Disruption of the Corticospinal Tract in Patients with Acute Lymphoblastic Leukemia: A Case Series
Abstract
:1. Introduction
2. Case Presentation
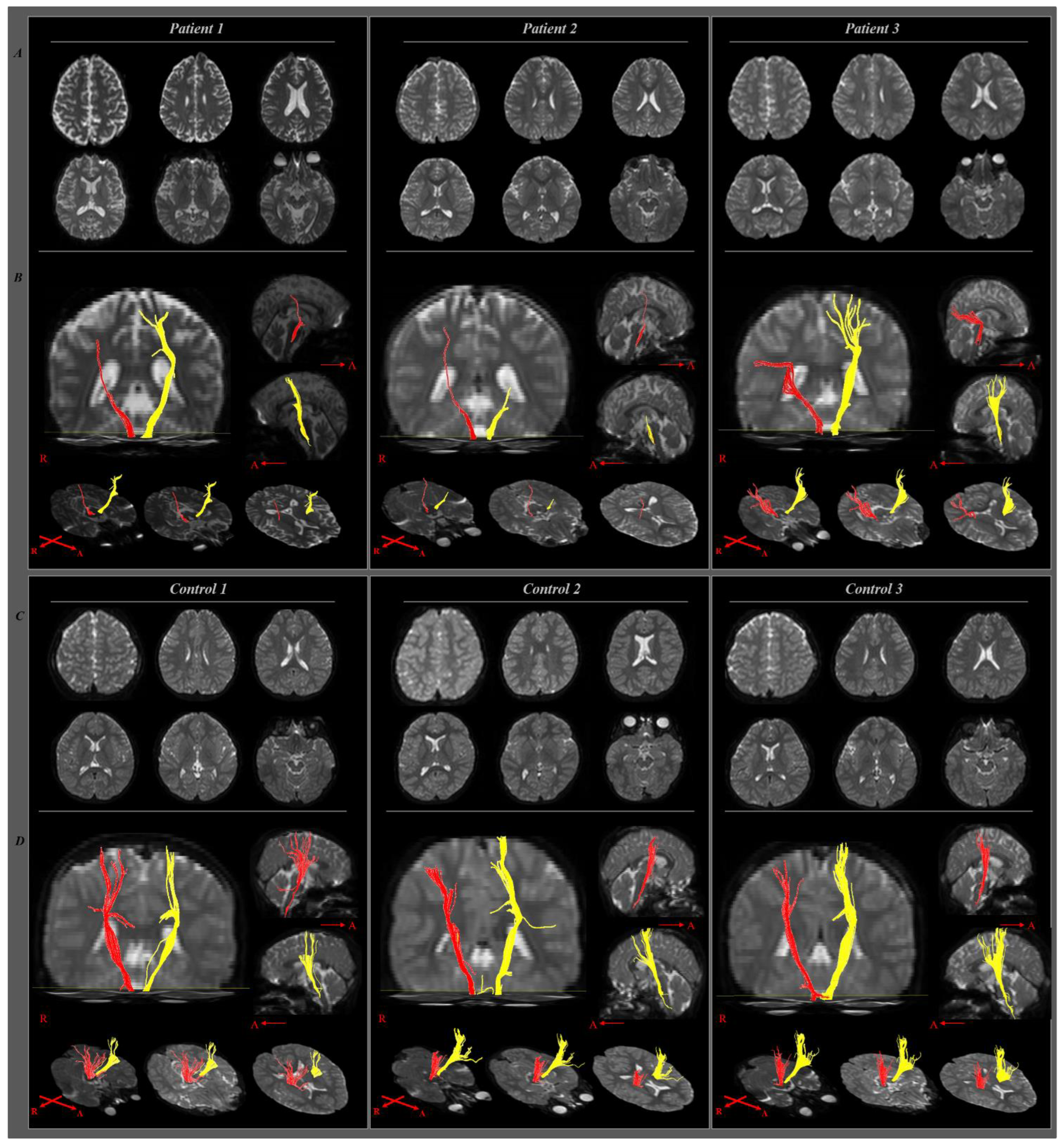
2.1. Case 1
2.2. Case 2
2.3. Case 3
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pui, C.H.; Campana, D.; Pei, D.; Bowman, W.P.; Sandlund, J.T.; Kaste, S.C.; Ribeiro, R.C.; Rubnitz, J.E.; Raimondi, S.C.; Onciu, M.; et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N. Engl. J. Med. 2009, 360, 2730–2741. [Google Scholar] [CrossRef]
- Spiegler, B.J.; Kennedy, K.; Maze, R.; Greenberg, M.L.; Weitzman, S.; Hitzler, J.K.; Nathan, P.C. Comparison of long-term neurocognitive outcomes in young children with acute lymphoblastic leukemia treated with cranial radiation or high-dose or very high-dose intravenous methotrexate. J. Clin. Oncol. 2006, 24, 3858–3864. [Google Scholar] [CrossRef]
- Krull, K.R.; Cheung, Y.T.; Liu, W.; Fellah, S.; Reddick, W.E.; Brinkman, T.M.; Kimberg, C.; Ogg, R.; Srivastava, D.; Pui, C.H.; et al. Chemotherapy pharmacodynamics and neuroimaging and neurocognitive outcomes in long-term survivors of childhood acute lymphoblastic leukemia. J. Clin. Oncol. 2016, 34, 2644–2653. [Google Scholar] [CrossRef]
- Campbell, L.K.; Scaduto, M.; Sharp, W.; Dufton, L.; Van Slyke, D.; Whitlock, J.A.; Compas, B. A meta-analysis of the neurocognitive sequelae of treatment for childhood acute lymphocytic leukemia. Pediatr. Blood Cancer 2007, 49, 65–73. [Google Scholar] [CrossRef]
- Aytaç, S.; Yetgin, S.; Tavil, B. Acute and long-term neurologic complications in children with acute lymphoblastic leukemia. Turk. J. Pediatr. 2006, 48, 1–7. [Google Scholar]
- Peng, L.; Yang, L.S.; Yam, P.; Lam, C.S.; Chan, A.S.; Li, C.K.; Cheung, Y.T. Neurocognitive and behavioral outcomes of chinese survivors of childhood lymphoblastic leukemia. Front. Oncol. 2021, 11, 655669. [Google Scholar] [CrossRef]
- Conklin, H.M.; Krull, K.R.; Reddick, W.E.; Pei, D.; Cheng, C.; Pui, C.H. Cognitive outcomes following contemporary treatment without cranial irradiation for childhood acute lymphoblastic leukemia. J. Natl. Cancer Inst. 2012, 104, 1386–1395. [Google Scholar] [CrossRef]
- Goebel, A.M.; Koustenis, E.; Rueckriegel, S.M.; Pfuhlmann, L.; Brandsma, R.; Sival, D.; Skarabis, H.; Schuelke, M.; Hernáiz Driever, P. Motor function in survivors of pediatric acute lymphoblastic leukemia treated with chemotherapy-only. Eur. J. Paediatr. Neurol. 2019, 23, 304–316. [Google Scholar] [CrossRef]
- Oswald, K.A.; Bo, J. Motor functioning and associated cognitive outcomes in pediatric survivors of acute lymphoblastic leukemia. Child Neuropsychol. 2020, 26, 597–611. [Google Scholar] [CrossRef]
- Vagace, J.M.; de la Maya, M.D.; Caceres-Marzal, C.; Gonzalez de Murillo, S.; Gervasini, G. Central nervous system chemotoxicity during treatment of pediatric acute lymphoblastic leukemia/lymphoma. Crit. Rev. Oncol. Hematol. 2012, 84, 274–286. [Google Scholar] [CrossRef]
- Green, J.L.; Knight, S.J.; McCarthy, M.; De Luca, C.R. Motor functioning during and following treatment with chemotherapy for pediatric acute lymphoblastic leukemia. Pediatr. Blood Cancer 2013, 60, 1261–1266. [Google Scholar] [CrossRef]
- Hanna, S.; Elshennawy, S.; El-Ayadi, M.; Abdelazeim, F. Investigating fine motor deficits during maintenance therapy in children with acute lymphoblastic leukemia. Pediatr. Blood Cancer 2020, 67, e28385. [Google Scholar] [CrossRef]
- Mori, H.; Masutani, Y.; Aoki, S.; Abe, O.; Hayashi, N.; Masumoto, T.; Yamada, H.; Yoshikawa, T.; Kunimatsu, A.; Ohtomo, K.; et al. Simple visualization of the corticospinal pathway using tractography: One-ROI and two-ROI methods. Nihon Igaku Hoshasen Gakkai Zasshi 2003, 63, 51–53. [Google Scholar]
- Kwon, Y.M.; Kwon, H.G.; Rose, J.; Son, S.M. The change of intra-cerebral CST location during childhood and adolescence; diffusion tensor tractography study. Front. Hum. Neurosci. 2016, 10, 638. [Google Scholar] [CrossRef]
- Son, S.M.; Ahn, Y.H.; Sakong, J.; Moon, H.K.; Ahn, S.H.; Lee, H.; Yu, I.K.; Shin, Y.J.; Jang, S.H. Diffusion tensor imaging demonstrates focal lesions of the corticospinal tract in hemiparetic patients with cerebral palsy. Neurosci. Lett. 2007, 420, 34–38. [Google Scholar] [CrossRef]
- Kim, S.H.; Jang, S.H.; Lee, E.; Kim, S.; Cho, Y.W.; Hong, J.H.; Son, S.M. Usefulness of diffusion tensor imaging in patients who showed sustained unexplainable clinical symptom of torticollis. Neurosci. Lett. 2012, 522, 25–29. [Google Scholar] [CrossRef]
- Cho, H.K.; Jang, S.H.; Lee, E.; Kim, S.Y.; Kim, S.; Kwon, Y.H.; Son, S.M. Diffusion tensor imaging-demonstrated differences between hemiplegic and diplegic cerebral palsy with symmetric periventricular leukomalacia. AJNR Am. J. Neuroradiol. 2013, 34, 650–654. [Google Scholar] [CrossRef]
- Jang, S.H.; Kim, K.; Kim, S.H.; Son, S.M.; Jang, W.H.; Kwon, H.G. The relation between motor function of stroke patients and diffusion tensor imaging findings for the corticospinal tract. Neurosci. Lett. 2014, 572, 1–6. [Google Scholar] [CrossRef]
- Choi, G.S.; Kim, O.L.; Kim, S.H.; Ahn, S.H.; Cho, Y.W.; Son, S.M.; Jang, S.H. Classification of cause of motor weakness in traumatic brain injury using diffusion tensor imaging. Arch. Neurol. 2012, 69, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Su, L.; Xu, J.; Xiang, L.; Wang, L.; Zhai, Z.; Zheng, S. Structural brain alteration in survivors of acute lymphoblastic leukemia with chemotherapy treatment: A voxel-based morphometry and diffusion tensor imaging study. Brain Res. 2017, 1658, 68–72. [Google Scholar] [CrossRef]
- Aukema, E.J.; Caan, M.W.; Oudhuis, N.; Majoie, C.B.; Vos, F.M.; Reneman, L.; Last, B.F.; Grootenhuis, M.A.; Schouten-van Meeteren, A.Y. White matter fractional anisotropy correlates with speed of processing and motor speed in young childhood cancer survivors. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Edelmann, M.N.; Krull, K.R.; Liu, W.; Glass, J.O.; Ji, Q.; Ogg, R.J.; Sabin, N.D.; Srivastava, D.K.; Robison, L.L.; Hudson, M.M.; et al. Diffusion tensor imaging and neurocognition in survivors of childhood acute lymphoblastic leukaemia. Brain 2014, 137, 2973–2983. [Google Scholar] [CrossRef] [PubMed]
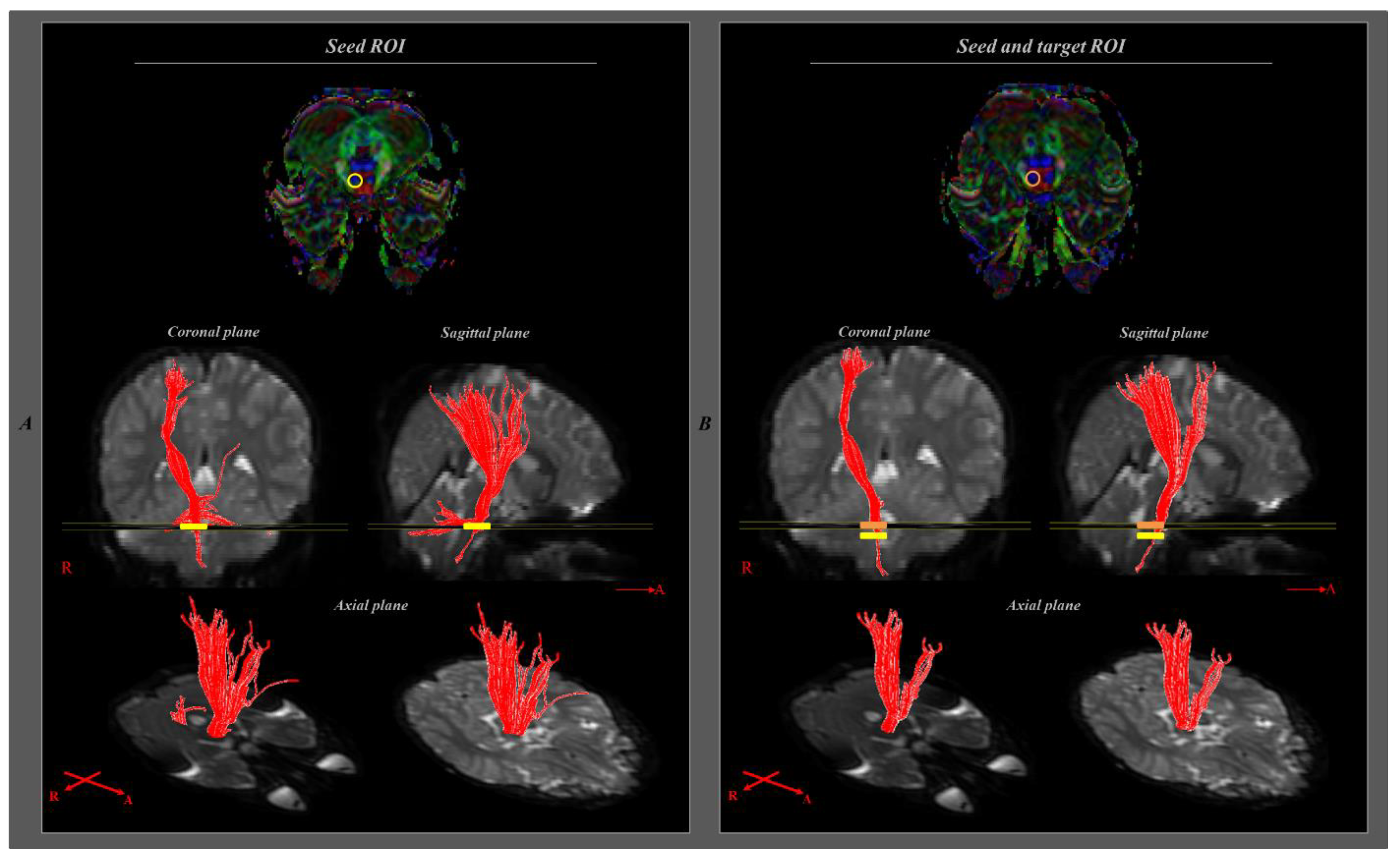
| P1 | P2 | P3 | C1 | C2 | C3 | |
|---|---|---|---|---|---|---|
| Age | 6 y 4 m | 6 y 2 m | 4 y 10 m | 6 y 3 m | 6 y 3 m | 4 y 11 m |
| Sex | Male | Male | Female | Male | Male | Female |
| Handedness | Right | Right | Right | Right | Right | Right |
| Period from diagnosis of ALL to examination | 4 m | 7 m | 20 m | 4 m | 7 m | 19 m |
| Purdue test | 12/8 | 6/8 | 8/5 | 11/10 | 12/10 | 8/7 |
| P1 | P2 | P3 | C1 | C2 | C3 | |
|---|---|---|---|---|---|---|
| WBC at diagnosis (/µL) | 2500 | 6860 | 2130 | 18,110 | 14,250 | 91,820 |
| immunophenotype | SR | SR | SR | B | B + myeloid | SR |
| CNS status | negative | negative | negative | negative | negative | negative |
| Protocol | Modified BFM | Modified BFM | Modified BFM | Modified BFM | Modified BFM | Modified BFM |
| Risk group | SR | SR | SR | SR | SR | HR |
| Cytogenetics | Hyperdiploidy | Hyperdiploidy | Hyperdiploidy | Hyperdiploidy | TEL/AML1 | MLL rearrangement |
| P1 | P2 | P3 | C1 | C2 | C3 | |
|---|---|---|---|---|---|---|
| FA affected * | 0.412 | 0.225 | 0.415 | 0.587 | 0.562 | 0.515 |
| Unaffected # | 0.481 | 0.356 | 0.472 | 0.601 | 0.570 | 0.491 |
| ADC affected * | 0.96 | 1.144 | 0.95 | 0.84 | 0.769 | 0.921 |
| unaffected # | 0.901 | 0.927 | 0.94 | 0.867 | 0.801 | 0.894 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.M.; Kim, J.B.; Byun, D.H.; Son, S.M. Disruption of the Corticospinal Tract in Patients with Acute Lymphoblastic Leukemia: A Case Series. Children 2022, 9, 1223. https://doi.org/10.3390/children9081223
Lee JM, Kim JB, Byun DH, Son SM. Disruption of the Corticospinal Tract in Patients with Acute Lymphoblastic Leukemia: A Case Series. Children. 2022; 9(8):1223. https://doi.org/10.3390/children9081223
Chicago/Turabian StyleLee, Jae Min, Jong Bum Kim, Dong Hyun Byun, and Su Min Son. 2022. "Disruption of the Corticospinal Tract in Patients with Acute Lymphoblastic Leukemia: A Case Series" Children 9, no. 8: 1223. https://doi.org/10.3390/children9081223
APA StyleLee, J. M., Kim, J. B., Byun, D. H., & Son, S. M. (2022). Disruption of the Corticospinal Tract in Patients with Acute Lymphoblastic Leukemia: A Case Series. Children, 9(8), 1223. https://doi.org/10.3390/children9081223





