Clinical Study of Continuous Non-Invasive Blood Pressure Monitoring in Neonates
Abstract
:1. Introduction
2. Materials and Methods
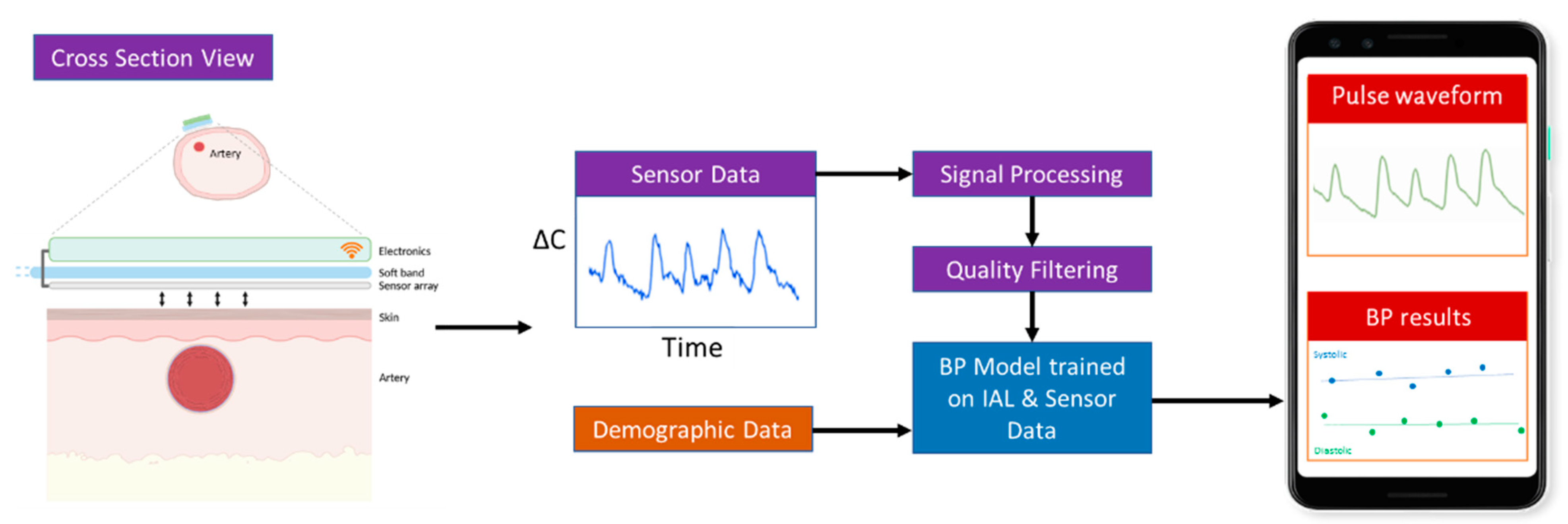
2.1. Device Design and Working Principle
2.1.1. Capacitive Sensing
2.1.2. Pipeline for Real-Time Processing of Pulse Waveforms
2.1.3. Data Quality
2.1.4. Signal Processing
2.1.5. Artifact Removal
2.1.6. BP Model
- A total of 306 infants under 5-years-old in the historical database and 95 patients collected in this study, no exclusions, both sexes.
- Up to 5000 pulse waveforms chosen randomly for each individual from a total of ~25,000 h of training data.
- Same quality preprocessing metrics as the data from the Boppli sensor.
3. Clinical Study Design
3.1. Arterial-Line Data Collection
Correlation and Accuracy Metrics
4. Results
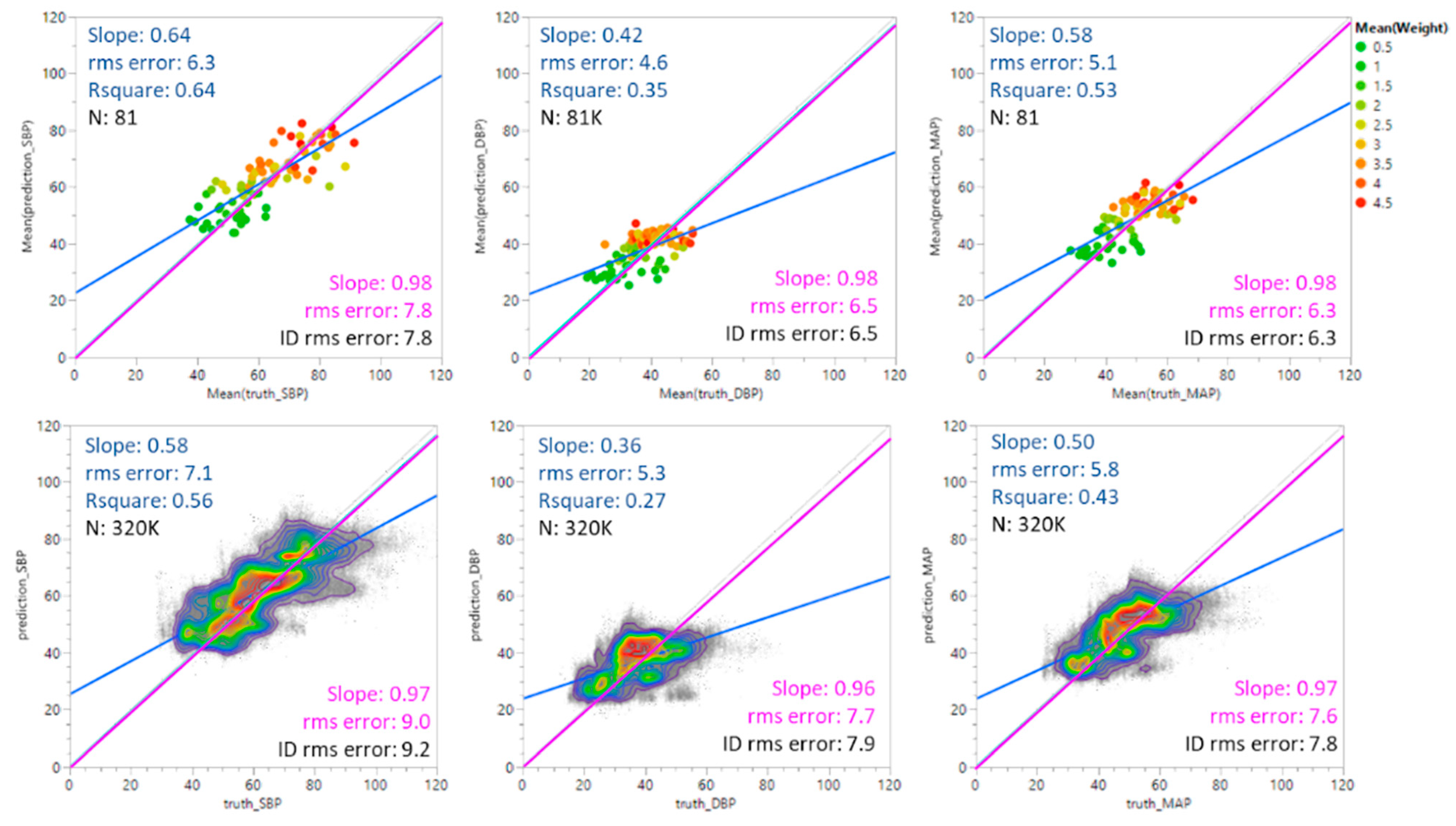
4.1. cNIBP (Sensor)
4.2. Degree of Agreement
4.3. Effect of Gestational Age
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Code Availability Statement
References
- Cuper, N.J.; de Graaff, J.C.; Hartman, B.J.; Verdaasdonk, R.M.; Kalkman, C.J. Difficult Arterial Cannulation in Children: Is a near-Infrared Vascular Imaging System the Answer? Br. J. Anaesth. 2012, 109, 420–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedro, V.; Burd, A.; Mehta, R.; Hiatt, M.; Hegyi, T. Resolution of Peripheral Artery Catheter-Induced Ischemic Injury Following Prolonged Treatment with Topical Nitroglycerin Ointment in a Newborn: A Case Report. J. Perinatol. 2003, 23, 348–350. [Google Scholar] [CrossRef]
- Furfaro, S. Arterial Catheter—Related Infections in Children A 1-Year Cohort Analysis. Am. J. Dis. Child. 1991, 145, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, G.; Burckhardt, J.; Hadley, A.; Kane, S.; Kor, D.; Marienau, M.S.; Schroeder, D.R.; Handlogten, K.; Wilson, G.; Oliver, W.C. Surgical and Patient Risk Factors for Severe Arterial Line Complications in Adults. Anesthesiology 2016, 124, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Takci, S.; Yigit, S.; Korkmaz, A.; Yurdakök, M. Comparison between Oscillometric and Invasive Blood Pressure Measurements in Critically Ill Premature Infants. Acta Paediatr. 2012, 101, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Shimokaze, T.; Akaba, K.; Saito, E. Oscillometric and Intra-arterial Blood Pressure in Preterm and Term Infants: Extent of Discrepancy and Factors Associated with Inaccuracy. Am. J. Perinatol. 2015, 32, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Tareerath, M.; Wongyingsinn, M. Comparison of the Incidences of Cuff-Related Trauma after Non-Invasive Arterial Blood Pressure Measurement with and without Padding in Patients Undergoing Elective Surgery. J. Med. Assoc. Thail. Chotmaihet Thangphaet 2018, 101, 1. [Google Scholar]
- Quan, X.; Liu, J.; Roxlo, T.; Siddharth, S.; Leong, W.; Muir, A.; Cheong, S.-M.; Rao, A. Advances in Non-Invasive Blood Pressure Monitoring. Sensors 2021, 21, 4273. [Google Scholar] [CrossRef]
- Andriessen, P.; Schoffelen, R.L.M.; Berendsen, R.C.M.; Beer, N.A.M.D.; Oei, S.G.; Wijn, P.F.F.; E Blanco, C. Noninvasive Assessment of Blood Pressure Variability in Preterm Infants. Pediatr. Res. 2004, 55, 220–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drouin, E.; Gournay, V.; Calamel, J.; Mouzard, A.; Rozé, J.C. Feasibility of Using Finger Arterial Pressure in Neonates. Arch. Dis. Childhood. Fetal Neonatal Ed. 1997, 77, F139–F140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemson, J.; Hofhuizen, C.M.; Schraa, O.; Settels, J.J.; Scheffer, G.J.; van der Hoeven, J.G. The Reliability of Continuous Noninvasive Finger Blood Pressure Measurement in Critically Ill Children. Anesth. Analg. 2009, 108, 814–821. [Google Scholar] [CrossRef] [PubMed]
- El Hajj, C.; Kyriacou, P.A. Recurrent Neural Network Models for Blood Pressure Monitoring Using PPG Morphological Features. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, New Orleans, LA, USA, 4–7 November 2021; IEEE Engineering in Medicine and Biology Society: Piscataway, NJ, USA, 2021; pp. 1865–1868. [Google Scholar]
- Almeida, V.; Vieira, J.; Santos, P.; Pereira, T.; Pereira, H.; Correia, C.; Pego, M.; Cardoso, J. Machine Learning Techniques for Arterial Pressure Waveform Analysis. J. Pers. Med. 2013, 3, 82–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Association for the Advancement of Medical Instrumentation. American National Standard. In Manual, Electronic or Automated Sphygmomanometers ANSI/AAMI SP10-2002. 3330 Washington Boulevard; AAMI: Arlington, VA, USA, 2003. [Google Scholar]
- Altman, D.G.; Bland, J.M. Measurement in Medicine: The Analysis of Method Comparison Studies. Statistician 1983, 32, 307–317. [Google Scholar] [CrossRef]
- Harrison, W.; Goodman, D. Epidemiologic Trends in Neonatal Intensive Care, 2007–2012. JAMA Pediatr. 2015, 169, 855–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visscher, M.; Ralf Adam, O.; Brink, S.; Odio, M. Newborn Infant Skin: Physiology, Development, and Care. Clin. Dermatol. 2015, 33, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Dannevig, I.; Dale, H.C.; Liestøl, K.; Lindemann, R. Blood Pressure in the Neonate: Three Non-Invasive Oscillometric Pressure Monitors Compared with Invasively Measured Blood Pressure. Acta Paediatr. 2005, 94, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Sonesson, S.E.; Broberger, U. Arterial Blood Pressure in the Very Low Birthweight Neonate. Evaluation of an Automatic Oscillometric Technique. Acta Paediatr. Scand. 1987, 76, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.; Sander, J.; Gräber, S.; Gottschling, S.; Gortner, L. Agreement of Invasive versus Non-Invasive Blood Pressure in Preterm Neonates Is Not Dependent on Birth Weight or Gestational Age. J. Paediatr. Child Health 2010, 46, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Akangire, G.; Sullivan, B.; Fairchild, K.; Sampath, V. Continuous Vital Sign Analysis for Predicting and Preventing Neonatal Diseases in the Twenty-First Century: Big Data to the Forefront. Pediatr. Res. 2020, 87, 210–220. [Google Scholar] [CrossRef] [PubMed]





| Method | Measurement | Example Companies | FDA | |
|---|---|---|---|---|
| Cuff | Finger, tabletop | Continuous | BMEYE, Finapres, ADI, Biopac, Edwards (ClearSight), CNAP | Yes |
| Finger, wearable | Continuous | Caretaker | Yes | |
| Wrist | Intermittent | Omron, H2Care | Yes | |
| Cuffless | PPG | Continuous | Aktiia, BioBeat, Apple, ASUS, Samsung, Sensifree | Yes |
| PWV, PTT | Continuous | Vital Insight, Quanttus, Scanadu, Blumio, Sibel | No | |
| Tonometer | Continuous | Tensys, HealthStat, LiveMetric | Yes | |
| Capacitance | Continuous | PyrAmes, Vena Vitals | Submitted |
| Modules | Steps | Relevance |
|---|---|---|
| A. Quality Model | Select data Exclude data segments Quality ranking Classification elements | Boppli Band has an array of four sensors. The algorithm chooses data from the best sensor. Infant moves ⇨ pulse waveform excluded if quality value is below the threshold. Automated PW quality rating (0—bad, 5—good) using ANN, correlation coefficient Boppli/IAL. Algorithm automatically detects if the pulse waveform is corrupted by HFOV. |
| B. Signal Processing | Noise filtering/↑SNR | If Classification element detects HFOV ⇨ then notch filter. Normalize pulse waveform. |
| C. BP Model | CNN trained on pulse waveform and IAL SHAPE | Determines systolic, diastolic, mean arterial blood pressure. |
| D. Training Data | Obtain Boppli/IAL data | Sources: Stanford and CNH patient data warehouse; Stanford Boppli/IAL data collection. |
| E. Data Curation | Clean IAL data Synchronize data | Removes artifacts due to motion, damping, and other IAL operational issues. Synchronize Boppli and IAL data taken simultaneously. |
| F. Training and Testing | Cross-validation (k-fold) Model Ranking | Inputs: pulse waveform data, age, and weight. Splits data into 10 groups. Takes one group as a test and the remainder as training. Recursively tests model using TensorFlow and proprietary code. Choose best model that minimizes MAE and SD while optimizing slope and correlation coefficient of regression fit of estimated vs. ground truth values. |
| Study Cohort | Patient Characteristics (N = 81) | ||
|---|---|---|---|
| Age (days) | Minimum Maximum Average Median | 1 150 17 4 |  |
| Gestational age (weeks) | Minimum Maximum Average Median | 24.14 41.29 34.33 37.00 |  |
| Weight (kg) | Minimum Maximum Average Median | 0.55 4.85 2.60 2.80 |  |
| Sex, n (%) | 60.5% male, 39.5% female | ||
| Race/Ethnicity |  | ||
| Primary diagnosis at time of measurement | Cardiac (34), gastrointestinal (3), hyperbilirubinemia (2), multisystem congenital (4), neurological (8), prematurity (21), respiratory (4), Trisomy 21 (1), combination issues (3), and pulmonary hypertension (1) issues | ||
| IAL MAP (mmHg) individual means | Minimum Maximum Average Median | 29 68 49 50 |  |
| IAL SBP (mmHg) individual means | Minimum Maximum Average Median | 38 91 63 61 |  |
| IAL DBP (mmHg) individual means | Minimum Maximum Average Median | 19 54 38 38 |  |
| IAL location(s) (Some patients had more than one IAL) |  |
| Boppli location(s) (Some patients wore more than one Boppli) |  |
| Systolic BP | Diastolic BP | Mean Arterial BP | FDA Guidelines | |||||
|---|---|---|---|---|---|---|---|---|
| MAE | SD | MAE | SD | MAE | SD | MAE | SD | |
| Individual averages (N = 81) | −0.1 | 7.9 | 0.1 | 6.6 | −0.1 | 6.4 | ≤±5 mmHg | <8 mmHg |
| All points (N = 327 K) | −0.6 | 9.2 | −0.4 | 7.9 | −0.6 | 7.8 | ||
| r2 | Slope | r2 | Slope | r2 | Slope | |||
| Individual averages (N = 81) | 0.64 | 0.64 | 0.35 | 0.42 | 0.53 | 0.58 | ||
| All points (N = 327 K) | 0.56 | 0.58 | 0.27 | 0.36 | 0.43 | 0.50 | ||
| GA | N | MAP | SBP | DBP | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MAE | SD | Letter Code * | MAE | SD | Letter Code * | MAE | SD | Letter Code * | ||
|
EPT: <28 wk | 15 | 0.2 | 6.9 | A | −0.0 | 7.3 | A | 0.4 | 7.3 | A |
|
MPT: 28–37 wk | 38 | 0.2 | 6.4 | A | 0.3 | 8.3 | A | 0.4 | 6.6 | A |
|
FT: ≥38 wk | 26 | −0.9 | 6.2 | A | −0.8 | 7.8 | A | −0.5 | 6.4 | A |
| N | MAP | SBP | DBP | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MAE | SD | Letter Code * | MAE | SD | Letter Code * | MAE | SD | Letter Code * | ||
| Female | 33 | 2.0 | 5.1 | A | 2.2 | 6.4 | A | 1.9 | 4.9 | A |
| Male | 48 | −1.6 | 6.8 | B | −1.7 | 8.4 | B | −1.1 | 7.3 | B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, A.; Eskandar-Afshari, F.; Weiner, Y.; Billman, E.; McMillin, A.; Sella, N.; Roxlo, T.; Liu, J.; Leong, W.; Helfenbein, E.; et al. Clinical Study of Continuous Non-Invasive Blood Pressure Monitoring in Neonates. Sensors 2023, 23, 3690. https://doi.org/10.3390/s23073690
Rao A, Eskandar-Afshari F, Weiner Y, Billman E, McMillin A, Sella N, Roxlo T, Liu J, Leong W, Helfenbein E, et al. Clinical Study of Continuous Non-Invasive Blood Pressure Monitoring in Neonates. Sensors. 2023; 23(7):3690. https://doi.org/10.3390/s23073690
Chicago/Turabian StyleRao, Anoop, Fatima Eskandar-Afshari, Ya’el Weiner, Elle Billman, Alexandra McMillin, Noa Sella, Thomas Roxlo, Junjun Liu, Weyland Leong, Eric Helfenbein, and et al. 2023. "Clinical Study of Continuous Non-Invasive Blood Pressure Monitoring in Neonates" Sensors 23, no. 7: 3690. https://doi.org/10.3390/s23073690
APA StyleRao, A., Eskandar-Afshari, F., Weiner, Y., Billman, E., McMillin, A., Sella, N., Roxlo, T., Liu, J., Leong, W., Helfenbein, E., Walendowski, A., Muir, A., Joseph, A., Verma, A., Ramamoorthy, C., Honkanen, A., Green, G., Drake, K., Govindan, R. B., ... Quan, X. (2023). Clinical Study of Continuous Non-Invasive Blood Pressure Monitoring in Neonates. Sensors, 23(7), 3690. https://doi.org/10.3390/s23073690






