Screening and Identification of Mitragynine and 7-Hydroxymitragynine in Human Urine by LC-MS/MS
Abstract
:1. Introduction
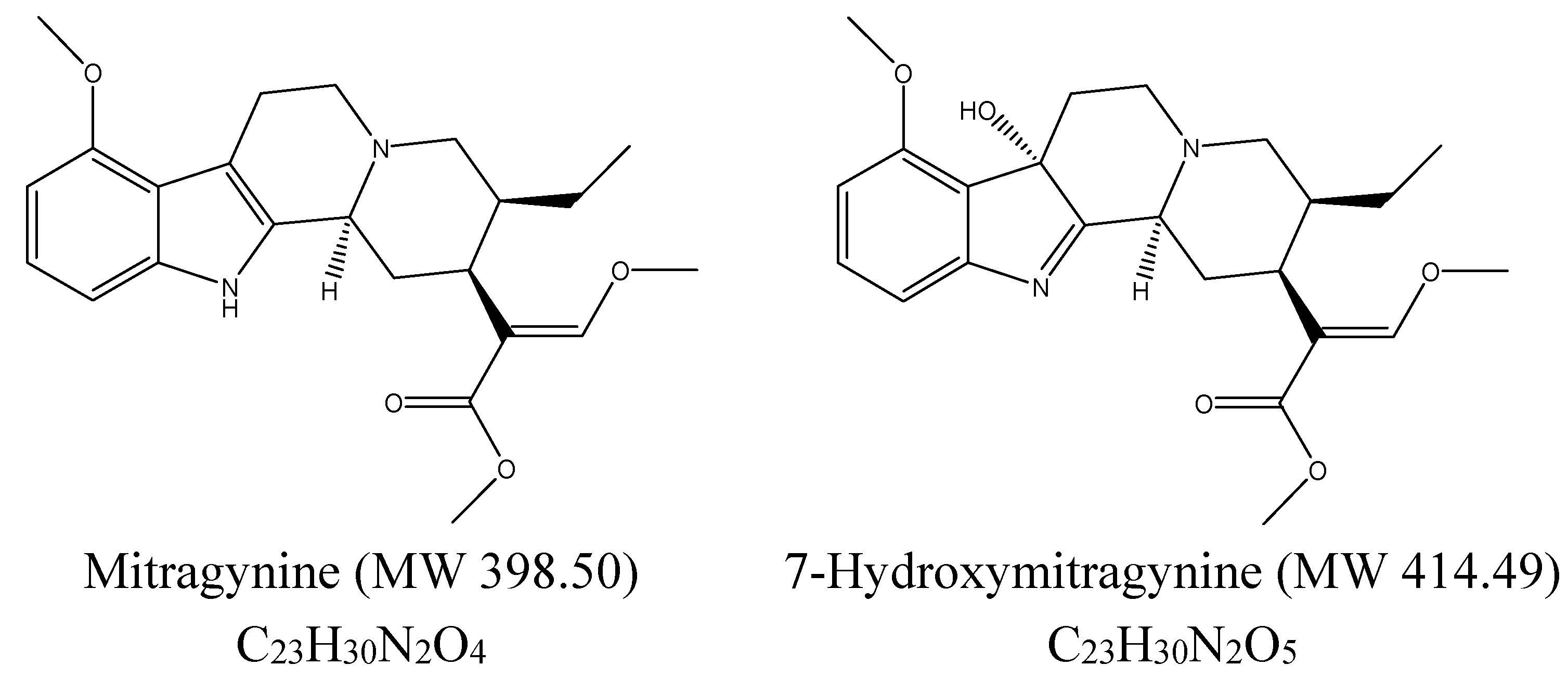
2. Experimental Section
2.1. Chemicals and Materials
2.2. Instrumentation
2.3. Standard Solutions
3. Results and Discussion
3.1. HPLC Conditions

3.2. Mass Spectrometry Parameters
| Ion Spray Voltage | 2500 V |
| Temperature | 600 °C |
| Collision Gas | Medium |
| Ion Source Gas 1 | 50.0 |
| Ion Source Gas 2 | 70.0 |
| Compound | Precursor ion (m/z) | Product ion (m/z) | Collision energy (volts) |
|---|---|---|---|
| Mitragynine | 399.3 | 174.1/159.0 | 40/55 |
| Mitragynine-D3 | 402.3 | 238.1 | 30 |
| 7-Hydroxymitragynine | 415.3 | 190.0/238.1 | 40/27 |
| 7-Hydroxymitragynine-D3 | 418.3 | 193.1 | 40 |
3.3. Method Validation
3.3.1. Linearity
3.3.2. Carryover
3.3.3. Sensitivity
3.3.4. Precision
3.3.5. Stability
3.3.5.1. Short-Term Stability (Autosampler Stability)
| Mitragynine | 7-hydroxymitragynine | |||
|---|---|---|---|---|
| Low QC | High QC | Low QC | High QC | |
| T0 | 0.1502 ng/mL | 1.0000 ng/mL | 0.7502 ng/mL | 4.9983 ng/mL |
| Tx | 0.1498 ng/mL | 0.9997 ng/mL | 0.7502 ng/mL | 5.0000 ng/mL |
| % Difference | 0.266% | 0.03% | 0% | −0.034% |
3.3.5.2. Long-Term Stability (Storage Stability)
| Mitragynine | 7-hydroxymitragynine | |||
|---|---|---|---|---|
| Low QC | High QC | Low QC | High QC | |
| T0 | 0.1500 ng/mL | 1.0017 ng/mL | 0.7500 ng/mL | 4.9967 ng/mL |
| Tx | 0.1503 ng/mL | 0.9990 ng/mL | 0.7500 ng/mL | 5.0000 ng/mL |
| % Difference | −0.20% | 0.27% | 0% | −0.066% |
3.3.6. Matrix Effect Study
3.4. Capability of the Established Method

| Analyte Peak Name (MRM Transition) | Mean Calculated Concentration (ng/mL) | Std. Deviation (ng/mL) | %CV | Number of Values Used | Mean Accuracy (%) |
|---|---|---|---|---|---|
| Mitragynine1 (399.300/174.100 Da) | 3.594 | 0.004 | 0.12 | 2 | 95.84 |
| Mitragynine 2 (399.300/159.000 Da) | 3.398 | 0.057 | 1.69 | 2 | 90.60 |
| 7-hydroxymitragynine 1 (415.300/190.000 Da) | 3.884 | 0.048 | 1.24 | 2 | 103.57 |
| 7-hydroxymitragynine 2 (415.300/238.100 Da) | 3.516 | 0.059 | 1.69 | 2 | 93.75 |
| mitragynine 1 (399.300/174.100 Da) | 24.616 | 0.353 | 1.44 | 2 | 98.46 |
| mitragynine 2 (399.300/159.000 Da) | 23.915 | 0.282 | 1.18 | 2 | 95.66 |
| 7-hydroxymitragynine 1 (415.300/190.000 Da) | 24.844 | 0.107 | 0.43 | 2 | 99.37 |
| 7-hydroxymitragynine 2 (415.300/238.100 Da) | 27.241 | 0.678 | 2.49 | 2 | 108.96 |
4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Ward, J.; Rosenbaum, C.; Hernon, C.; McCurdy, C.; Boyer, E. Herbal medicines for the management of opioid addiction. CNS Drugs 2011, 25, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Le, D.; Goggin, M.M.; Janis, G.C. Analysis of mitragynine and metabolites in human urine for detecting the use of the psychoactive plant kratom. J. Anal. Toxicol. 2012. [Google Scholar] [CrossRef]
- Rosenbaum, C.; Carreiro, S.; Babu, K. Here today, gone tomorrow…and back again? A review of herbal marijuana alternatives (k2, spice), synthetic cathinones (bath salts), kratom, salvia divinorum, methoxetamine, and piperazines. J. Med. Toxicol. 2012, 8, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Assanangkornchai, S.; Muekthong, A.; Sam-angsri, N.; Pattanasattayawong, U. The use of mitragynine speciosa (“krathom”), an addictive plant, in thailand. Subst. Use Misuse 2007, 42, 2145–2157. [Google Scholar] [CrossRef] [PubMed]
- Arndt, T.; Claussen, U.; Güssregen, B.; Schröfel, S.; Stürzer, B.; Werle, A.; Wolf, G. Kratom alkaloids and o-desmethyltramadol in urine of a “krypton” herbal mixture consumer. Forensic Sci. Int. 2011, 208, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Harizal, S.N.; Mansor, S.M.; Hasnan, J.; Tharakan, J.K.J.; Abdullah, J. Acute toxicity study of the standardized methanolic extract of mitragyna speciosa korth in rodent. J. Ethnopharm. 2010, 131, 404–409. [Google Scholar] [CrossRef]
- Kumarnsit, E.; Keawpradub, N.; Nuankaew, W. Effect of mitragyna speciosa aqueous extract on ethanol withdrawal symptoms in mice. Fitoterapia 2007, 78, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Holler, J.M.; Vorce, S.P.; McDonough-Bender, P.C.; Magluilo, J.; Solomon, C.J.; Levine, B. A drug toxicity death involving propylhexedrine and mitragynine. J. Anal. Toxicol. 2011, 35, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Kronstrand, R.; Roman, M.; Thelander, G.; Eriksson, A. Unintentional fatal intoxications with mitragynine and o-desmethyltramadol from the herbal blend krypton. J. Anal. Toxicol. 2011, 35, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Vicknasingam, B.; Narayanan, S.; Beng, G.T.; Mansor, S.M. The informal use of ketum (mitragyna speciosa) for opioid withdrawal in the northern states of peninsular malaysia and implications for drug substitution therapy. Int. J. Drug Policy 2010, 21, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K.L.R.; Prast, C.J. Psychoactive properties of mitragynine (kratom). J. Psychoact. Drugs 1988, 20, 455–457. [Google Scholar] [CrossRef]
- Babu, K.M.; McCurdy, C.R.; Boyer, E.W. Opioid receptors and legal highs: Salvia divinorum and kratom. Clin. Toxicol. 2008, 46, 146–152. [Google Scholar] [CrossRef]
- Schmidt, M.M.; Sharma, A.; Schifano, F.; Feinmann, C. “Legal highs” on the net—Evaluation of uk-based websites, products and product information. Forensic Sci. Int. 2011, 206, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Boyer, E.W.; Babu, K.M.; Macalino, G.E.; Compton, W. Self-treatment of opioid withdrawal with a dietary supplement, kratom. Am. J. Addict. 2007, 16, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Boyer, E.W.; Babu, K.M.; Adkins, J.E.; McCurdy, C.R.; Halpern, J.H. Self-treatment of opioid withdrawal using kratom (mitragynia speciosa korth). Addiction 2008, 103, 1048–1050. [Google Scholar] [CrossRef] [PubMed]
- Phongprueksapattana, S.; Putalun, W.; Keawpradub, N.; Wungsintaweekul, J. Mitragyna speciosa: Hairy root culture for triterpenoid production and high yield of mitragynine by regenerated plants. Zeitschrift für Naturforschung. C 2008, 63, 691–698. [Google Scholar] [CrossRef]
- Zhao, C.; Pelaez, M.; Dionysiou, D.D.; Pillai, S.C.; Byrne, J.A.; O'Shea, K.E. Uv and visible light activated tio2 photocatalysis of 6-hydroxymethyl uracil, a model compound for the potent cyanotoxin cylindrospermopsin. Catal. Today 2014, 224, 70–76. [Google Scholar] [CrossRef]
- O'Shea, K.; Zhao, C.; Dionysiou, D.D.; Pelaez, M.; Song, W.; Byrne, J.A.; Pillai, S.C. Advanced Oxidation of Cylindrospermopsin (Cyanobacterial Toxin): Mechanistic and Practical Considerations. In Abstracts of Papers of the American Chemical Society; American Chemical Society: Washington, DC, USA, 2013. [Google Scholar]
- Kong, W.M.; Chik, Z.; Ramachandra, M.; Subramaniam, U.; Aziddin, R.E.R.; Mohamed, Z. Evaluation of the effects of mitragyna speciosa alkaloid extract on cytochrome p450 enzymes using a high throughput assay. Molecules 2011, 16, 7344–7356. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Mizowaki, M.; Suchitra, T.; Takayama, H.; Sakai, S.-I.; Aimi, N.; Watanabe, H. Antinociceptive action of mitragynine in mice: Evidence for the involvement of supraspinal opioid receptors. Life Sci. 1996, 59, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- de la Cruz, A.A.; Hiskia, A.; Kaloudis, T.; Chernoff, N.; Hill, D.; Antoniou, M.G.; He, X.; Loftin, K.; O'Shea, K.; Zhao, C.; et al. A review on cylindrospermopsin: The global occurrence, detection, toxicity and degradation of a potent cyanotoxin. Environ. Sci. 2013, 15, 1979–2003. [Google Scholar]
- Luzi, M.; Zhao, C.; O'Shea, K. Synthesis and Oxidative Degradation 6-(Hydroxymethyl) Uracil as a Model Compound for Cylindrospermopsin (cyn), A Potent Cyanotoxin. In Abstracts of Papers of the American Chemical Society; American Chemical Society: Washington, DC, USA, 2013. [Google Scholar]
- Stolt, A.-C.; Schröder, H.; Neurath, H.; Grecksch, G.; Höllt, V.; Meyer, M.; Maurer, H.; Ziebolz, N.; Havemann-Reinecke, U.; Becker, A. Behavioral and neurochemical characterization of kratom (mitragyna speciosa) extract. Psychopharmacology 2014, 231, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Cocores, J.A.; Gold, M.S. The salted food addiction hypothesis may explain overeating and the obesity epidemic. Med. Hypotheses 2009, 73, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Merola, G.; Fu, H.; Tagliaro, F.; Macchia, T.; McCord, B.R. Chiral separation of 12 cathinone analogs by cyclodextrin-assisted capillary electrophoresis with uv and mass spectrometry detection. Electrophoresis 2014, 35, 3231–3241. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Arroyo-Mora, L.E.; DeCaprio, A.P.; Sharma, V.K.; Dionysiou, D.D.; O'Shea, K.E. Reductive and oxidative degradation of iopamidol, iodinated x-ray contrast media, by fe(iii)-oxalate under uv and visible light treatment. Water Res. 2014, 67, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Arroyo-Mora, L.E.; DeCaprio, A.P.; Dionysiou, D.D.; O’Shea, K.E. Photodegradation of Iodinated X-ray Contrast Media Iopamidol by fe(iii)-oxalate System with the Composition of h2o2. In Abstracts of Papers of the American Chemical Society; American Chemical Society: Washington, DC, USA, 2014. [Google Scholar]
- Maruyama, T.; Kawamura, M.; Kikura-Hanajiri, R.; Takayama, H.; Goda, Y. The botanical origin of kratom (mitragyna speciosa; rubiaceae) available as abused drugs in the japanese markets. J. Nat. Med. 2009, 63, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, C.; Dionysiou, D.D.; O’Shea, K.E. TiO2 photocatalytic degradation and detoxification of cylindrospermopsin. J. Photochem. Photobiol. A 2015, 307–308, 115–122. [Google Scholar] [CrossRef]
- Chittrakarn, S.; Keawpradub, N.; Sawangjaroen, K.; Kansenalak, S.; Janchawee, B. The neuromuscular blockade produced by pure alkaloid, mitragynine and methanol extract of kratom leaves (mitragyna speciosa korth.). J. Ethnopharm. 2010, 129, 344–349. [Google Scholar] [CrossRef]
- Suwanlert, S. A study of kratom eaters in thailand. Bull. Narcotics 1975, 27, 21–27. [Google Scholar]
- Zhao, C.; Sharma, V.; Dionysiou, D.; O'Shea, K. Oxidation of Cylindrospermopsin and Its Model Compound 6-Hydroxymethyl Uracil by Ferrate (vi). In Abstracts of Papers of the American Chemical Society; American Chemical Society: Washington, DC, USA, 2014. [Google Scholar]
- Zhao, C.; Pelaez, M.; Dionysiou, D.D.; Pillai, S.C.; Byrne, J.A.; O'Shea, K. Visible Light Activated (vla) tio2 Photocatalysis of 6-Hydroxymethyl Uracil as a Model Compound for the Cylindrospermopsin. In Abstracts of Papers of the American Chemical Society; American Chemical Society: Washington, DC, USA, 2013. [Google Scholar]
- Fan, M.; Yang, D.; Wang, X.; Liu, W.; Fu, H. Doss-based qails: As both neat lubricants and lubricant additives with excellent tribological properties and good detergency. Ind. Eng. Chem. Res. 2014, 53, 17952–17960. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, Y.; Hu, X.; O'Shea, K.; Ma, L.; Zhao, C.; Ma, F.; Wu, M. Control of microcystis aeruginosa growth and the associated microcystin cyanotoxin remediation by electron beam irradiation (ebi). RSC Adv. 2015, 5, 31292–31297. [Google Scholar] [CrossRef]
- Yang, D.; Li, Z.; Diwu, Y.A.; Fu, H.; Liao, J.; Wei, C.; Diwu, Z. A novel fluorogenic coumarin substrate for monitoring acid phosphatase activity at low ph environment. Curr. Chem. Genom. 2008, 2, 48–50. [Google Scholar] [CrossRef]
- Philipp, A.; Meyer, M.; Wissenbach, D.; Weber, A.; Zoerntlein, S.; Zweipfenning, P.M.; Maurer, H. Monitoring of kratom or krypton intake in urine using gc-ms in clinical and forensic toxicology. Anal. Bioanal. Chem. 2011, 400, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Meyer, G.M.J.; Meyer, M.R.; Wissenbach, D.K.; Maurer, H.H. Studies on the metabolism and toxicological detection of glaucine, an isoquinoline alkaloid from glaucium flavum (papaveraceae), in rat urine using gc-ms, lc-msn and lc-high-resolution msn. J. Mass Spectrom. 2013, 48, 24–41. [Google Scholar] [CrossRef] [PubMed]
- Kapp, F.; Maurer, H.; Auwärter, V.; Winkelmann, M.; Hermanns-Clausen, M. Intrahepatic cholestasis following abuse of powdered kratom (mitragyna speciosa). J. Med. Toxicol. 2011, 7, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Kikura-Hanajiri, R.; Kawamura, M.; Maruyama, T.; Kitajima, M.; Takayama, H.; Goda, Y. Simultaneous analysis of mitragynine, 7-hydroxymitragynine, and other alkaloids in the psychotropic plant “kratom” (mitragyna speciosa) by lc-esi-ms. Forensic Toxicol. 2009, 27, 67–74. [Google Scholar] [CrossRef]
- Philipp, A.A.; Wissenbach, D.K.; Zoerntlein, S.W.; Klein, O.N.; Kanogsunthornrat, J.; Maurer, H.H. Studies on the metabolism of mitragynine, the main alkaloid of the herbal drug kratom, in rat and human urine using liquid chromatography-linear ion trap mass spectrometry. J. Mass Spectrom. 2009, 44, 1249–1261. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, N.V.; Moretti, R.A.C.; Furr Iii, E.B.; McCurdy, C.R.; Lanchote, V.L. Determination of mitragynine in rat plasma by lc–ms/ms: Application to pharmacokinetics. J. Chromatogr. B 2009, 877, 2593–2597. [Google Scholar] [CrossRef]
- Posch, T.N.; Müller, A.; Schulz, W.; Pütz, M.; Huhn, C. Implementation of a design of experiments to study the influence of the background electrolyte on separation and detection in non-aqueous capillary electrophoresis–mass spectrometry. Electrophoresis 2012, 33, 583–598. [Google Scholar] [CrossRef] [PubMed]
- Al-Saffar, Y.; Stephanson, N.N.; Beck, O. Multicomponent lc–ms/ms screening method for detection of new psychoactive drugs, legal highs, in urine—experience from the swedish population. J. Chromatogr. B 2013, 930, 112–120. [Google Scholar] [CrossRef]
- Fu, H.; McCord, B.R. Separation and Identification of Select Drugs by Monolith Capillary Electrochromatography. In Abstracts of Papers of the American Chemical Society; American Chemical Society: Washington, DC, USA, 2013. [Google Scholar]
- Fu, H. Development of advanced capillary electrophoresis techniques with uv and mass spectrometry detection for forensic, pharmaceutical and environmental applications. 2014; paper 1531. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, H.; Cid, F.X.; Dworkin, N.; Cocores, J.; Shore, G. Screening and Identification of Mitragynine and 7-Hydroxymitragynine in Human Urine by LC-MS/MS. Chromatography 2015, 2, 253-264. https://doi.org/10.3390/chromatography2020253
Fu H, Cid FX, Dworkin N, Cocores J, Shore G. Screening and Identification of Mitragynine and 7-Hydroxymitragynine in Human Urine by LC-MS/MS. Chromatography. 2015; 2(2):253-264. https://doi.org/10.3390/chromatography2020253
Chicago/Turabian StyleFu, Hanzhuo, Frank X. Cid, Nat Dworkin, James Cocores, and Gloria Shore. 2015. "Screening and Identification of Mitragynine and 7-Hydroxymitragynine in Human Urine by LC-MS/MS" Chromatography 2, no. 2: 253-264. https://doi.org/10.3390/chromatography2020253





