver Disease in Obese Children. Eur. J. Clin. Nutr. 2007, 61, 877–883. [Google Scholar] [CrossRef] [Green Version]
- Della Corte, C.; Alisi, A.; Saccari, A.; De Vito, R.; Vania, A.; Nobili, V. Nonalcoholic Fatty Liver in Children and Adolescents: An Overview. J. Adolesc. Health 2012, 51, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Ajmera, V.; Perito, E.R.; Bass, N.M.; Terrault, N.A.; Yates, K.P.; Gill, R.; Loomba, R.; Diehl, A.M.; Aouizerat, B.E.; NASH Clinical Research Network. Novel Plasma Biomarkers Associated with Liver Disease Severity in Adults with Nonalcoholic Fatty Liver Disease. Hepatology 2017, 65, 65–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petäjä, E.; Yki-Järvinen, H. Definitions of Normal Liver Fat and the Association of Insulin Sensitivity with Acquired and Genetic NAFLD—A Systematic Review. Int. J. Mol. Sci. 2016, 17, 633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awai, H.I.; Newton, K.P.; Sirlin, C.B.; Behling, C.; Schwimmer, J.B. Evidence and Recommendations for Imaging Liver Fat in Children, Based on Systematic Review. Clin. Gastroenterol. Hepatol. 2014, 12, 765–773. [Google Scholar] [CrossRef] [Green Version]
- Park, S.H.; Kim, P.N.; Kim, K.W.; Lee, S.W.; Yoon, S.E.; Park, S.W.; Ha, H.K.; Lee, M.-G.; Hwang, S.; Lee, S.-G.; et al. Macrovesicular Hepatic Steatosis in Living Liver Donors: Use of CT for Quantitative and Qualitative Assessment. Radiology 2006, 239, 105–112. [Google Scholar] [CrossRef]
- Fishbein, M.; Castro, F.; Cheruku, S.; Jain, S.; Webb, B.; Gleason, T.; Stevens, W.R. Hepatic MRI for Fat Quantitation: Its Relationship to Fat Morphology, Diagnosis, and Ultrasound. J. Clin. Gastroenterol. 2005, 39, 619–625. [Google Scholar] [CrossRef]
- Saadeh, S.; Younossi, Z.M.; Remer, E.M.; Gramlich, T.; Ong, J.P.; Hurley, M.; Mullen, K.D.; Cooper, J.N.; Sheridan, M.J. The Utility of Radiological Imaging in Nonalcoholic Fatty Liver Disease. Gastroenterology 2002, 123, 745–750. [Google Scholar] [CrossRef]
- Xiao, G.; Zhu, S.; Xiao, X.; Yan, L.; Yang, J.; Wu, G. Comparison of Laboratory Tests, Ultrasound, or Magnetic Resonance Elastography to Detect Fibrosis in Patients with Nonalcoholic Fatty Liver Disease: A Meta-analysis. Hepatology 2017, 66, 1486–1501. [Google Scholar] [CrossRef] [Green Version]
- Chan, W.; Nik Mustapha, N.R.; Wong, G.L.; Wong, V.W.; Mahadeva, S. Controlled Attenuation Parameter Using the FibroScan® XL Probe for Quantification of Hepatic Steatosis for Non-alcoholic Fatty Liver Disease in an Asian Population. United Eur. Gastroenterol. J. 2017, 5, 76–85. [Google Scholar] [CrossRef]
- Nadeau, K.J.; Ehlers, L.B.; Zeitler, P.S.; Love-Osborne, K. Treatment of Non-Alcoholic Fatty Liver Disease with Metformin versus Lifestyle Intervention in Insulin-Resistant Adolescents. Pediatr. Diabetes 2009, 10, 5–13. [Google Scholar] [CrossRef]
- Shah, A.G.; Lydecker, A.; Murray, K.; Tetri, B.N.; Contos, M.J.; Sanyal, A.J. Comparison of Noninvasive Markers of Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2009, 7, 1104–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratziu, V.; Massard, J.; Charlotte, F.; Messous, D.; Imbert-Bismut, F.; Bonyhay, L.; Tahiri, M.; Munteanu, M.; Thabut, D.; Cadranel, J.F.; et al. Diagnostic Value of Biochemical Markers (FibroTest-FibroSURE) for the Prediction of Liver Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease. BMC Gastroenterol. 2006, 6, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunt, E.M.; Janney, C.G.; Di Bisceglie, A.M.; Neuschwander-Tetri, B.A.; Bacon, B.R. Nonalcoholic Steatohepatitis: A Proposal for Grading and Staging the Histological Lesions. Am. J. Gastroenterol. 1999, 94, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and Validation of a Histological Scoring System for Nonalcoholic Fatty Liver Disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Bedossa, P.; Poitou, C.; Veyrie, N.; Bouillot, J.-L.; Basdevant, A.; Paradis, V.; Tordjman, J.; Clement, K. Histopathological Algorithm and Scoring System for Evaluation of Liver Lesions in Morbidly Obese Patients. Hepatology 2012, 56, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Kohut, T.; Panganiban, J. Lifestyle Intervention as the Primary Treatment for Pediatric Nonalcoholic Fatty Liver Disease. Clin. Liver Dis. 2021, 17, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Anania, C.; Perla, F.M.; Olivero, F.; Pacifico, L.; Chiesa, C. Mediterranean Diet and Nonalcoholic Fatty Liver Disease. World J. Gastroenterol. 2018, 24, 2083–2094. [Google Scholar] [CrossRef]
- Della Corte, C.; Mosca, A.; Vania, A.; Alterio, A.; Iasevoli, S.; Nobili, V. Good Adherence to the Mediterranean Diet Reduces the Risk for NASH and Diabetes in Pediatric Patients with Obesity: The Results of an Italian Study. Nutrition 2017, 39–40, 8–14. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; Ugalde-Nicalo, P.; Welsh, J.A.; Angeles, J.E.; Cordero, M.; Harlow, K.E.; Alazraki, A.; Durelle, J.; Knight-Scott, J.; Newton, K.P.; et al. Effect of a Low Free Sugar Diet vs Usual Diet on Nonalcoholic Fatty Liver Disease in Adolescent Boys: A Randomized Clinical Trial. JAMA 2019, 321, 256. [Google Scholar] [CrossRef] [Green Version]
- Nobili, V.; Manco, M.; Devito, R.; Ciampalini, P.; Piemonte, F.; Marcellini, M. Effect of Vitamin E on Aminotransferase Levels and Insulin Resistance in Children with Non-Alcoholic Fatty Liver Disease. Aliment. Pharmacol. Ther. 2006, 24, 1553–1561. [Google Scholar] [CrossRef]
- Nobili, V.; Manco, M.; Devito, R.; Di Ciommo, V.; Comparcola, D.; Sartorelli, M.R.; Piemonte, F.; Marcellini, M.; Angulo, P. Lifestyle Intervention and Antioxidant Therapy in Children with Nonalcoholic Fatty Liver Disease: A Randomized, Controlled Trial. Hepatology 2008, 48, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Conjeevaram Selvakumar, P.K.; Kabbany, M.N.; Alkhouri, N. Nonalcoholic Fatty Liver Disease in Children: Not a Small Matter. Pediatr. Drugs 2018, 20, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Clerc, P.; Mouzaki, M.; Goldman, R.D. Omega-3 for Nonalcoholic Fatty Liver Disease in Children. Can. Fam. Physician Med. Fam. Can. 2019, 65, 34–38. [Google Scholar]
- Gao, X.; Zhu, Y.; Wen, Y.; Liu, G.; Wan, C. Efficacy of Probiotics in Non-Alcoholic Fatty Liver Disease in Adult and Children: A Meta-Analysis of Randomized Controlled Trials: Meta-Analysis. Hepatol. Res. 2016, 46, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Furthner, D.; Weghuber, D.; Dalus, C.; Lukas, A.; Stundner-Ladenhauf, H.N.; Mangge, H.; Pixner, T. Nonalcoholic Fatty Liver Disease in Children with Obesity: Narrative Review and Research Gaps. Horm. Res. Paediatr. 2022, 95, 167–176. [Google Scholar] [CrossRef]
- Leoni, S.; Tovoli, F.; Napoli, L.; Serio, I.; Ferri, S.; Bolondi, L. Current Guidelines for the Management of Non-Alcoholic Fatty Liver Disease: A Systematic Review with Comparative Analysis. World J. Gastroenterol. 2018, 24, 3361–3373. [Google Scholar] [CrossRef]
- Alves, J.G.B.; Alves, G.V. Effects of Physical Activity on Children’s Growth. J. Pediatr. Rio J. 2019, 95 (Suppl. S1), 72–78. [Google Scholar] [CrossRef]
- Veldman, S.L.C.; Paw, C.A.; Mai, J.M.; Altenburg, T.M. Physical Activity and Prospective Associations with Indicators of Health and Development in Children Aged <5 Years: A Systematic Review. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 6. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 Guidelines on Physical Activity and Sedentary Behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- McMurray, R.G.; Berry, D.C.; Schwartz, T.A.; Hall, E.G.; Neal, M.N.; Li, S.; Lam, D. Relationships of Physical Activity and Sedentary Time in Obese Parent-Child Dyads: A Cross-Sectional Study. BMC Public Health 2016, 16, 124. [Google Scholar] [CrossRef] [Green Version]
- Whiting, S.; Buoncristiano, M.; Gelius, P.; Abu-Omar, K.; Pattison, M.; Hyska, J.; Duleva, V.; Musić Milanović, S.; Zamrazilová, H.; Hejgaard, T.; et al. Physical Activity, Screen Time, and Sleep Duration of Children Aged 6–9 Years in 25 Countries: An Analysis within the WHO European Childhood Obesity Surveillance Initiative (COSI) 2015–2017. Obes. Facts 2021, 14, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.; Evans, R. Physical Activity, Aerobic Fitness and Obesity in Children. Med. Exerc. Nutr. Health 1995, 4, 3–16. [Google Scholar]
- Guthold, R.; Stevens, G.A.; Riley, L.M.; Bull, F.C. Worldwide Trends in Insufficient Physical Activity from 2001 to 2016: A Pooled Analysis of 358 Population-Based Surveys with 1·9 million Participants. Lancet Glob. Health 2018, 6, e1077–e1086. [Google Scholar] [CrossRef] [Green Version]
- Vandoni, M.; Carnevale Pellino, V.; De Silvestri, A.; Lovecchio, N.; Rovida, A.; Gatti, A.; Biagioli, V.; Zuccotti, G.; Calcaterra, V. The Temporal Association between Body Characteristics and Speed Performance over Twenty-Five Years in Italian Adolescents. Child. Basel Switz. 2022, 9, 521. [Google Scholar] [CrossRef]
- Schwarzfischer, P.; Gruszfeld, D.; Stolarczyk, A.; Ferre, N.; Escribano, J.; Rousseaux, D.; Moretti, M.; Mariani, B.; Verduci, E.; Koletzko, B.; et al. Physical Activity and Sedentary Behavior From 6 to 11 Years. Pediatrics 2019, 143, e20180994. [Google Scholar] [CrossRef] [Green Version]
- Vandoni, M.; Lovecchio, N.; Carnevale Pellino, V.; Codella, R.; Fabiano, V.; Rossi, V.; Zuccotti, G.V.; Calcaterra, V. Self-Reported Physical Fitness in Children and Adolescents with Obesity: A Cross-Sectional Analysis on the Level of Alignment with Multiple Adiposity Indexes. Children 2021, 8, 476. [Google Scholar] [CrossRef]
- Yu, J.J.; Capio, C.M.; Abernethy, B.; Sit, C.H.P. Moderate-to-Vigorous Physical Activity and Sedentary Behavior in Children with and without Developmental Coordination Disorder: Associations with Fundamental Movement Skills. Res. Dev. Disabil. 2021, 118, 104070. [Google Scholar] [CrossRef]
- Babic, M.J.; Morgan, P.J.; Plotnikoff, R.C.; Lonsdale, C.; White, R.L.; Lubans, D.R. Physical Activity and Physical Self-Concept in Youth: Systematic Review and Meta-Analysis. Sports Med. 2014, 44, 1589–1601. [Google Scholar] [CrossRef]
- Lovecchio, N.; Zago, M. Fitness Differences According to BMI Categories: A New Point of View. J. Sports Med. Phys. Fit. 2019, 59, 298–303. [Google Scholar] [CrossRef]
- Stodden, D.; Goodway, J.; Langendorfer, S.; Roberton, M.A.; Rudisill, M.; Garcia, C.; Garcia, L. A Developmental Perspective on the Role of Motor Skill Competence in Physical Activity: An Emergent Relationship. Quest 2008, 60, 290–306. [Google Scholar] [CrossRef]
- Gatti, A.; Pugliese, L.; Carnevale Pellino, V.; Del Bianco, M.; Vandoni, M.; Lovecchio, N. Self-Declared Physical Activity Levels and Self-Reported Physical Fitness in a Sample of Italian Adolescents during the COVID-19 Pandemic. Eur. J. Investig. Health Psychol. Educ. 2022, 12, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Fuemmeler, B.F.; Anderson, C.B.; Mâsse, L.C. Parent-Child Relationship of Directly Measured Physical Activity. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinkley, T.; Crawford, D.; Salmon, J.; Okely, A.D.; Hesketh, K. Preschool Children and Physical Activity: A Review of Correlates. Am. J. Prev. Med. 2008, 34, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Nettle, H.; Sprogis, E. Pediatric Exercise: Truth and/or Consequences. Sports Med. Arthrosc. Rev. 2011, 19, 75–80. [Google Scholar] [CrossRef]
- Riddoch, C.J.; Bo Andersen, L.; Wedderkopp, N.; Harro, M.; Klasson-Heggebø, L.; Sardinha, L.B.; Cooper, A.R.; Ekelund, U. Physical Activity Levels and Patterns of 9- and 15-Yr-Old European Children. Med. Sci. Sports Exerc. 2004, 36, 86–92. [Google Scholar] [CrossRef] [Green Version]
- Garaulet, M.; Martínez, A.; Victoria, F.; Pérez–Llamas, F.; Ortega, R.M.; Zamora, S. Differences in Dietary Intake and Activity Level between Normal-Weight and Overweight or Obese Adolescents. J. Pediatr. Gastroenterol. Nutr. 2000, 30, 253–258. [Google Scholar] [CrossRef]
- Haverly, K.; Davison, K.K. Personal Fulfillment Motivates Adolescents to Be Physically Active. Arch. Pediatr. Adolesc. Med. 2005, 159, 1115–1120. [Google Scholar] [CrossRef] [Green Version]
- Trost, S.G.; Pate, R.R.; Ward, D.S.; Saunders, R.; Riner, W. Correlates of Objectively Measured Physical Activity in Preadolescent Youth. Am. J. Prev. Med. 1999, 17, 120–126. [Google Scholar] [CrossRef]
- Deldin, A.R.; Lee, S. Role of Physical Activity in the Treatment of Nonalcoholic Fatty Liver Disease in Children and Adolescents. Appl. Physiol. Nutr. Metab. 2013, 38, 805–812. [Google Scholar] [CrossRef]
- Isoura, Y.; Cho, Y.; Fujimoto, H.; Hamazaki, T.; Tokuhara, D. Effects of Obesity Reduction on Transient Elastography–Based Parameters in Pediatric Non-Alcoholic Fatty Liver Disease. Obes. Res. Clin. Pract. 2020, 14, 473–478. [Google Scholar] [CrossRef]
- Babu, A.F.; Csader, S.; Lok, J.; Gómez-Gallego, C.; Hanhineva, K.; El-Nezami, H.; Schwab, U. Positive Effects of Exercise Intervention without Weight Loss and Dietary Changes in NAFLD-Related Clinical Parameters: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3135. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Park, S.; Koyanagi, A.; Jacob, L.; Yon, D.K.; Lee, S.W.; Kim, M.S.; Kim, S.U.; Kim, B.K.; Shin, J.I.; et al. The Effect of Pharmacological Treatment and Lifestyle Modification in Patients with Nonalcoholic Fatty Liver Disease: An Umbrella Review of Meta-analyses of Randomized Controlled Trials. Obes. Rev. 2022, e13464. [Google Scholar] [CrossRef] [PubMed]
- Perseghin, G.; Lattuada, G.; De Cobelli, F.; Ragogna, F.; Ntali, G.; Esposito, A.; Belloni, E.; Canu, T.; Terruzzi, I.; Scifo, P.; et al. Habitual Physical Activity Is Associated with Intrahepatic Fat Content in Humans. Diabetes Care 2007, 30, 683–688. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Song, J.; Shang, X.; Chawla, N.; Yang, Y.; Meng, X.; Wang, H.; Ma, J. Physical Activity and Sedentary Behavior Can Modulate the Effect of the PNPLA3 Variant on Childhood NAFLD: A Case-Control Study in a Chinese Population. BMC Med. Genet. 2016, 17, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. WHO Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Katsagoni, C.N.; Papachristou, E.; Sidossis, A.; Sidossis, L. Effects of Dietary and Lifestyle Interventions on Liver, Clinical and Metabolic Parameters in Children and Adolescents with Non-Alcoholic Fatty Liver Disease: A Systematic Review. Nutrients 2020, 12, 2864. [Google Scholar] [CrossRef]
- Lee, S.; Deldin, A.R.; White, D.; Kim, Y.; Libman, I.; Rivera-Vega, M.; Kuk, J.L.; Sandoval, S.; Boesch, C.; Arslanian, S. Aerobic Exercise but Not Resistance Exercise Reduces Intrahepatic Lipid Content and Visceral Fat and Improves Insulin Sensitivity in Obese Adolescent Girls: A Randomized Controlled Trial. Am. J. Physiol.-Endocrinol. Metab. 2013, 305, E1222–E1229. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Bacha, F.; Hannon, T.; Kuk, J.L.; Boesch, C.; Arslanian, S. Effects of Aerobic Versus Resistance Exercise Without Caloric Restriction on Abdominal Fat, Intrahepatic Lipid, and Insulin Sensitivity in Obese Adolescent Boys. Diabetes 2012, 61, 2787–2795. [Google Scholar] [CrossRef] [Green Version]
- Van Der Heijden, G.-J.; Wang, Z.J.; Chu, Z.; Toffolo, G.; Manesso, E.; Sauer, P.J.J.; Sunehag, A.L. Strength Exercise Improves Muscle Mass and Hepatic Insulin Sensitivity in Obese Youth. Med. Sci. Sports Exerc. 2010, 42, 1973–1980. [Google Scholar] [CrossRef] [Green Version]
- van der Heijden, G.-J.; Wang, Z.J.; Chu, Z.D.; Sauer, P.J.J.; Haymond, M.W.; Rodriguez, L.M.; Sunehag, A.L. A 12-Week Aerobic Exercise Program Reduces Hepatic Fat Accumulation and Insulin Resistance in Obese, Hispanic Adolescents. Obesity 2010, 18, 384–390. [Google Scholar] [CrossRef]
- González-Ruiz, K.; Ramírez-Vélez, R.; Correa-Bautista, J.E.; Peterson, M.D.; García-Hermoso, A. The Effects of Exercise on Abdominal Fat and Liver Enzymes in Pediatric Obesity: A Systematic Review and Meta-Analysis. Child. Obes. 2017, 13, 272–282. [Google Scholar] [CrossRef]
- De Piano, A.; de Mello, M.T.; Sanches, P. de L.; da Silva, P.L.; Campos, R.M.S.; Carnier, J.; Corgosinho, F.; Foschini, D.; Masquio, D.L.; Tock, L.; et al. Long-Term Effects of Aerobic plus Resistance Training on the Adipokines and Neuropeptides in Nonalcoholic Fatty Liver Disease Obese Adolescents. Eur. J. Gastroenterol. Hepatol. 2012, 24, 1313–1324. [Google Scholar] [CrossRef] [PubMed]
- Iraji, H.; Minasian, V.; Kelishadi, R. Changes in Liver Enzymes and Metabolic Profile in Adolescents with Fatty Liver Following Exercise Interventions. Pediatr. Gastroenterol. Hepatol. Nutr. 2021, 24, 54. [Google Scholar] [CrossRef] [PubMed]
- Logan, G.R.M.; Harris, N.; Duncan, S.; Schofield, G. A Review of Adolescent High-Intensity Interval Training. Sports Med. 2014, 44, 1071–1085. [Google Scholar] [CrossRef] [PubMed]
- Van der Windt, D.J.; Sud, V.; Zhang, H.; Tsung, A.; Huang, H. The Effects of Physical Exercise on Fatty Liver Disease. Gene Expr. 2018, 18, 89–101. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.-J.; Kim, K.M.; Hwang, S.; Lee, S.G.; Ha, T.-Y.; Song, G.-W.; Jung, D.-H.; Kim, K.-H.; Yu, E.; Shim, J.H.; et al. Exercise and Diet Modification in Non-Obese Non-Alcoholic Fatty Liver Disease: Analysis of Biopsies of Living Liver Donors: Lifestyle Modification in Non-Obese NAFLD. J. Gastroenterol. Hepatol. 2012, 27, 1341–1347. [Google Scholar] [CrossRef]
- Eckard, C.; Cole, R.; Lockwood, J.; Torres, D.M.; Williams, C.D.; Shaw, J.C.; Harrison, S.A. Prospective Histopathologic Evaluation of Lifestyle Modification in Nonalcoholic Fatty Liver Disease: A Randomized Trial. Ther. Adv. Gastroenterol. 2013, 6, 249–259. [Google Scholar] [CrossRef] [Green Version]
- Farzanegi, P.; Dana, A.; Ebrahimpoor, Z.; Asadi, M.; Azarbayjani, M.A. Mechanisms of Beneficial Effects of Exercise Training on Non-Alcoholic Fatty Liver Disease (NAFLD): Roles of Oxidative Stress and Inflammation. Eur. J. Sport Sci. 2019, 19, 994–1003. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Wang, T.-Y.; Targher, G.; Byrne, C.D.; Zheng, M.-H. Lifestyle Interventions for Non-Obese Patients Both with, and at Risk, of Non-Alcoholic Fatty Liver Disease. Diabetes Metab. J. 2022, 46, 391–401. [Google Scholar] [CrossRef]
- Berzigotti, A.; Saran, U.; Dufour, J.-F. Physical Activity and Liver Diseases. Hepatology 2016, 63, 1026–1040. [Google Scholar] [CrossRef]
- Cigrovski Berkovic, M.; Bilic-Curcic, I.; Mrzljak, A.; Cigrovski, V. NAFLD and Physical Exercise: Ready, Steady, Go! Front. Nutr. 2021, 8, 734859. [Google Scholar] [CrossRef]
- Pan, X.; Han, Y.; Zou, T.; Zhu, G.; Xu, K.; Zheng, J.; Zheng, M.; Cheng, X. Sarcopenia Contributes to the Progression of Nonalcoholic Fatty Liver Disease- Related Fibrosis: A Meta-Analysis. Dig. Dis. 2018, 36, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Farzanegi, P.; Abbaszadeh, H.; Abbassi Daloii, A.; Kazemi, M.; Sabbaghian, M.; Shoeibi, A.; Nabipour, R.; Abuhosseini, Z.; Azarbayjani, M.A. Effects of Aerobic Exercise on Histopathology and Toxicology of ZnO and Nano ZnO in Male Rats. Toxicol. Environ. Chem. 2018, 100, 103–114. [Google Scholar] [CrossRef]
- Cui, J.; Guo, Y.; Yang, W.; Zhao, X.; Yu, F.; Tang, W.; Pang, B.; Su, X. Effects of Exercise on Learning and Memory, Oxidative Stress and NNOS Expression in Marginal Division of Striatum of Ovariectomized Rats. J. Sports Med. Phys. Fit. 2018, 58, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, N.; Yano, H.; Mizokami, T.; Takahashi, M.; Oyanagi, E.; Suzuki, K. Exercise Training Attenuates Hepatic Inflammation, Fibrosis and Macrophage Infiltration during Diet Induced-Obesity in Mice. Brain. Behav. Immun. 2012, 26, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Del Chierico, F.; Nobili, V.; Vernocchi, P.; Russo, A.; De Stefanis, C.; Gnani, D.; Furlanello, C.; Zandonà, A.; Paci, P.; Capuani, G.; et al. Gut Microbiota Profiling of Pediatric Nonalcoholic Fatty Liver Disease and Obese Patients Unveiled by an Integrated Meta-omics-based Approach. Hepatology 2017, 65, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Moran-Ramos, S.; Lopez-Contreras, B.E.; Villarruel-Vazquez, R.; Ocampo-Medina, E.; Macias-Kauffer, L.; Martinez-Medina, J.N.; Villamil-Ramirez, H.; León-Mimila, P.; Del Rio-Navarro, B.E.; Ibarra-Gonzalez, I.; et al. Environmental and Intrinsic Factors Shaping Gut Microbiota Composition and Diversity and Its Relation to Metabolic Health in Children and Early Adolescents: A Population-Based Study. Gut Microbes 2020, 11, 900–917. [Google Scholar] [CrossRef]
- Tokuhara, D. Role of the Gut Microbiota in Regulating Non-Alcoholic Fatty Liver Disease in Children and Adolescents. Front. Nutr. 2021, 8, 700058. [Google Scholar] [CrossRef]
- Monga Kravetz, A.; Testerman, T.; Galuppo, B.; Graf, J.; Pierpont, B.; Siebel, S.; Feinn, R.; Santoro, N. Effect of Gut Microbiota and PNPLA3 Rs738409 Variant on Nonalcoholic Fatty Liver Disease (NAFLD) in Obese Youth. J. Clin. Endocrinol. Metab. 2020, 105, e3575–e3585. [Google Scholar] [CrossRef]
- Stanislawski, M.A.; Lozupone, C.A.; Wagner, B.D.; Eggesbø, M.; Sontag, M.K.; Nusbacher, N.M.; Martinez, M.; Dabelea, D. Gut Microbiota in Adolescents and the Association with Fatty Liver: The EPOCH Study. Pediatr. Res. 2018, 84, 219–227. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; Johnson, J.S.; Angeles, J.E.; Behling, C.; Belt, P.H.; Borecki, I.; Bross, C.; Durelle, J.; Goyal, N.P.; Hamilton, G.; et al. Microbiome Signatures Associated with Steatohepatitis and Moderate to Severe Fibrosis in Children With Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 157, 1109–1122. [Google Scholar] [CrossRef] [Green Version]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and Associated Dietary Extremes Impact on Gut Microbial Diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med. Sci. Sports Exerc. 2018, 50, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, R.; Nistal, E.; Estébanez, B.; Porras, D.; Juárez-Fernández, M.; Martínez-Flórez, S.; García-Mediavilla, M.V.; de Paz, J.A.; González-Gallego, J.; Sánchez-Campos, S.; et al. Exercise Training Modulates the Gut Microbiota Profile and Impairs Inflammatory Signaling Pathways in Obese Children. Exp. Mol. Med. 2020, 52, 1048–1061. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calcaterra, V.; Magenes, V.C.; Vandoni, M.; Berardo, C.; Marin, L.; Bianchi, A.; Cordaro, E.; Silvestro, G.S.; Silvestri, D.; Carnevale Pellino, V.; et al. Benefits of Physical Exercise as Approach to Prevention and Reversion of Non-Alcoholic Fatty Liver Disease in Children and Adolescents with Obesity. Children 2022, 9, 1174. https://doi.org/10.3390/children9081174
Calcaterra V, Magenes VC, Vandoni M, Berardo C, Marin L, Bianchi A, Cordaro E, Silvestro GS, Silvestri D, Carnevale Pellino V, et al. Benefits of Physical Exercise as Approach to Prevention and Reversion of Non-Alcoholic Fatty Liver Disease in Children and Adolescents with Obesity. Children. 2022; 9(8):1174. https://doi.org/10.3390/children9081174
Chicago/Turabian StyleCalcaterra, Valeria, Vittoria Carlotta Magenes, Matteo Vandoni, Clarissa Berardo, Luca Marin, Alice Bianchi, Erika Cordaro, Giustino Simone Silvestro, Dario Silvestri, Vittoria Carnevale Pellino, and et al. 2022. "Benefits of Physical Exercise as Approach to Prevention and Reversion of Non-Alcoholic Fatty Liver Disease in Children and Adolescents with Obesity" Children 9, no. 8: 1174. https://doi.org/10.3390/children9081174
APA StyleCalcaterra, V., Magenes, V. C., Vandoni, M., Berardo, C., Marin, L., Bianchi, A., Cordaro, E., Silvestro, G. S., Silvestri, D., Carnevale Pellino, V., Cereda, C., & Zuccotti, G. (2022). Benefits of Physical Exercise as Approach to Prevention and Reversion of Non-Alcoholic Fatty Liver Disease in Children and Adolescents with Obesity. Children, 9(8), 1174. https://doi.org/10.3390/children9081174








