C14 Laves Phase Metal Hydride Alloys for Ni/MH Batteries Applications
Abstract
:1. Introduction
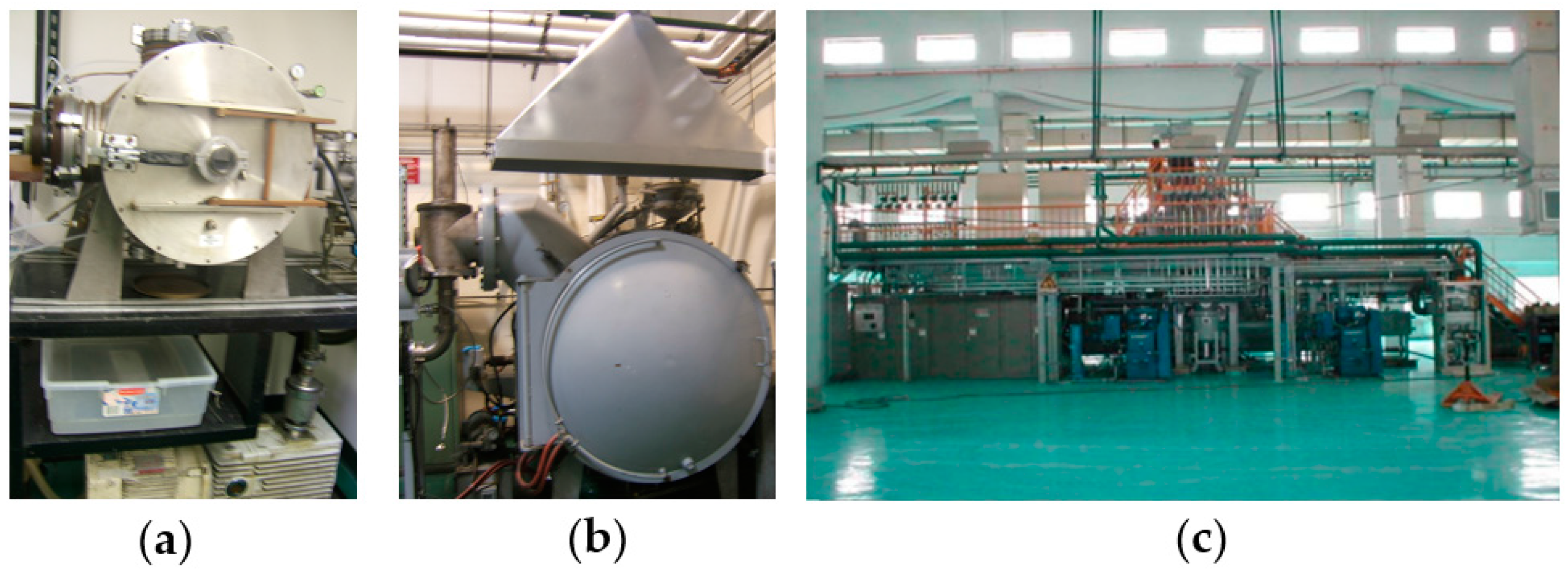
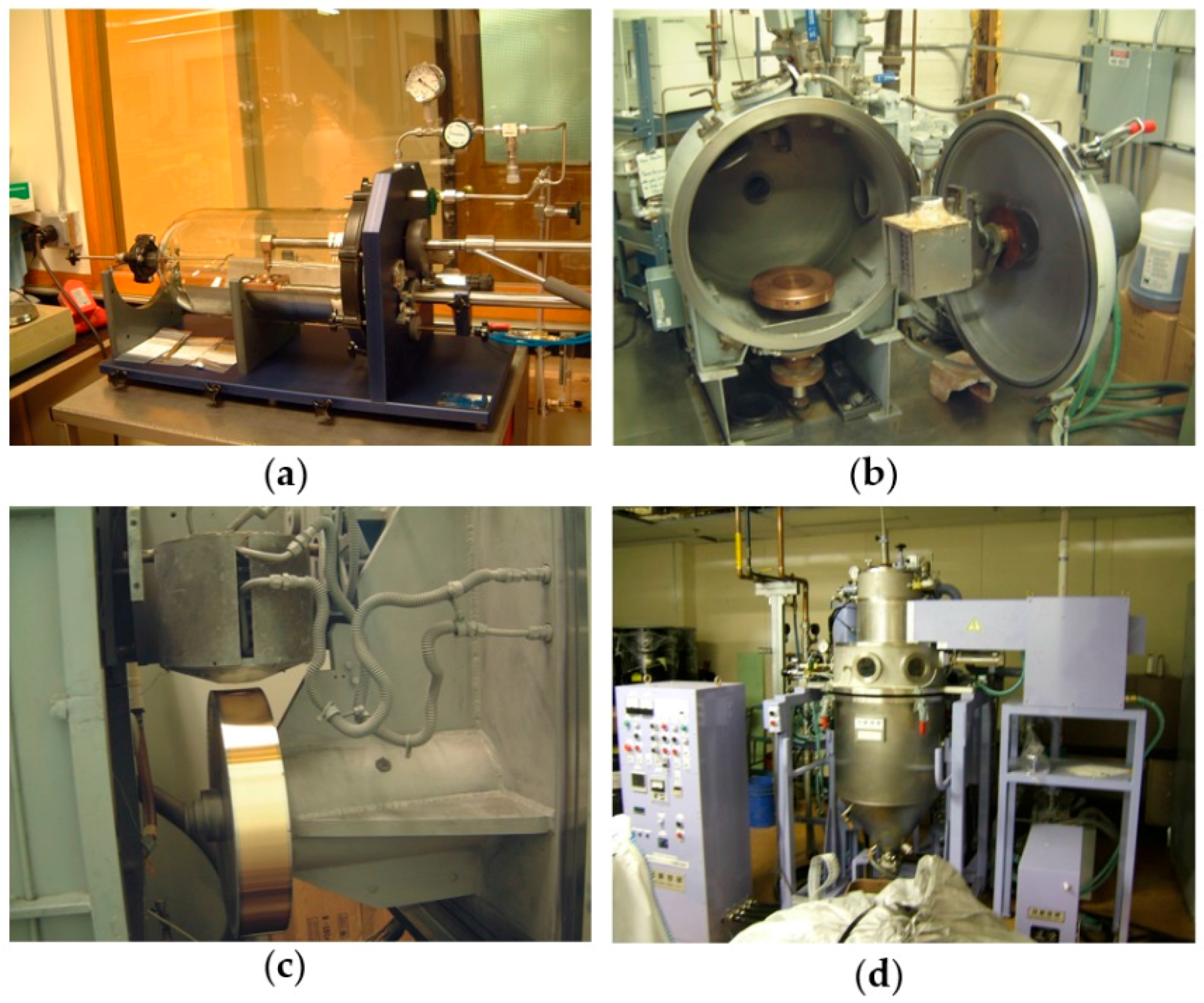
2. Alloys Preparation
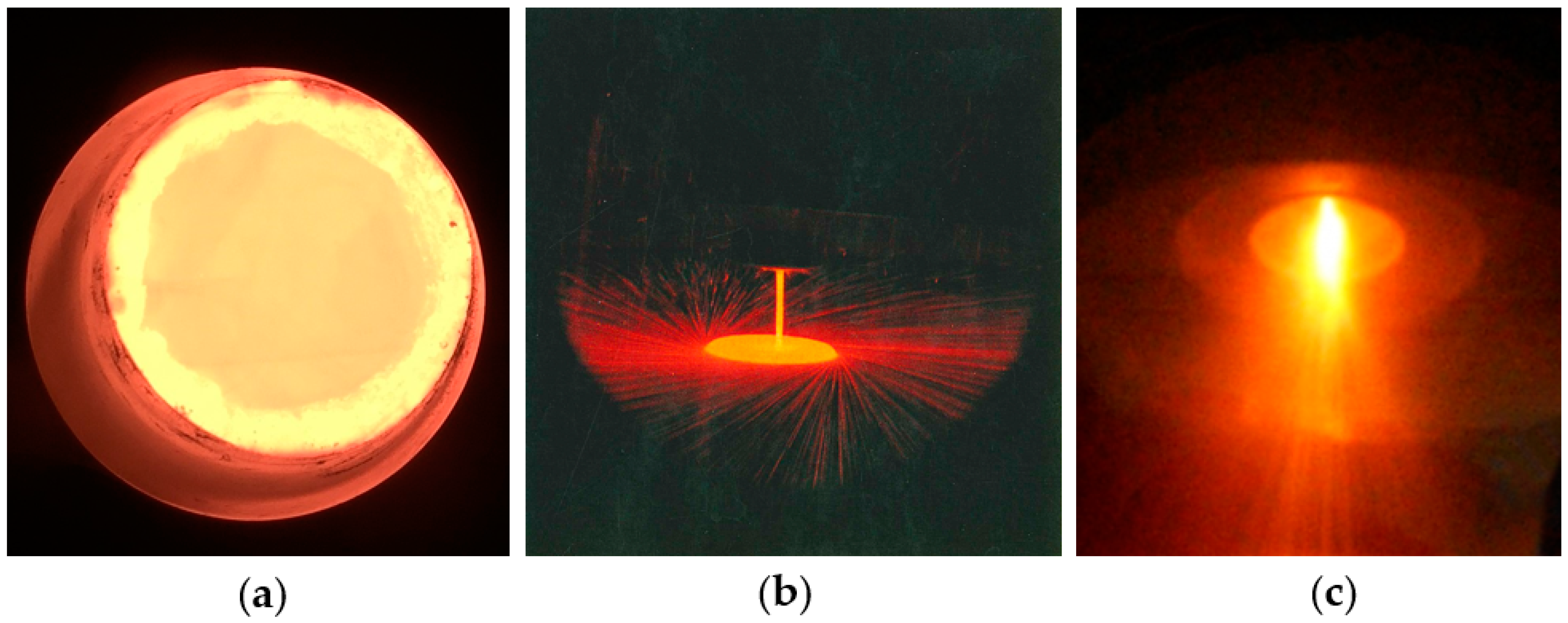
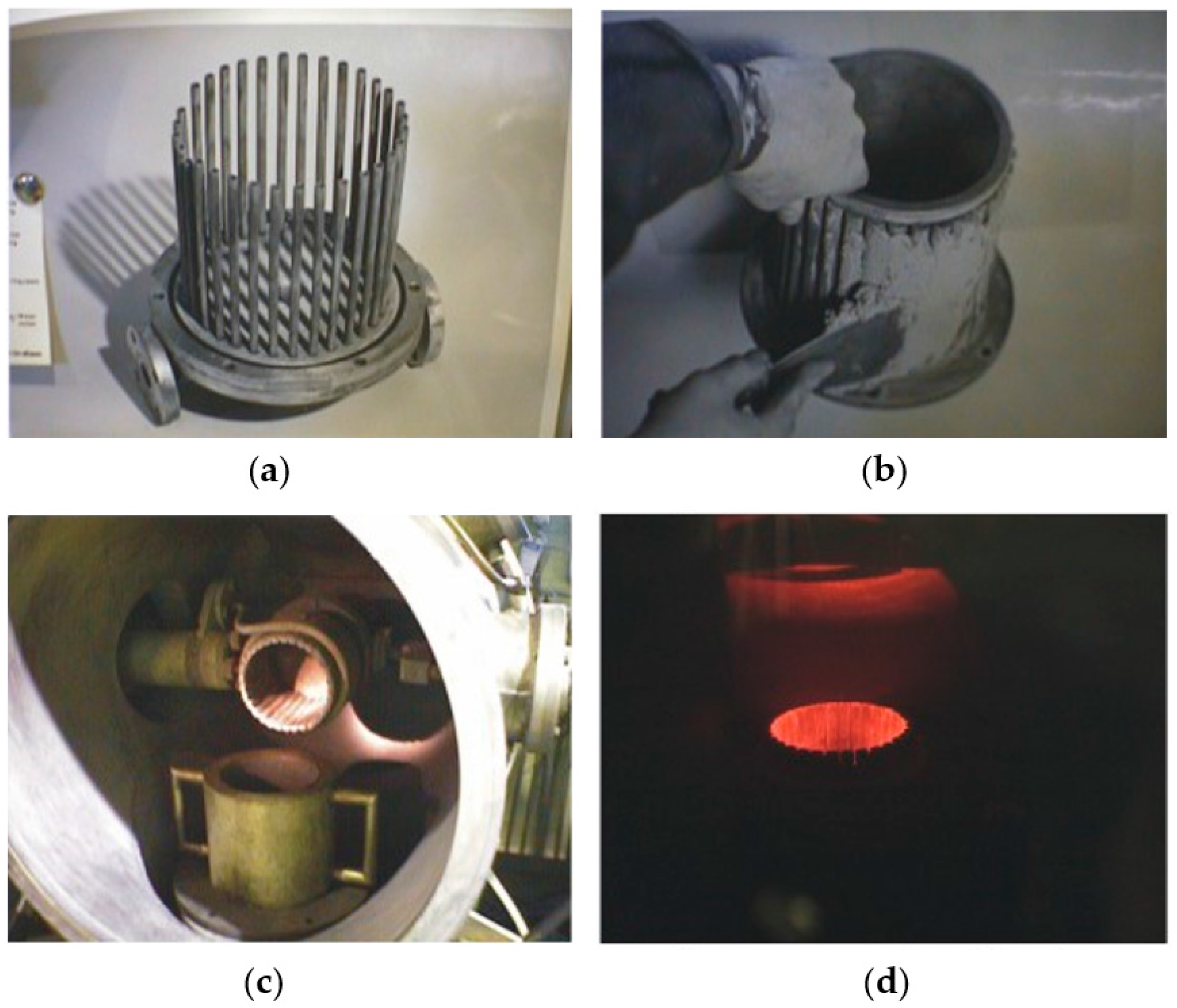
2.1. Melting and Casting
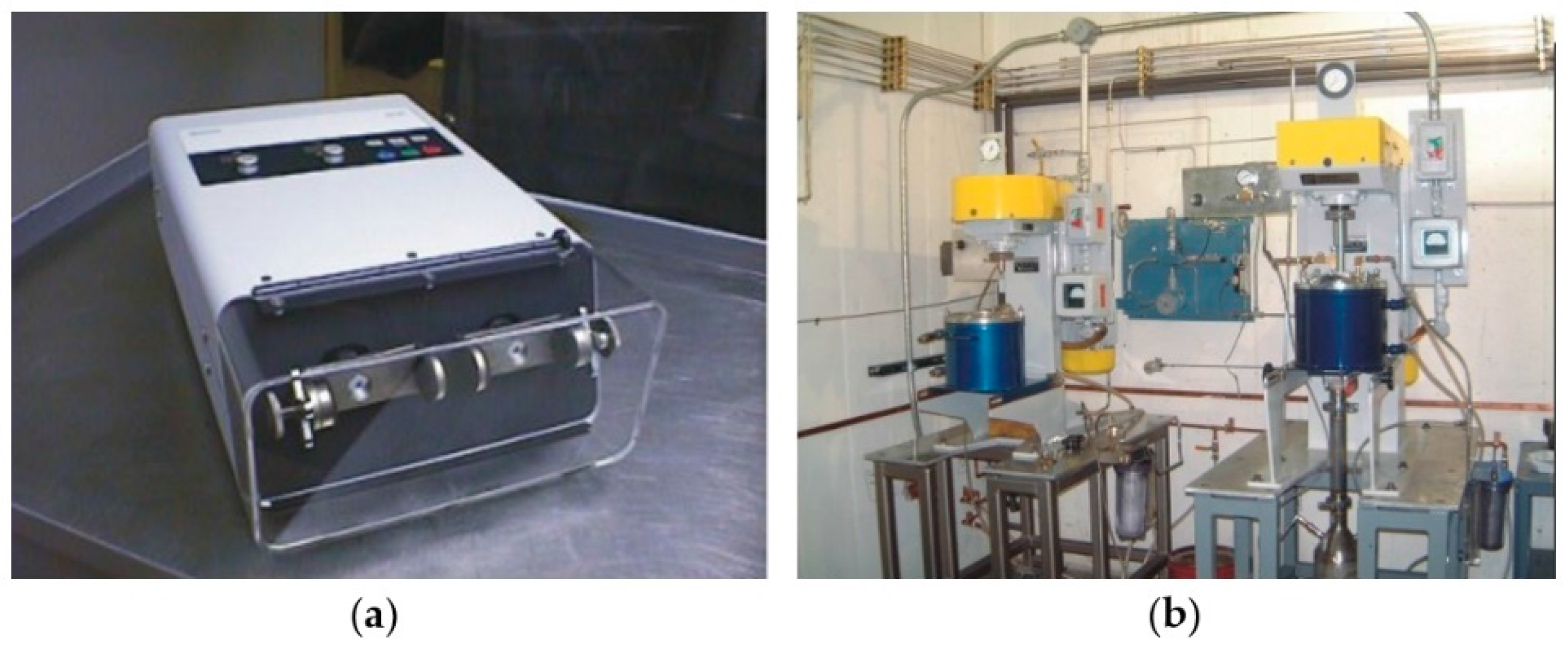
2.2. Particle Size Reduction
2.3. Annealing
2.4. Surface Treatment
3. Performance Criteria
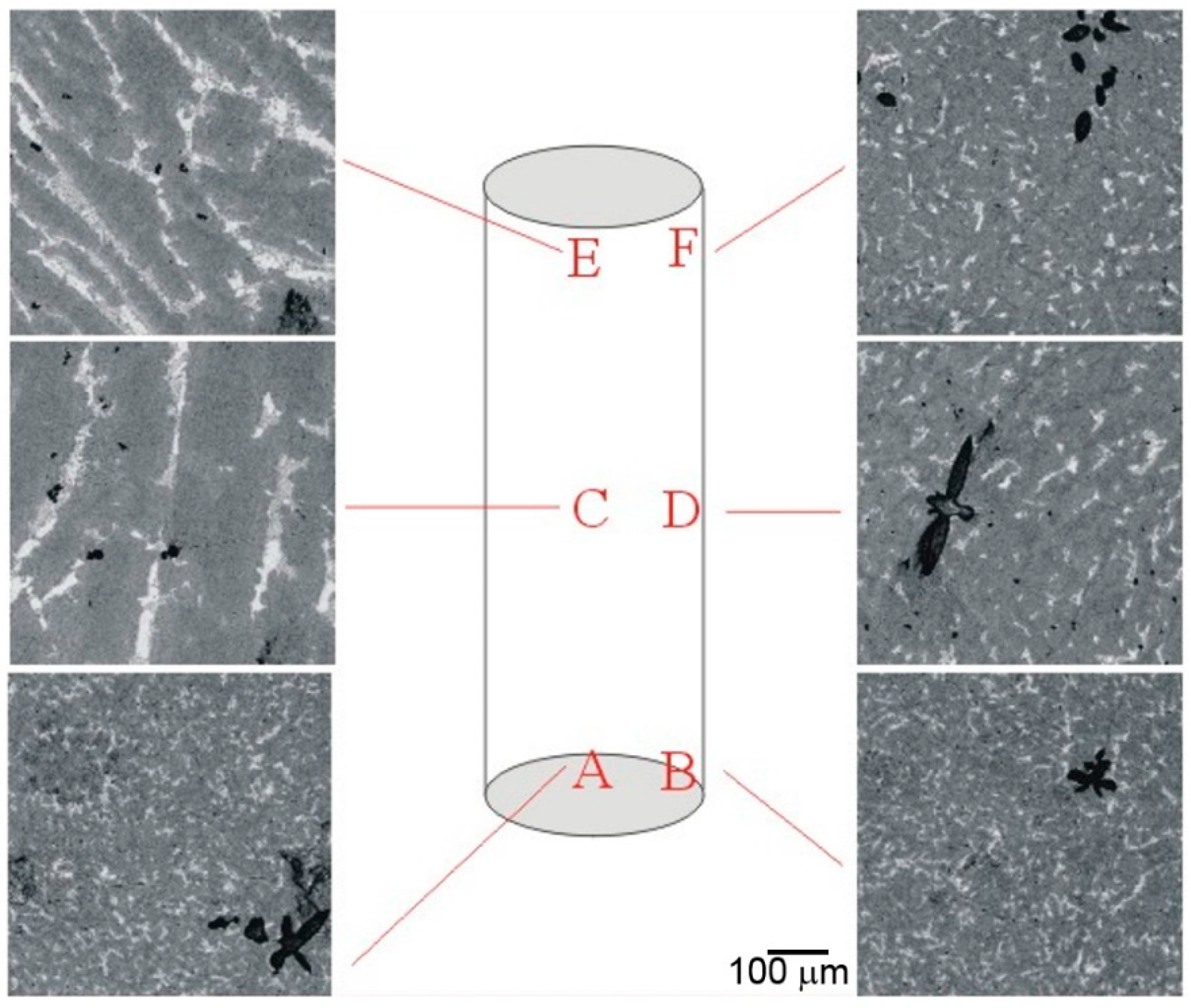
4. Failure Mechanism
4.1. Oxidation
4.2. Pulverization
4.3. Amorphization
5. Non-Laves Secondary Phases
5.1. TiNi
5.2. ZrxNiy
5.3. V-Based bcc Solid Solution
5.4. ZrO2
5.5. Other Secondary Phases
6. Selections of Element
6.1. A-Site Element
6.1.1. Titanium
6.1.2. Zirconium
6.1.3. Hafnium
6.1.4. Niobium
6.1.5. Palladium
6.1.6. Scandium
6.2. B-Site Element
6.2.1. Vanadium
6.2.2. Chromium
6.2.3. Manganese
6.2.4. Iron
6.2.5. Cobalt
6.2.6. Nickel
6.2.7. Copper
6.2.8. Zinc
6.2.9. Second Row Transition Metals (Mo)
6.2.10. Third Row Transition Metals (W, Pt)
6.2.11. Group 13 Elements (B, Al)
6.2.12. Group 14 Elements (C, Si, Ge, Sn)
6.2.13. Group 16 Elements (O, S, Se)
6.3. Secondary Phase Promoter
6.3.1. Group 1 Elements (Li, K)
6.3.2. Group 2 Elements (Mg)
6.3.3. Rare Earth (RE) Metals
6.4. Summary of Modifier Studies
7. Stoichiometry
7.1. Stoichiometric Alloy
7.2. Hypo-Stoichiometry
7.3. Hyper-Stoichiometry
8. Discussions
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Ni/MH | Nickel/metal hydride |
| MH | Metal hydride |
| H-storage | Hydrogen-strorage |
| VIM | Vacuum induction melting |
| AM | Arc melting |
| CC | Centrifugal casting |
| MS | Melt spinning |
| GA | Gas atomization |
| MA | Mechannical alloying |
| PS | Plasma spray |
| SEM | Scanning electron microscope |
| bcc | Bady-centered cubic |
| HRD | High-rate dischargeability |
| M-H | metal-hydrogen |
| RE | Rare-earth elements |
| PCT | Pressure-concomposition-temperature |
| EV | Electric vehicle |
| HEV | Hybrid electric vehicle |
| N/P | Negative/Positive |
| HIA | Hydrogen-induced amorphization |
| IMC | Intermettalic compound |
| TEM | Transmission electron microscope |
References
- Cai, X.; Young, K.; Chang, S. Reviews on the Chinese Patent regarding nickel/metal hydride battery. Batteries 2017, 3, 24. [Google Scholar] [CrossRef]
- Ogawa, K. Toyota to Start Local Production of NiMH Battery Cells in China by End of 2016. Available online: http://techon.nikkeibp.co.jp/atclen/news_en/15mk/030900433/ (accessed on 2 November 2016).
- Zelinsky, M.; Koch, J.; Fetcenko, M. Heat Tolerant NiMH Batteries for Stationary Power. Available online: www.battcon.com/PapersFinal2010/ZelinskyPaper2010Final_12.pdf (accessed on 28 March 2016).
- Zelinsky, M.; Koch, J. Batteries and Heat—A Recipe for Success? Available online: www.battcon.com/PapersFinal2013/16-Mike%20Zelinsky%20-%20Batteries%20and%20Heat.pdf (accessed on 28 March 2016).
- Teraoka, H. Development of Ni-MH ESS with Lifetime and Performance Estimation Technology. In Proceedings of the 34th International Battery Seminar & Exhibit, Fort Lauderdale, FL, USA, 20–23 March 2017. [Google Scholar]
- Teraoka, H. Ni-MH Stationary Energy Storage: Extreme Temperature & Long Life Developments. In Proceedings of the 33th International Battery Seminar & Exhibit, Fort Lauderdale, FL, USA, 21–24 March 2016. [Google Scholar]
- Teraoka, H. Development of Highly Durable and Long Life Ni-MH Batteries for Energy Storage Systems. In Proceedings of the 32th International Battery Seminar & Exhibit, Fort Lauderdale, FL, USA, 9–12 March 2015. [Google Scholar]
- Young, K.; Ouchi, T.; Nei, J.; Yasuoka, S. Fe-substitution for Ni in misch metal-based superlattice hydrogen absorbing alloys—Part 1. Structural, hydrogen storage, and electrochemical properties. Batteries 2016, 2, 34. [Google Scholar] [CrossRef]
- Kai, T.; Ishida, J.; Yasuoka, S.; Takeno, K. The Effect of Nickel-Metal Hydride Battery’s Characteristics with Structure of the Alloy. In Proceedings of the 54th Battery Symposium, Osaka, Japan, 7–9 October 2013; p. 210. [Google Scholar]
- Fetcenko, M.A.; Ovshinsky, S.A.; Young, K.; Reichman, B.; Fierro, C.; Koch, J.; Martin, F.; Mays, W.; Ouchi, T.; Sommers, B.; et al. High catalytic activity disordered VTiZrNiCrCoMnAlSn hydrogen storage alloys for nickel–metal hydride batteries. J. Alloys Compd. 2002, 330, 752–759. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Koch, J.; Fetcenko, M.A. The role of Mn in C14 Laves phase multi-component alloys for NiMH battery application. J. Alloys Compd. 2009, 477, 749–758. [Google Scholar] [CrossRef]
- Young, K.; Yasuoka, S. Past, Present, and Future of Metal Hydride Alloys in Nickel-Metal Hydride Batteries. In Proceedings of the MH2014 Conference, Salford, UK, 20–25 July 2014. [Google Scholar]
- Chang, S.; Young, K.; Nei, J.; Fierro, C. Reviews on the U.S. Patents regarding nickel/metal hydride batteries. Batteries 2016, 2, 10. [Google Scholar] [CrossRef]
- Young, K.; Nei, J. The current status of hydrogen storage alloy development for electrochemical applications. Materials 2013, 6, 4574–4608. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Geng, M.; Northwood, D.O. Electrochemical behaviour of intermetallic-based metal hydrides used in Ni/metal hydride (MH) batteries: A review. Int. J. Hydrogen Energy 2001, 26, 725–734. [Google Scholar] [CrossRef]
- Kleperis, J.; Wójcik, G.; Czerwinski, A.; Skowronski, J.; Kopxzyk, M.; Beltowska-Brzezinska, M. Electrochemical behavior of metal hydrides. J. Solid State Electrochem. 2001, 5, 229–249. [Google Scholar] [CrossRef]
- Han, S.; Zhao, M.; Wu, L.; Zheng, Y. Effect of additive elements on electrochemical properties of AB2-type Laves phase alloys. Chem. J. Chin. Univ. 2003, 24, 2256–2259. (In Chinese) [Google Scholar]
- Wang, J.; Yu, R.; Liu, Q. Effects of alloying side B on Ti-based AB2 hydrogen storage alloys. J. Harbin Inst. Technol. (New Ser.) 2004, 11, 485–492. [Google Scholar]
- Zhao, X.; Ma, L. Recent progress in hydrogen storage alloys for nickel/metal hydride secondary batteries. Int. J. Hydrogen Energy 2009, 34, 4788–4796. [Google Scholar] [CrossRef]
- Ouyang, L.; Huang, J.; Wang, H.; Liu, J.; Zhu, M. Progress of hydrogen storage alloys for Ni-MH rechargeable power batteries in electric vehicles: A review. Mater. Chem. Phys. 2017, 200, 164–178. [Google Scholar] [CrossRef]
- Stein, F.; Palm, M.; Sauthoff, G. Structure and stability of Laves phases. Part I. Critical assessment of factors controlling Laves phase stability. Intermetallics 2011, 12, 713–720. [Google Scholar] [CrossRef]
- Kumar, K.S.; Hazzledine, P.M. Polytypic transformations in Laves phases. Intermetallics 2004, 12, 763–770. [Google Scholar] [CrossRef]
- Chisholm, M.F.; Kumar, S.; Hazzledine, P. Dislocations in complex materials. Sciences 2005, 307, 701–703. [Google Scholar] [CrossRef] [PubMed]
- Baumann, W.; Leineweber, A.; Mittemeijer, E.J. The kinetics of a polytypic Laves phase transformation in TiCr2. Intermetallics 2011, 19, 526–535. [Google Scholar] [CrossRef]
- Scudino, S.; Donnadieu, P.; Surreddi, K.B.; Nikolowski, K.; Stoica, M.; Eckert, J. Microstructure and mechanical properties of Laves phase-reinforced Fe–Zr–Cr alloys. Intermetallics 2009, 17, 532. [Google Scholar] [CrossRef]
- Abraham, D.P.; Richardson, J.W., Jr.; McDeavitt, S.M. Laves intermetallics in stainless steel–zirconium alloys. Mater. Sci. Eng. A 1997, 239–240, 658–664. [Google Scholar] [CrossRef]
- Young, K.; Nei, J.; Wan, C.; Yartys, V. Comparison of C14- and C15-predominated AB2 metal hydride alloys for electrochemical applications. Batteries 2017, 3, 22. [Google Scholar] [CrossRef]
- Laves Crystal Structure of Laves Phase. Available online: http://www.geocities.jp/ohba_lab_ob_page/structure5.html (accessed on 26 April 2017).
- Young, K. Metal Hydride. In Elsevier Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Reedijk, J., Ed.; Elsevier: Waltham, MA, USA, 2013. [Google Scholar]
- Joubert, J.-M.; Latroche, M.; Percheron-Guégan, A.; Bouet, J. Improvement of the electrochemical activity of Zr–Ni–Cr Laves phase hydride electrodes by secondary phase precipitation. J. Alloys Compd. 1996, 240, 219–228. [Google Scholar] [CrossRef]
- Lee, H.-H.; Lee, K.-Y.; Lee, J.-Y. The hydrogenation characteristics of Ti-Zr-V-Mn-Ni C14 type Laves phase alloys for metal hydride electrodes. J. Alloys Compd. 1997, 253, 601–604. [Google Scholar] [CrossRef]
- Shu, K.Y.; Lei, Y.Q.; Yang, X.G.; Zhang, S.K.; Chen, L.S.; Lu, G.L.; Wang, Q.D. A comparative study on the electrochemical performance of rapidly solidified and conventionally cast hydride electrode alloy Zr(NiMnM)2.1. J. Alloys Compd. 1999, 290, 124–128. [Google Scholar] [CrossRef]
- Chen, L.; Wu, F.; Tong, M.; Chen, D.M.; Long, R.B.; Shang, Z.Q.; Liu, H.; Sun, W.S.; Yang, Y.; Wang, L.B.; et al. Advanced nanocrystalline Zr-based AB2 hydrogen storage electrode materials for NiMH EV batteries. J. Alloys Compd. 1999, 293, 508–520. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.; Hua, F.; Li, L. Effect of rapid solidification on the structural and electrochemical properties of the Ti–V-based hydrogen storage electrode alloy. J. Alloys Compd. 2008, 463, 528–532. [Google Scholar] [CrossRef]
- Huang, S.S.; Chuang, H.J.; Chan, S.L.I. Effects of fluorination on the hydriding and electrochemical properties of a gas-atomized Zr-based hydrogen storage alloy. J. Alloys Compd. 2002, 330, 617–621. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, H.; Hwang, K.T.; Han, J.S. Hydriding behavior in Zr-based AB2 alloy by gas atomization process. Int. J. Hydrogen Energy 2009, 34, 9424–9430. [Google Scholar] [CrossRef]
- Jung, C.B.; Lee, K.S. Electrode characteristics of metal hydride electrodes prepared by mechanical alloying. J. Alloys Compd. 1997, 253, 605–608. [Google Scholar] [CrossRef]
- Qiu, S.J.; Chu, H.L.; Zhang, Y.; Sun, L.X.; Xu, F.; Cao, Z. The electrochemical performances of Ti–V-based hydrogen storage composite electrodes prepared by ball milling method. Int. J. Hydrogen Energy 2008, 33, 7471–7478. [Google Scholar] [CrossRef]
- Kazemipour, M.; Salimijazi, H.; Saidi, A.; Saatchi, A. Hydrogen storage properties of Ti0.72Zr0.28Mn1.6V0.4 alloy prepared by mechanical alloying and copper boat induction melting. Int. J. Hydrogen Energy 2014, 39, 12784–12788. [Google Scholar] [CrossRef]
- Kazemipour, M.; Salimijazi, H.; Saidi, A.; Saatchi, A.; Mostaghimi, J.; Pershin, L. The electrochemical hydrogen storage properties of Ti0.72Zr0.28Mn1.6V0.4 alloy synthesized by vacuum plasma spraying and vacuum copper boat induction melting: A comparative study. Int. J. Hydrogen Energy 2015, 40, 15569–15577. [Google Scholar] [CrossRef]
- Young, K.; Fetcenko, M.A.; Li, F.; Ouchi, T. Structural, thermodynamic, and electrochemical properties of TixZr1−x(VNiCrMnCoAl)2 C14 Laves phase alloys. J. Alloys Compd. 2008, 464, 238–247. [Google Scholar] [CrossRef]
- Izumi, Y.; Moriwaki, Y.; Yamashita, K.; Tokuhiro, T. Nickel-Metal Hydride Storage Battery and Alloy for Configuring Negative Electrode of the Same. U.S. Patent 5,962,156, 5 October 1999. [Google Scholar]
- Young, K.; Koch, J.; Ouchi, T.; Banik, A.; Fetcenko, M.A. Study of AB2 alloy electrodes for Ni/MH battery prepared by centrifugal casting and gas atomization. J. Alloys Compd. 2010, 496, 669–677. [Google Scholar] [CrossRef]
- Ouchi, T.; Young, K.; Moghe, D. Reviews on the Japanese Patent Applications regarding nickel/metal hydride batteries. Batteries 2016, 2, 21. [Google Scholar] [CrossRef]
- Young, K. Stoichiometry in inter-metallic compounds for hydrogen storage applications. In Stoichiometry and Materials Science—When Numbers Matter; Innocenti, A., Kamarulzaman, N., Eds.; InTech: Rijeka, Crotia, 2012. [Google Scholar]
- Humana, R.M.; Thomas, J.E.; Ruiz, F.; Real, S.G.; Castro, E.B.; Visintin, A. Electrochemical behavior of metal hydride electrode with different particle size. Int. J. Hydrogen Energy 2012, 37, 14966–14971. [Google Scholar] [CrossRef]
- Matsuoka, M.; Tamura, K. Effects of mechanical modification on electrochemical performance of Zr–Ti-based Laves-phase alloy electrode. J. Electrochem. Soc. 2007, 154, A119–A122. [Google Scholar] [CrossRef]
- Fetcenko, M.A.; Kaatz, T.; Sumner, S.P.; LaRocca, J. Hydride Reactor Apparatus for Hydrogen Comminution of Metal Hydride Hydrogen Storage Materials. U.S. Patent 4,893,756, 16 January 1990. [Google Scholar]
- Young, K.; Fetcenko, M.A. Method for Powder Formation of a Hydrogen Storage Alloy. U.S. Patent 6,120,936, 19 September 2000. [Google Scholar]
- Kim, D.M.; Lee, H.; Cho, K.; Lee, J.Y. Effect of Cu powder as an additive material on the inner pressure of a sealed-type Ni–MH rechargeable battery using a Zr-based alloy as an anode. J. Alloys Compd. 1999, 282, 261–267. [Google Scholar] [CrossRef]
- Tan, S.; Shen, Y.; Şahin, E.O.; Noréus, D.; Öztürk, T. Activation behavior of an AB2 type metal hydride alloy for NiMH batteries. Int. J. Hydrogen Energy 2016, 41, 9948–9953. [Google Scholar] [CrossRef]
- Liu, B.H.; Li, Z.P.; Okutsu, A.; Suda, S. A wet ball milling treatment of Zr-based AB2 alloys as negative electrode materials. J. Alloys Compd. 2000, 296, 148–151. [Google Scholar] [CrossRef]
- Great Powder Website. Available online: http://www.greatpowder.com/yingwen/ (accessed on 10 May 2017).
- Higuchi, E.; Li, Z.P.; Suda, S.; Nohara, S.; Inoue, H.; Iwakura, C. Structural and electrochemical characterization of fluorinated AB2-type Laves phase alloys obtained by different pulverization methods. J. Alloys Compd. 2002, 335, 241–245. [Google Scholar] [CrossRef]
- Hu, W.K.; Kim, D.M.; Jeon, S.W.; Lee, J.Y. Effect of annealing treatment on electrochemical properties of Mm-based hydrogen storage alloys for Ni/MH batteries. J. Alloys Compd. 1998, 270, 255–264. [Google Scholar] [CrossRef]
- Young, K.; Nei, J.; Ouchi, T.; Fetcenko, M.A. Phase abundances in AB2 metal hydride alloys and their correlations to various properties. J. Alloys Compd. 2011, 509, 2277–2284. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Meng, T.; Wong, D.F. Studies on the synergetic effects in multi-phase metal hydride alloys. Batteries 2016, 2, 15. [Google Scholar] [CrossRef]
- Züttel, A.; Meli, F.; Chartouni, D.; Schlapbach, L.; Lichtenberg, F.; Friedrich, B. Properties of Zr(V0.25Ni0.75)2 metal hydride as active electrode material. J. Alloys Compd. 1996, 239, 175–282. [Google Scholar] [CrossRef]
- Zhang, Q.A.; Lei, Y.Q.; Yang, X.G.; Ren, K.; Wang, Q.D. Annealing treatment of AB2-type hydrogen storage alloys: I. crystal structures. J. Alloys Compd. 1999, 292, 236–240. [Google Scholar] [CrossRef]
- Zhang, Q.A.; Lei, Y.Q.; Yang, X.G.; Du, Y.L.; Wang, Q.D. Effects of annealing treatment on phase structures, hydrogen absorption–desorption characteristics and electrochemical properties of a V3TiNi0.56Hf0.24Mn0.15Cr0.1 alloy. J. Alloys Compd. 2000, 305, 125–129. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Huang, B.; Chao, B.; Fetcenko, M.A.; Bendersky, L.A.; Wang, K.; Chiu, C. The correlation of C14/C15 phase abundance and electrochemical properties in the AB2 alloys. J. Alloys Compd. 2010, 506, 841–848. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Banik, A.; Koch, J.; Fetcenko, M.A. Improvement in the electrochemical properties of gas atomized AB2 metal hydride alloys by hydrogen annealing. Int. J. Hydrogen Energy 2011, 36, 3547–3555. [Google Scholar] [CrossRef]
- Chuang, H.J.; Huang, S.S.; Ma, C.Y.; Chan, S.L.I. Effect of annealing heat treatment on an atomized AB2 hydrogen storage alloy. J. Alloys Compd. 1999, 285, 284–291. [Google Scholar] [CrossRef]
- Liu, H.; Li, R. Effect of variation of constituent on microstructure and electrochemical properties of AB2 hydrogen storage alloys. Foundry Technol. 2007, 29, 179–183. (In Chinese) [Google Scholar]
- Klein, B.; Simon, N.; Klyamkine, S.; Latroche, M.; Percheron-Guégan, A. Improvement of the thermodynamical and electrochemical properties of multicomponent Laves phase hydrides by thermal annealing. J. Alloys Compd. 1998, 280, 284–289. [Google Scholar] [CrossRef]
- Züttel, A.; Meli, F.; Schlapbach, L. Effects of pretreatment on the activation behavior of Zr(V0.25Ni0.75)2 metal hydride electrodes in alkaline solution. J. Alloys Compd. 1994, 209, 99–105. [Google Scholar] [CrossRef]
- Liu, F.J.; Kitayama, K.; Suda, S. La and Ce-incorporation effects on the surface properties of the fluorinated (Ti,Xr)(Mn,Cr,Ci)2 hydriding alloys. Vacuum 1996, 47, 903–906. [Google Scholar] [CrossRef]
- Liu, B.H.; Li, Z.P.; Higuchi, E.; Suda, S. Improvement of the electrochemical properties of Zr-based AB2 alloys by an advanced fluorination technique. J. Alloys Compd. 1999, 293, 702–706. [Google Scholar] [CrossRef]
- Li, Z.P.; Higuchi, E.; Liu, B.H.; Suda, S. Effects of fluorination temperature on surface structure and electrochemical properties of AB2 electrode alloys. Electrochim. Acta 2000, 45, 1773–1779. [Google Scholar] [CrossRef]
- Park, H.Y.; Cho, W.I.; Cho, B.W.; Lee, S.R.; Yun, K.S. Effect of fluorination on the lanthanum-doped AB2-type metal hydride electrodes. J. Power Sources 2001, 92, 149–156. [Google Scholar] [CrossRef]
- Li, Z.P.; Liu, B.H.; Hitaka, K.; Suda, S. Effects of surface structure of fluorinated AB2 alloys on their electrodes and battery performances. J. Alloys Compd. 2002, 330, 776–781. [Google Scholar] [CrossRef]
- Young, K.H.; Fetcenko, M.A.; Ovshinsky, S.R.; Ouchi, T.; Reichman, B.; Mays, W.C. Improved surface catalysis of Zr-based Laves phase alloys for NiMH batteries. In Hydrogen at Surface and Interfaces; Jerkiewicz, G., Feliu, J.M., Popov, B.N., Eds.; Electrochemical Society: Pennington, NJ, USA, 2000. [Google Scholar]
- Reichman, B.; Venkatesan, S.; Fetcenko, M.A.; Jeffries, K.; Stahl, S.; Bennett, C. Activated Rechargeable Hydrogen Storage Electrode and Method. U.S. Patent 4,716,088, 29 December 1987. [Google Scholar]
- Jung, J.H.; Liu, B.H.; Lee, J.Y. Activation behavior of Zr0.7Ti0.3Cr0.3Mn0.3V0.4Ni alloy electrode modified by the hot-charging treatment. J. Alloys Compd. 1998, 264, 306–310. [Google Scholar] [CrossRef]
- Liu, B.; Jung, J.; Lee, H.; Lee, K.; Lee, J. Improved electrochemical performance of AB2-type metal hydride electrode activated by the hot-charging process. J. Alloys Compd. 1996, 245, 132–141. [Google Scholar] [CrossRef]
- Cao, J.; Gao, X.; Lin, D.; Zhou, X.; Yuan, H.; Song, D.; Shen, P. Activation behavior of the Zr-based Laves phase alloy electrode. J. Power Sources 2011, 93, 141–144. [Google Scholar]
- Şahin, E.O. Development of Rare Earth-Free Negative Electrode Materials for NiMH Batteries. Proceeding of the 18th International Metallurgy & Materials Congress, Istanbul, Turkey, 29 September–1 October 2016; pp. 770–773. [Google Scholar]
- Jung, J.H.; Lee, H.H.; Kim, D.M.; Jang, K.J.; Lee, J.Y. Degradation behavior of Cu-coated Ti–Zr–V–Mn–Ni metal hydride electrodes. J. Alloys Compd. 1998, 266, 266–270. [Google Scholar] [CrossRef]
- Sun, D.; Latroche, M.; Percheron-Guégan, A. Activation behaviour of mechanically Ni-coated Zr-based laves phase hydride electrode. J. Alloys Compd. 1997, 257, 302–305. [Google Scholar] [CrossRef]
- Jurczyk, M.; Rajewski, W.; Majchrzycki, W.; Wojcik, G. Synthesis and electrochemical properties of high-energy ball-milled Laves phase (Zr,Ti)(V,Mn,Cr)2 alloys with nickel powder. J. Alloys Compd. 1998, 274, 299–302. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.; Yu, J.S.; Fateev, G.A.; Lee, J.Y. The activation characteristics of a Zr-based hydrogen storage alloy electrode surface-modified by ball-milling process. J. Alloys Compd. 1999, 292, 258–265. [Google Scholar] [CrossRef]
- Li, S.; Zhao, M.; Wang, Y.; Zhai, J. Structure and electrochemical property of ball-milled Ti0.26Zr0.07Mn0.1Ni0.33V0.24 alloy. Mater. Chem. Phys. 2009, 118, 51–56. [Google Scholar] [CrossRef]
- Chu, H.L.; Zhang, Y.; Sun, L.X.; Qiu, S.J.; Qi, Y.N.; Xu, F.; Yuan, H. Structure and electrochemical properties of composite electrodes synthesized by mechanical milling Ni-free TiMn2-based alloy with La-based alloy. J. Alloys Compd. 2007, 446–447, 614–619. [Google Scholar] [CrossRef]
- Li, P.; Wang, X.; Wu, J.; Zhao, W.; Li, R.; Ma, N. Present status on research of AB2-type Laves phase hydrogen storage electrode materials. Met. Funct. Mater. 2000, 7, 7–12. (In Chinese) [Google Scholar]
- Young, K. Electrochemical applications of metal hydride. In Compendium of Hydrogen Energy Volume 3: Hydrogen Energy Conversion; Barbir, F., Basile, A., Veziroglu, T.N., Eds.; Woodhead Publishing: Cambridge, UK, 2016. [Google Scholar]
- Hong, K. The development of hydrogen storage alloys and the progress of nickel hydride batteries. J. Alloys Compd. 2001, 321, 307–313. [Google Scholar] [CrossRef]
- Young, K.; Huang, B.; Regmi, R.K.; Lawes, G.; Liu, Y. Comparisons of metallic clusters imbedded in the surface oxide of AB2, AB5, and A2B7 alloys. J. Alloys Compd. 2010, 506, 831–840. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Fetcenko, M.A. Pressure–composition–temperature hysteresis in C14 Laves phase alloys: Part 1. Simple ternary alloys. J. Alloys Compd. 2009, 480, 428–433. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Mays, W.; Reichman, B.; Fetcenko, M.A. Pressure–composition–temperature hysteresis in C14 Laves phase alloys: Part 2. Applications in NiMH batteries. J. Alloys Compd. 2009, 480, 434–439. [Google Scholar] [CrossRef]
- Young, K.; Yasuoka, S. Capacity degradation mechanisms in nickel/metal hydride batteries. Batteries 2016, 2, 3. [Google Scholar] [CrossRef]
- Knosp, B.; Vallet, L.; Blanchard, P. Performance of an AB2 alloy in sealed Ni–MH batteries for electric vehicles: Quantification of corrosion rate and consequences on the battery performance. J. Alloys Compd. 1999, 293, 770–774. [Google Scholar] [CrossRef]
- Young, K.; Wong, D.F.; Yasuoka, S.; Ishida, J.; Nei, J.; Koch, J. Different failure modes for V-containing and V-free AB2 metal hydride alloys. J. Power Sources 2014, 251, 170–177. [Google Scholar] [CrossRef]
- Fetcenko, M.A.; Ovshinsky, S.R.; Reichman, B.; Young, K.; Fierro, C.; Koch, J.; Zallen, A.; Mays, W.; Ouchi, T. Recent advances in NiMH battery technology. J. Power Sources 2007, 165, 544–551. [Google Scholar] [CrossRef]
- Young, K.; Chao, B.; Liu, Y.; Nei, J. Microstructures of the oxides on the activated AB2 and AB5 metal hydride alloys surface. J. Alloys Compd. 2014, 606, 97–104. [Google Scholar] [CrossRef]
- Young, K.; Chao, B.; Pawlik, D.; Shen, H.T. Transmission electron microscope studies in the surface oxide on the La-containing AB2 metal hydride alloy. J. Alloys Compd. 2016, 672, 356–365. [Google Scholar] [CrossRef]
- Zhu, Y.; Pan, H.; Gao, M.; Liu, Y.; Li, R.; Lei, Y.; Wang, Q. Degradation mechanisms of Ti–V-based multiphase hydrogen storage alloy electrode. Int. J. Hydrogen Energy 2004, 29, 313–318. [Google Scholar] [CrossRef]
- Liu, B.H.; Li, Z.P.; Matsuyama, Y.; Kitani, R.; Suda, S. Corrosion and degradation behavior of Zr-based AB2 alloy electrodes during electrochemical cycling. J. Alloys Compd. 2000, 296, 201–208. [Google Scholar] [CrossRef]
- Chuang, H.J.; Chan, S.L.I. Study of the performance of Ti–Zr based hydrogen storage alloys. J. Power Sources 1999, 77, 159–163. [Google Scholar] [CrossRef]
- Aoki, K.; Yamamoto, T.; Masumoto, T. Hydrogen induced amorphization in RNi2 laves phases. Scr. Metall. 1987, 21, 27–31. [Google Scholar] [CrossRef]
- Aoki, K. Amorphous phase formation by hydrogen absorption. Mater. Sci. Eng. A 2001, 304–306, 45–53. [Google Scholar] [CrossRef]
- Aoki, K.; Masumoto, T. Hydrogen-induced amorphization of intermetallics. J. Alloys Compd. 1995, 231, 20–28. [Google Scholar] [CrossRef]
- Züttel, A.; Chartouni, D.; Nützenadel, C.; Gross, K.; Schlapbach, L. Bulk and surface properties of crystalline and amorphous Zr36(V0.33Ni0.66)64 alloy as active electrode material. J. Alloys Compd. 1998, 266, 321–326. [Google Scholar] [CrossRef]
- Hu, W.K.; Zhang, Y.S.; Song, D.Y.; Shen, P.W. Electrochemical hydrogen storage properties of amorphous and crystalline MI-Ni alloy films. Int. J. Hydrogen Energy 1996, 21, 651–656. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, L.; Gou, Y.; Shen, X. Structure, morphology and hydrogen desorption characteristics of amorphous and crystalline Ti-Ni alloys. Mater. Sci. Eng. A 2009, 516, 50–53. [Google Scholar] [CrossRef]
- Yang, X.; Lei, Y.; Wang, C.; Zhu, G.; Zhang, W.; Wang, Q. Influence of amorphization on electrode performances of AB2 type hydrogen storage alloys. J. Alloys Compd. 1998, 265, 264–268. [Google Scholar] [CrossRef]
- Boettinger, W.J.; Newbury, D.E.; Wang, K.; Bendersky, L.A.; Chiu, C.; Kattner, U.R.; Young, K.; Chao, B. Examination of multiphase (Zr,Ti)(V,Cr,Mn,Ni)2 Ni-MH electrode alloys: Part I. Dendritic solidification structure. Metall. Mater. Trans. A 2010, 41, 2033–2047. [Google Scholar] [CrossRef]
- Bendersky, L.A.; Wang, K.; Boettinger, W.J.; Newbury, D.E.; Young, K.; Chao, B. Examination of multiphase (Zr,Ti)(V,Cr,Mn,Ni)2 Ni-MH electrode alloys: Part II. Solid-state transformation of the interdendritic B2 phase. Metall. Mater. Trans. A 2010, 41, 1891–1906. [Google Scholar] [CrossRef]
- Liu, Y.; Young, K. Microstructure investigation on metal hydride alloys by electron backscatter Diffraction Technique. Batteries 2016, 2, 26. [Google Scholar] [CrossRef]
- Bendersky, L.A.; Wang, K.; Levin, I.; Newbury, D.; Young, K.; Chao, B.; Creuziger, A. Ti12.5Zr21V10Cr8.5MnxCo1.5Ni46.5−x AB2-type metal hydride alloys for electrochemical storage application: Part 1. Structural characteristics. J. Power Sources 2012, 218, 474–486. [Google Scholar] [CrossRef]
- Tokunaga, T.; Motsumoto, S.; Ohtani, H.; Hasebe, M. Thermodynamic calculation of phase equilibria in the Nb-Ni-Ti-Zr quaternary system. Mater. Trans. 2007, 48, 89–96. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Reichman, B.; Mays, W.; Regmi, R.; Lawes, G.; Fetcenko, M.A.; Wu, A. Optimization of Co-content in C14 Laves phase multi-component alloys for NiMH battery application. J. Alloys Compd. 2010, 489, 202–210. [Google Scholar] [CrossRef]
- Young, K.; Reichman, B.; Fetcenko, M.A. Electrochemical performance of AB2 metal hydride alloys measured at −40 °C. J. Alloys Compd. 2013, 580, S349–S352. [Google Scholar] [CrossRef]
- Young, K.; Wong, D.F.; Nei, J.; Reichman, B. Electrochemical properties of hypo-stoichiometric Y-doped AB2 metal hydride alloys at ultra-low temperature. J. Alloys Compd. 2015, 643, 17–27. [Google Scholar] [CrossRef]
- Zhang, Q.A.; Lei, Y.Q.; Wang, C.S.; Wang, F.S.; Wang, Q.D. Structure of the secondary phase and its effects on hydrogen-storage properties in a Ti0.7Zr0.2V0.1Ni alloy. J. Power Sources 1998, 75, 288–291. [Google Scholar] [CrossRef]
- Saldan, I.; Burtovyy, R.; Becker, H.W.; Ader, V.; Wöll, C. Ti–Ni alloys as MH electrodes in Ni–MH accumulators. Int. J. Hydrog. Energy 2008, 33, 7177–7184. [Google Scholar] [CrossRef]
- Nei, J.; Young, K. Gaseous phase and electrochemical hydrogen storage properties of Ti50Zr1Ni44X5 (X = Ni, Cr, Mn, Fe, Co, or Cu) for nickel metal hydride battery applications. Batteries 2016, 2, 24. [Google Scholar] [CrossRef]
- Emami, H.; Cuevas, F.; Latroche, M. Ti(Ni,Cu) pseudobinary compounds as efficient negative electrodes for Ni–MH batteries. J. Power Sources 2014, 265, 182–191. [Google Scholar] [CrossRef]
- Shu, K.Y.; Yang, X.G.; Zhang, S.K.; Lü, G.L.; Lei, Y.Q.; Wang, Q.D. Effect of Cr and Co additives on microstructure and electrochemical performance of Zr(NiVMn)2M0.1 alloys. J. Alloys Compd. 2000, 306, 122–126. [Google Scholar] [CrossRef]
- Young, K.; Chao, B.; Bendersky, L.A.; Wang, K. Ti12.5Zr21V10Cr8.5MnxCo1.5Ni46.5−x AB2-type metal hydride alloys for electrochemical storage application: Part 2. Hydrogen storage and electrochemical properties. J. Power Sources 2012, 218, 487–494. [Google Scholar] [CrossRef]
- Ji, S.; Li, S.; Sun, J. Effect of alloys with Mn, V on phase structures and electrochemical properties of Zr-Cr-Ni based AB2 hydrogen storage electrode alloys. Chin. J. Rare Met. 2004, 28, 657–661. (In Chinese) [Google Scholar]
- Nei, J.; Young, K.; Regmi, R.; Lawes, G.; Salley, S.O.; Ng, K.Y.S. Gaseous phase hydrogen storage and electrochemical properties of Zr8Ni21, Zr7Ni10, Zr9Ni11, and ZrNi metal hydride alloys. Int. J. Hydrogen Energy 2012, 37, 16042–16055. [Google Scholar] [CrossRef]
- Matsuyama, A.; Mizutani, H.; Kozuka, T.; Inoue, H. Effect of Ti substitution on electrochemical properties of ZrNi alloy electrode for use in nickel-metal hydride batteries. Int. J. Hydrogen Energy 2017. [Google Scholar] [CrossRef]
- Matsuyama, A.; Mizutani, H.; Kozuka, T.; Inoue, H. Crystal structure and hydrogen absorption-desorption properties of Zr1–xTixNi (0.05 ≤ x ≤ 0.5) alloys. J. Alloys Compd. 2017, 714, 467–475. [Google Scholar] [CrossRef]
- Ruiz, F.C.; Castro, E.B.; Real, S.G.; Peretti, H.A.; Visintin, A.; Triaca, W.E. Electrochemical characterization of AB2 alloys used for negative electrodes in Ni/MH batteries. Int. J. Hydrogen Energy 2008, 33, 3576–3580. [Google Scholar] [CrossRef]
- Ruiz, F.C.; Castro, E.B.; Peretti, H.A.; Visintin, A. Study of the different ZrxNiy phases of Zr-based AB2 materials. Int. J. Hydrogen Energy 2010, 35, 9879–9887. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Liu, Y.; Reichman, B.; Mays, W.; Fetcenko, M.A. Structural and electrochemical properties of TixZr7−xNi10. J. Alloys Compd. 2009, 480, 521–528. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Fetcenko, M.A.; Mays, W.; Reichman, B. Structural and electrochemical properties of Ti1.5Zr5.5VxNi10−x. Int. J. Hydrogen Energy 2009, 34, 8695–8706. [Google Scholar] [CrossRef]
- Nei, J.; Young, K.; Salley, S.O.; Ng, K.Y.S. Effects of annealing on Zr8Ni19X2 (X = Ni, Mg, Al, Sc, V, Mn, Co, Sn, La, and Hf): Hydrogen storage and electrochemical properties. Int. J. Hydrogen Energy 2012, 37, 8418–8427. [Google Scholar] [CrossRef]
- Young, M.; Chang, S.; Young, K.; Nei, J. Hydrogen storage properties of ZrVxNi3.5−x (x = 0.0–0.9) metal hydride alloys. J. Alloys Compd. 2013, 580, S171–S174. [Google Scholar] [CrossRef]
- Young, K.; Young, M.; Chang, S.; Huang, B. Synergetic effects in electrochemical properties of ZrVxNi4.5−x (x = 0.0, 0.1, 0.2, 0.3, 0.4, and 0.5) metal hydride alloys. J. Alloys Compd. 2013, 560, 33–41. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Pan, H.G.; Wang, G.Y.; Gao, M.X.; Ma, J.X.; Chen, C.P.; Wang, Q.D. Phase structure, crystallography and electrochemical properties of Laves phase compounds Ti0.8Zr0.2V1.6Mn0.8−xMxNi0.6 (M = Fe, Al, Cr, Co). Int. J. Hydrogen Energy 2001, 26, 807–816. [Google Scholar] [CrossRef]
- Du, Y.L.; Yang, X.G.; Lei, Y.Q.; Zhang, M.S. Hydrogen storage properties of Zr0.8Ti0.2(Ni0.6Mn0.3−xV0.1+xCr0.05)2 (x = 0.0, 0.05, 0.15, 0.2) alloys. Int. J. Hydrogen Energy 2002, 27, 695–697. [Google Scholar] [CrossRef]
- Li, S.; Zhao, M.; Zhai, J.; Wang, F. Effect of substitution of chromium for nickel on structure and electrochemical characteristics of Ti0.26Zr0.07V0.24Mn0.1Ni0.33 multi-phase hydrogen storage alloy. Mater. Chem. Phys. 2009, 113, 96–102. [Google Scholar]
- Young, K.; Nei, J.; Wong, D.F.; Wang, L. Structural, hydrogen storage, and electrochemical properties of Laves phase-related body-centered-cubic solid solution metal hydride alloys. Int. J. Hydrogen Energy 2014, 39, 21489–21499. [Google Scholar] [CrossRef]
- Young, K.; Ng, K.Y.S.; Bendersky, L.A. A technical report of the robust affordable next generation energy storage system-BASF program. Batteries 2016, 2, 2. [Google Scholar] [CrossRef]
- Young, K.; Regmi, R.; Lawes, G.; Ouchi, T.; Reichman, B.; Fetcenko, M.A.; Wu, A. Effects of aluminum substitution in C14-rich multi-component alloys for NiMH battery application. J. Alloys Compd. 2010, 490, 282–292. [Google Scholar] [CrossRef]
- Iba, H.; Akiba, E. The relation between microstructure and hydrogen absorbing property in Laves phase-solid solution multiphase alloys. J. Alloys Compd. 1995, 231, 508–512. [Google Scholar] [CrossRef]
- Young, K.; Young, M.; Ouchi, T.; Reichman, B.; Fetcenko, M.A. Improvement in high-rate dischargeability, activation, and low-temperature performance in multi-phase AB2 alloys by partial substitution of Zr with Y. J. Power Sources 2012, 215, 279–287. [Google Scholar] [CrossRef]
- Young, K.; Wong, D.F.; Ouchi, T.; Huang, B.; Reichman, B. Effects of La-addition to the structure, hydrogen storage, and electrochemical properties of C14 metal hydride alloys. Electrochim. Acta 2015, 174, 815–825. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Nei, J.; Moghe, D. The importance of rare-earth additions in Zr-based AB2 metal hydride alloys. Batteries 2016, 2, 25. [Google Scholar] [CrossRef]
- Wong, D.F.; Young, K.; Nei, J.; Wang, L.; Ng, K.Y.S. Effects of Nd-addition on the structural, hydrogen storage, and electrochemical properties of C14 metal hydride alloys. J. Alloys Compd. 2015, 647, 507–518. [Google Scholar] [CrossRef]
- Yoshida, M.; Ishibashi, H.; Susa, K.; Ogura, T.; Akiba, E. The multiphase effect on crystal structure, hydrogen absorbing properties and electrode performance of Sc-Zr based Laves phase alloys. J. Alloys Compd. 1995, 230, 100–108. [Google Scholar] [CrossRef]
- Chang, S.; Young, K.; Ouchi, T.; Meng, T.; Nei, J.; Wu, X. Studies on incorporation of Mg in Zr-based AB2 metal hydride alloys. Batteries 2016, 2, 11. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Huang, B.; Fetcenko, M.A. Effects of B, Fe, Gd, Mg, and C on the structure, hydrogen storage, and electrochemical properties of vanadium-free AB2 metal hydride alloy. J. Alloys Compd. 2012, 511, 242–250. [Google Scholar] [CrossRef]
- Liu, H.; Li, R. Effect of Sn content on properties of AB2 hydrogen storage alloy. Foundry Technol. 2006, 27, 503–505. (In Chinese) [Google Scholar]
- Pottgen, R.; Dronskowski, R. Structure and properties of Zr2Ni2In and Zr2Ni2Sn. J. Solid State Chem. 1997, 128, 289–294. [Google Scholar] [CrossRef]
- Pearson, W.B. The Crystal Chemistry and Physics of Metals and Alloys; Wiley—Interscience: New York, NY, USA, 1972; p. 59. [Google Scholar]
- Nihon, K.G. Hi Kagaku Ryouronteki Kinzokukan Kagoubutsu; Maruzen: Tokyo, Japan, 1975; p. 296. (In Japanese) [Google Scholar]
- Yukawa, H.; Nakatsuka, K.; Morinaga, M. Design of hydrogen storage alloys in view of chemical bond between atoms. Sol. Energy Mater. Sol. Cells 2000, 62, 75–80. [Google Scholar] [CrossRef]
- Liu, S.; Sun, D. The current research and the development trend of hydrogen storage alloy. Rare Met. Cem. Carbides 2005, 33, 46–51. (In Chinese) [Google Scholar]
- Kim, D.M.; Jeon, S.W.; Lee, J.Y. A study of the development of a high capacity and high performance Zr–Ti–Mn–V–Ni hydrogen storage alloy for Ni–MH rechargeable batteries. J. Alloys Compd. 1998, 279, 209–214. [Google Scholar] [CrossRef]
- Kim, S.R.; Lee, J.Y.; Park, H.H. A study of the activation behaviour of Zr-Cr-Ni-La metal hydride electrodes in alkaline solution. J. Alloys Compd. 1994, 205, 225–229. [Google Scholar] [CrossRef]
- Shu, K.; Zhang, S.; Lei, Y.; Lü, G.; Wang, Q. Effect of Ti on the structure and electrochemical performance of Zr-based AB2 alloys for nickel-metal rechargeable batteries. J. Alloys Compd. 2003, 349, 237–241. [Google Scholar] [CrossRef]
- Lee, H.H.; Lee, K.Y.; Lee, J.Y. Degradation mechanism of Ti-Zr-V-Mn-Ni metal hydride electrodes. J. Alloys Compd. 1997, 260, 201–207. [Google Scholar] [CrossRef]
- Züttel, A.; Meli, F.; Schlapbach, L. Surface and bulk properties of the TiyZr1−y(VxNi1−x)2 alloy system as active electrode material in alkaline electrolyte. J. Alloys Compd. 1995, 231, 645–649. [Google Scholar] [CrossRef]
- Visintin, A.; Peretti, H.A.; Tori, C.A.; Triaca, W.E. Hydrogen absorption characteristics and electrochemical properties of Ti substituted Zr-based AB2 alloys. Int. J. Hydrogen Energy 2001, 26, 683–689. [Google Scholar] [CrossRef]
- Sun, J.C.; Li, S.; Ji, S.J. Phase composition and electrochemical performances of the Zr1−xTixCr0.4Mn0.2 V0.1Ni1.3 alloys with 0.1 ≤ x ≤ 0.3. J. Alloys Compd. 2005, 404, 687–690. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Pan, H.G.; Gao, M.X.; Ma, J.X.; Li, S.Q.; Wang, Q.D. The effect of Zr substitution for Ti on the microstructures and electrochemical properties of electrode alloys Ti1−xZrxV1.6Mn0.32Cr0.48Ni0.6. Int. J. Hydrogen Energy 2002, 27, 287–293. [Google Scholar] [CrossRef]
- Hariprakash, B.; Martha, S.K.; Shukla, A.K. Effect of copper additive on Zr0.9Ti0.1V0.2Mn0.6Cr0.05Co0.05Ni1.2 alloy anode for nickel-metal hydride batteries. J. Appl. Electrochem. 2003, 33, 497–504. [Google Scholar] [CrossRef]
- Huot, J.; Akiba, E.; Ogura, T.; Ishido, Y. Crystal structure, phase abundance and electrode performance of Laves phase compounds (Zr, A)V0.5Ni1.1Mn0.2Fe0.2 (A≡Ti, Nb or Hf). J. Alloys Compd. 1995, 218, 101–109. [Google Scholar] [CrossRef]
- Huot, J.; Akiba, E.; Ishido, Y. Crystal structure of multiphase alloys (Zr,Ti)(Mn,V)2. J. Alloys Compd. 1995, 231, 85–89. [Google Scholar] [CrossRef]
- Nei, J.; Young, K.; Salley, S.O.; Ng, K.Y.S. Determination of C14/C15 phase abundance in Laves phase alloys. Mater. Chem. Phys. 2012, 136, 520–527. [Google Scholar] [CrossRef]
- Kandavel, M.; Bhat, V.V.; Rougier, A.; Aymard, L.; Nazri, G.A.; Tarascon, J.M. Improvement of hydrogen storage properties of the AB2 Laves phase alloys for automotive application. Int. J. Hydrogen Energy 2008, 33, 3754–3761. [Google Scholar] [CrossRef]
- Wakao, S.; Sawa, H.; Furukawa, J. Effects of partial substitution and anodic oxidation treatment of Zr–V–Ni alloys on electrochemical properties. J. Less Common Met. 1991, 172, 1219–1226. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J. Activation behaviour of ZrCrNiM0.05 metal hydride electrode (M = La, Mm (misch metal), Nd). J. Alloys Compd. 1992, 185, L1–L4. [Google Scholar] [CrossRef]
- Rönnebro, E.; Noréus, D.; Sakai, T.; Tsukahara, M. Structural studies of a new Laves phase alloy (Hf,Ti)(Ni,V)2 and its very stable hydride. J. Alloys Compd. 1995, 231, 90–94. [Google Scholar] [CrossRef]
- Huot, J.; Akiba, E.; Orura, O.; Ishido, Y. Crystal structure of electrode materials (Zr,A)V0.5Ni1.1Mn0.2Fe0.2 (A=Ti, Nb and Hf) for Ni-hydrogen rechargeable batteries. Trans. Mater. Res. Soc. Jpn. 1994, 18B, 1197–1200. [Google Scholar]
- Zhou, O.; Yao, Q.; Sun, X.; Gu, Q.; Sun, J. Lattice-substitution of alloying elements and its influences on mechanical properties of ZrCr2 Laves phase. Chin. J. Nonferr. Met. 2006, 16, 1603–1607. (In Chinese) [Google Scholar]
- Santos, A.R.; Ambrosio, R.C.; Ticianelli, E.A. Electrochemical and structural studies on nonstoichiometric AB2-type metal hydride alloys. Int. J. Hydrogen Energy 2004, 29, 1253–1261. [Google Scholar]
- Young, K.; Ouchi, T.; Nei, J.; Chang, S. Increase in the Surface Catalytic Ability by Addition of Palladium in C14 Metal Hydride Alloy. Batteries 2017, 3, 26. [Google Scholar] [CrossRef]
- Yang, X.G.; Zhang, W.K.; Lei, Y.Q.; Wang, Q.D. Electrochemical properties of Zr-V-Ni system hydrogen storage alloys. J. Electrochem. Soc. 1999, 146, 1245–1250. [Google Scholar] [CrossRef]
- Ovshinsky, S.R.; Young, R. High Power Nickel-Metal Hydride Batteries and High Power Alloys/Electrodes for Use Therein. U.S. Patent 6,413,670 B1, 2 July 2002. [Google Scholar]
- Yoshida, M.; Akiba, E. Hydrogen absorbing properties of ScM2 Laves phase alloys (M = Fe, Co and Ni). J. Alloys Compd. 1995, 226, 75–80. [Google Scholar] [CrossRef]
- Yoshida, M.; Ishibashi, H.; Susa, K.; Ogura, T.; Akiba, E. Crystal structure, hydrogen absorbing properties and electrode performances of Sc-based Laves phase alloys. J. Alloys Compd. 1995, 226, 161–165. [Google Scholar] [CrossRef]
- Li, K.; Luo, Y.; Wang, W.; Qiu, J.; Kang, L. Effects of scandium on hydrogen storage and electrochemical properties of AB2-type Zr1−xScxMn0.6V0.2Ni1.2Co0.1 (x = 0~1) alloys. J. Chin. Rare Earth Soc. 2013, 31, 442–449. (In Chinese) [Google Scholar]
- Liu, X.J.; Yang, S.Y.; Huang, Y.X.; Zhang, J.B.; Wang, C.P. Experimental investigation of isothermal sections (100, 1200 °C) in the Ni-Ti-Zr system. J. Ph. Equilib. Diffus. 2015, 36, 414–421. [Google Scholar] [CrossRef]
- Young, K.; Fetcenko, M.A.; Li, F.; Ouchi, T.; Koch, J. Effect of vanadium substitution in C14 Laves phase alloys for NiMH battery application. J. Alloys Compd. 2009, 468, 482–492. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Koch, J.; Fetcenko, M.A. Compositional optimization of vanadium-free hypo-stoichiometric AB2 metal hydride alloy for Ni/MH battery application. J. Alloys Compd. 2012, 510, 97–106. [Google Scholar] [CrossRef]
- Wang, J.; Yu, R.; Liu, Q. Relation of element substitution to discharge capacity and activation of Ti-Zr based AB2-type hydrogen storage electrode alloys. Mater. Sci. Technol. 2005, 13, 166–170. (In Chinese) [Google Scholar]
- Yang, H.W.; Wang, Y.Y.; Wan, C.C. Studies of electrochemical properties of Ti0.35Zr0.65NixV2−x−yMny alloys with C14 Laves phases for nickel/metal hydride batteries. J. Electrochem. Soc. 1996, 143, 429–435. [Google Scholar] [CrossRef]
- Hagström, M.T.; Klyamkin, S.N.; Lund, P.D. Effect of substitution on hysteresis in some high-pressure AB2 and AB5 metal hydrides. J. Alloys Compd. 1999, 293, 67–73. [Google Scholar] [CrossRef]
- Lee, S.F.; Wang, Y.Y.; Wan, C.C. Effect of adding chromium to Ti-Zr-Ni-V-Mn alloy on its cycle life as an Ni/metal-hydride battery material. J. Power Sources 1997, 66, 165–168. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S.; Lee, J. Activation characteristics of multiphase Zr-based hydrogen storage alloys for Ni/MH rechargeable batteries. J. Electrochem. Soc. 1999, 146, 3666–3671. [Google Scholar] [CrossRef]
- Park, H.Y.; Chang, I.; Cho, W.I.; Cho, B.W.; Jang, H.; Lee, S.R.; Yun, K.S. Electrode characteristics of the Cr and La doped AB2-type hydrogen storage alloys. Int. J. Hydrogen Energy 2001, 26, 949–955. [Google Scholar] [CrossRef]
- Liu, B.H.; Li, Z.P.; Kitani, R.; Suda, S. Improvement of electrochemical cyclic durability of Zr-based AB2 alloy electrodes. J. Alloys Compd. 2002, 330, 825–830. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Fetcenko, M.A. Roles of Ni, Cr, Mn, Sn, Co, and Al in C14 Laves phase alloys for NiMH battery application. J. Alloys Compd. 2009, 476, 774–781. [Google Scholar] [CrossRef]
- Peretti, H.A.; Visintin, A.; Mogni, L.V.; Corso, H.L.; Gamboa, J.A.; Serafini, D.; Triaca, W.E. Hydrogen absorption behavior of multicomponent zirconium based AB2 alloys with different chromium–vanadium ratio. J. Alloys Compd. 2003, 354, 181–186. [Google Scholar] [CrossRef]
- Wang, G.Y.; Xu, Y.H.; Pan, H.G.; Wang, Q.D. Effect of substitution of chromium for manganese on structure discharge characteristics of Ti-Zr-V-Mn-Ni-type multi-phase hydrogen storage electrode alloys. Int. J. Hydrogen Energy 2003, 28, 499–508. [Google Scholar] [CrossRef]
- Pan, H.; Li, R.; Gao, M.; Liu, Y.; Wang, Q. Effects of Cr on the structural and electrochemical properties of TiV-based two-phase hydrogen storage alloys. J. Alloys Compd. 2005, 404, 669–674. [Google Scholar] [CrossRef]
- Yu, J.; Lee, S.; Cho, K.; Lee, J. The cycle life of Ti0.8Zr0.2V0.5Mn0.5–xCrxNi0.8 (x = 0 to 0.5) alloys for metal hydride electrodes of Ni-metal hydride rechargeable battery. J. Electrochem. Soc. 2000, 147, 2013–2017. [Google Scholar] [CrossRef]
- Xu, Y.H.; Chen, C.P.; Wang, X.L.; Lei, Y.Q.; Wang, Q.D. The cycle life and surface properties of Ti-based AB2 metal hydride electrodes. J. Alloys Compd. 2002, 337, 214–220. [Google Scholar] [CrossRef]
- Shinyama, K.; Magari, Y.; Akita, H.; Kumagae, K.; Nakamura, H.; Matsuta, S.; Nohma, T.; Takee, M.; Ishiwa, K. Investigation into the deterioration in storage characteristics of nickel-metal hydride batteries during cycling. J. Power Sources 2005, 143, 265–269. [Google Scholar] [CrossRef]
- Chen, J.; Dou, S.X.; Liu, H.K. Hydrogen desorption and electrode properties of Zr0.8Ti0.2(V0.3Ni0.6M0.1)2. J. Alloys Compd. 1997, 256, 40–44. [Google Scholar] [CrossRef]
- Chai, Y.J.; Zhao, M.S.; Wang, N. Crystal structural and electrochemical properties of Ti0.17Zr0.08V0.35Cr0.10Ni0.30−xMnx (x = 0–0.12) alloys. Mater. Sci. Eng. B 2008, 147, 47–51. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Huang, B.; Reichman, B.; Fetcenko, M.A. The structure, hydrogen storage, and electrochemical properties of Fe-doped C14-predominating AB2 metal hydride alloys. Int. J. Hydrogen Energy 2011, 36, 12296–12304. [Google Scholar] [CrossRef]
- Song, M.Y.; Ahn, D.; Kwon, I.; Lee, R.; Rim, H. Development of AB2-type Zr–Ti–Mn–V–Ni–Fe hydride electrodes for Ni–MH secondary batteries. J. Alloys Compd. 2000, 298, 254–260. [Google Scholar] [CrossRef]
- Song, M.Y.; Kown, I.H.; Ahh, D.S.; Sohn, M.S. Improvement in the electrochemical properties of ZrMn2 hydrides by substitution of elements. Met. Mater. Int. 2001, 7, 257–263. [Google Scholar] [CrossRef]
- Kim, S.R.; Lee, J.Y. Electrode characteristics of C14-type Zr-based laves phase alloys. J. Alloys Compd. 1994, 210, 109–113. [Google Scholar] [CrossRef]
- Song, M.Y.; Ahn, D.; Kwon, I.H.; Chough, S.H. Development of AB2-type Zr-Ti-Mn-V-Ni-M hydride electrode for Ni/MH secondary battery. J. Electrochem. Soc. 2001, 148, A1041–A1044. [Google Scholar] [CrossRef]
- Honda, N.; Furukawa, N.; Fujitani, S.; Yonezu, I. Hydrogen Absorbing Modified ZrMn2-Type Alloys. U.S. Patent 4,913,879, 3 April 1990. [Google Scholar]
- Ovshinsky, S.R.; Fetcenko, M.A.; Ross, J. A nickel metal hydride battery for electric vehicles. Science 1993, 260, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Lee, K.Y.; Lee, J.Y. Improved low-temperature dischargeability of C14-type Zr–Cr–Ni Laves phase alloy. J. Alloys Compd. 1995, 223, 22–27. [Google Scholar] [CrossRef]
- Kwon, I.; Park, H.; Song, M. Electrochemical properties of ZrMnNi1+x hydrogen-storage alloys. Int. J. Hydrogen Energy 2002, 27, 171–176. [Google Scholar] [CrossRef]
- Lee, H.H.; Lee, K.Y.; Lee, J.Y. The Ti-based metal hydride electrode for NiMH rechargeable batteries. J. Alloys Compd. 1996, 239, 63–70. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Huang, B.; Reichman, B.; Fetcenko, M.A. Studies of copper as a modifier in C14-predominant AB2 metal hydride alloys. J. Power Sources 2012, 204, 205–212. [Google Scholar] [CrossRef]
- Yamamura, Y.; Seri, H.; Tsuji, Y.; Owada, N.; Iwaki, T. Hydrogen Storage Alloy and Electrode Therefrom. U.S. Patent 5,532,076, 2 July 1996. [Google Scholar]
- Young, K.; Ouchi, T.; Lin, X.; Reichman, B. Effects of Zn-addition to C14 metal hydride alloys and comparisons to Si, Fe, Cu, Y, and Mo-additives. J. Alloys Compd. 2016, 655, 50–59. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, D.; Yuan, H.; Wang, G.; Zhou, Z.; Song, D.; Zhang, Y. Preparation and electrochemical properties of MI(NiCuAlZn)5 hydrogen storage alloys. Acta Sci. Bat. Univ. Nankaiensis 2000, 33, 120–123. (In Chinese) [Google Scholar]
- Young, K.; Ouchi, T.; Huang, B.; Reichman, B.; Fetcenko, M.A. Effect of molybdenum content on structural, gaseous storage, and electrochemical properties of C14-predominant AB2 metal hydride alloys. J. Power Sources 2011, 196, 8815–8821. [Google Scholar] [CrossRef]
- Au, M.; Pourarian, F.; Sankar, S.G.; Wallace, W.E.; Zhang, L. TiMn2-bases alloys as high hydrogen materials. Mater. Sci. Eng. B 1995, 33, 53–57. [Google Scholar] [CrossRef]
- Huang, T.; Wu, Z.; Xia, B.; Xu, N. Influence of stoichiometry and alloying elements on the crystallography and hydrogen sorption properties of TiCr based alloys. Mater. Sci. Eng. A 2005, 397, 284–287. [Google Scholar]
- Erika, T.; Ricardo, F.; Fabricio, R.; Fernando, Z.; Verónica, D. Electrochemical and metallurgical characterization of ZrCr1−xNiMox AB2 metal hydride alloys. J. Alloys Compd. 2015, 649, 267–274. [Google Scholar] [CrossRef]
- Doi, H.; Yabuki, R. Hydrogen Absorbing Ni, Zr-Based Alloy and Rechargeable Alkaline Battery. U.S. Patent 4,898,794, 6 February 1990. [Google Scholar]
- Ruiz, F.C.; Peretti, H.A.; Visintin, A.; Triaca, W.E. A study on ZrCrNiPtx alloys as negative electrode components for NiMH batteries. Int. J. Hydrogen Energy 2011, 36, 901–906. [Google Scholar] [CrossRef]
- Luan, B.; Cui, N.; Zhao, H.J.; Liu, H.K.; Dou, S.X. Effects of potassium-boron addition on the performance of titanium based hydrogen storage alloy electrodes. Int. J. Hydrogen Energy 1996, 21, 373–379. [Google Scholar] [CrossRef]
- Reddy, A.L.M.; Ramaprabhu, S. Hydrogen diffusion studied in Zr-based Laves phase AB2 alloys. J. Alloys Compd. 2008, 460, 268–271. [Google Scholar] [CrossRef]
- Reddy, A.L.M.; Ramaprabhu, S. Structural and hydrogen absorption kinetics studies of polymer dispersed and boron added Zr-based AB2 alloy. Int. J. Hydrogen Energy 2006, 31, 867–876. [Google Scholar]
- Li, S.; Wen, B.; Li, X. Structure and electrochemical property of ball-milled Ti0.26Zr0.07Mn0.1Ni0.33V0.24 alloy with 3 mass% B. J. Alloys Compd. 2016, 654, 580–585. [Google Scholar] [CrossRef]
- Young, K.; Fetcenko, M.A.; Koch, J.; Morii, K.; Shimizu, T. Studies of Sn, Co, Al, and Fe additives in C14/C15 Laves alloys for NiMH battery application by orthogonal arrays. J. Alloys Compd. 2009, 486, 559–569. [Google Scholar] [CrossRef]
- Fetcenko, M.A.; Young, K.; Ovshinky, S.R.; Reichman, B.; Koch, J.; Mays, W. Modified Electrochemical Hydrogen Storage Alloy Having Increased Capacity, Rate Capability and Catalytic Activity. U.S. Patent 6,270,719, 7 August 2001. [Google Scholar]
- Gamo, T.; Moriwaki, Y.; Iwaki, T. Alloy for Hydrogen Storage Electrodes. U.S. Patent 4,946,646, 7 August 1990. [Google Scholar]
- Doi, H.; Yabuki, R. Hydrogen Absorbing Ni-Based Alloy and Rechargeable Alkaline Battery. U.S. Patent 4,983,474, 9 January 1991. [Google Scholar]
- Yu, X.B.; Wu, Z.; Huang, T.Z.; Chen, J.Z.; Xia, B.J.; Xu, N.X. Effect of carbon addition on activation and hydrogen sorption characteristics of TiMn1.25Cr0.25 alloy. Mater. Chem. Phys. 2004, 83, 273–277. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Huang, B.; Reichman, B.; Blankenship, R. Improvement in −40 °C electrochemical properties of AB2 metal hydride alloy by silicon incorporation. J. Alloys Compd. 2013, 575, 65–72. [Google Scholar] [CrossRef]
- Han, S.; Zhoa, M.; Liu, B. Microstructure and high-temperature electrochemical characteristics of Zr0.9Ti0.1Ni1.0Mn0.7V0.3Six (x = 0.05, 0.10, 0.15, 0.20) alloy. Mater. Chem. Phys. 2005, 89, 221–227. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Zhu, J. Effect of Si-element on electrochemical properties of Ti-based hydrogen storage alloys. Titan. Ind. Prog. 2007, 24, 10–22. (In Chinese) [Google Scholar]
- Young, K.; Chao, B.; Nei, J. Microstructures of the activated Si-containing AB2 metal hydride alloy surface by transmission electron microscope. Batteries 2016, 2, 4. [Google Scholar] [CrossRef]
- Drašner, A.; Blaẑina, Ẑ. The influence of Si and Ge on the hydrogen sorption properties of the intermetallic compound ZrCr2. J. Alloys Compd. 1993, 199, 101–104. [Google Scholar]
- Xu, L.; Xiao, Y.; Sandwijk, A.; Xu, Q.; Yang, Y. Separation of zirconium and hafnium: A review. In Energy Materials 2014; Springer: Heidelberg, Germany, 2014; p. 451. [Google Scholar]
- Yamanaka, S.; Higuchi, K.; Miyake, M. Hydrogen solubility in zirconium alloys. J. Alloys Compd. 1995, 231, 503–507. [Google Scholar] [CrossRef]
- Young, K.; Fetcenko, M.A.; Ouchi, T.; Li, F.; Koch, J. Effect of Sn-substitution in C14 Laves phase alloys for NiMH battery application. J. Alloys Compd. 2009, 469, 406–416. [Google Scholar] [CrossRef]
- Morita, Y.; Gamo, T.; Kuranaka, S. Effects of nonmetal addition on hydriding properties for Ti–Mn Laves phase alloys. J. Alloys Compd. 1997, 253, 29–33. [Google Scholar] [CrossRef]
- Giza, K.; Isasieczko, W.; Pavlyuk, V.V.; Bala, H.; Drulis, H.; Adamczyk, L. Hydrogen absorption and corrosion resistance of LaNi4.8Al0.2 and LaNi4.8Al0.1Li0.1 alloy. J. Alloys Compd. 2007, 429, 352–356. [Google Scholar] [CrossRef]
- Wei, X.; Tang, R.; Liu, Y.; Zhang, P.; Yu, G.; Zhu, J. Effect of small amounts of Li on microstructures and electrochemical properties of non-stoichiometric low-Co AB5-type alloys. Int. J. Hydrogen Energy 2006, 31, 1365–1371. [Google Scholar] [CrossRef]
- Wang, L.; Yuan, H.; Yang, H.; Zhou, K.; Song, D.; Zhang, Y. Study of the multi-composition AB2 alloys including Li, made by the diffusion method, and their electrodes. J. Alloys Compd. 2000, 302, 65–69. [Google Scholar] [CrossRef]
- Cracco, D.; Percheron-Guégan, A. Morphology and hydrogen absorption properties of an AB2 type alloy ball milled with Mg2Ni. J. Alloys Compd. 1998, 268, 248–255. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, M. Electrochemical hydrogen storage characteristics of Ti0.10Zr0.15V0.35Cr0.10Ni0.30−10% LaNi3 composite and its synergetic effect. Trans. Nonferr. Met. Soc. China 2012, 22, 2000. (In Chinese) [Google Scholar] [CrossRef]
- Chu, H.; Zhang, Y.; Sun, L.; Qiu, S.; Xu, F.; Yuan, H.; Wang, Q.; Dong, C. Structure, morphology and hydrogen storage properties of composites prepared by ball milling Ti0.9Zr0.2Mn1.5Cr0.3V0.3 with La–Mg-based alloy. Int. J. Hydrogen Energy 2007, 32, 3363–3369. [Google Scholar] [CrossRef]
- Liu, F.J.; Sandrock, G.; Suda, S. Surface and metallographic microstructure of the La-added AB2 compound (Ti, Zr)(Mn, Cr, Ni)2. J. Alloys Compd. 1995, 231, 392–396. [Google Scholar] [CrossRef]
- Sun, D.; Latroche, M.; Percheron-Guégan, A. Effects of lanthanum or cerium on the equilibrium of ZrNi1.2Mn0.6V0.2Cr0.1 and its related hydrogenation properties. J. Alloys Compd. 1997, 248, 215–219. [Google Scholar] [CrossRef]
- Yang, X.G.; Lei, Y.Q.; Shu, K.Y.; Lin, G.F.; Zhang, Q.A.; Zhang, W.K.; Zhang, X.B.; Lu, G.L.; Wang, Q.D. Contribution of rare-earths to activation property of Zr-based hydride electrode. J. Alloys Compd. 1999, 293, 632–636. [Google Scholar] [CrossRef]
- Sun, J.C.; Li, S.; Ji, S.J. The effects of the substitution of Ti and La for Zr in ZrMn0.7V0.2Co0.1Ni1.2 hydrogen storage alloys on the phase structure and electrochemical properties. J. Alloys Compd. 2007, 446, 630–634. [Google Scholar] [CrossRef]
- Chen, W.X. Effects of addition of rare-earth element on electrochemical characteristics of ZrNi1.1Mn0.5V0.3Cr0.1 hydrogen storage alloy electrodes. J. Alloys Compd. 2001, 319, 119–123. [Google Scholar] [CrossRef]
- Jung, J.H.; Lee, K.Y.; Lee, J.Y. The activation mechanism of Zr-based alloy electrodes. J. Alloys Compd. 1995, 226, 166–169. [Google Scholar] [CrossRef]
- Joubert, J.M.; Sun, D.; Latroche, M.; Percheron-Guegan, A. Electrochemical performances of ZrM2 (M=V, Cr, Mn, Ni) Laves phases and the relation to microstructures and thermodynamical properties. J. Alloys Compd. 1997, 253, 564–569. [Google Scholar] [CrossRef]
- Han, S.M.; Zhang, Z.; Zhao, M.S.; Zheng, Y.Z. Electrochemical characteristics and microstructure of Zr0.9Ti0.1Ni1.1Mn0.6V0.3–LaNi5 composite hydrogen storage alloys. Int. J. Hydrogen Energy 2006, 31, 563–567. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, M.; Li, S.; Wang, L. Structure and electrochemical characteristics of melted composite Ti0.10Zr0.15V0.35Cr0.10Ni0.30–LaNi5 hydrogen storage alloys. Electrochim. Acta 2008, 53, 7831–7837. [Google Scholar] [CrossRef]
- Lu, Z.; Qin, M.; Jiang, W.; Qing, P.; Liu, S. Effect of AB2-based alloy addition on structure and electrochemical properties of La0.5Pr0.2Zr0.1Mg0.2Ni2.75Co0.45Fe0.1Al0.2 hydrogen storage alloy. J. Rare Earths 2013, 31, 386–394. [Google Scholar] [CrossRef]
- HEFA Rare Earth. Available online: http://mineralprices.com (accessed on 8 May 2017).
- Griessen, R.; Riesterer, T. Heat of Formation Models. In Hydrogen in Intermetallic Compounds I; Schlapbach, L., Ed.; Springer: Berlin/Heidelberg, Germany, 1988. [Google Scholar]
- Zhu, J.H.; Pike, L.M.; Liu, C.T.; Liaw, P.K. Point defects in Binary NbCr2 Laves-phase alloys. Scr. Mater. 1998, 39, 833–838. [Google Scholar] [CrossRef]
- Kanazawa, S.; Kaneno, Y.; Inoue, H.; Kim, W.Y.; Takasugi, T. Microstructures and defect structures in ZrCr2 Laves phase based intermetallic compounds. Intermetallics 2002, 10, 783–792. [Google Scholar] [CrossRef]
- Wong, D.F.; Young, K.; Ouchi, T.; Ng, K.Y.S. First-principles point defect models for Zr7Ni10 and Zr2Ni7 phases. Batteries 2016, 2, 23. [Google Scholar] [CrossRef]
- Massalski, T.B. Binary Alloy Phase Diagrams, 2nd ed.; ASM International: Russell, OH, USA, 1990. [Google Scholar]
- Notten, P.H.L.; Einerhand, R.E.F.; Daams, J.L.C. How to achieve long-term electrochemical cycling stability with hydride-forming electrode materials. J. Alloys Compd. 1995, 231, 604–610. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Yang, J.; Fetcenko, M.A. Studies of off-stoichiometric AB2 metal hydride alloy: Part 1. Structural characteristics. Int. J. Hydrogen Energy 2011, 36, 11137–11145. [Google Scholar] [CrossRef]
- Young, K.; Nei, J.; Huang, B.; Fetcenko, M.A. Studies of off-stoichiometric AB2 metal hydride alloy: Part 2. Hydrogen storage and electrochemical properties. Int. J. Hydrogen Energy 2011, 36, 11146–11154. [Google Scholar] [CrossRef]
- Liu, B.H.; Li, Z.P.; Suda, S. Electrochemical cycle life of Zr-based Laves phase alloys influenced by alloy stoichiometry and composition. J. Electrochem. Soc. 2002, 149, A537–A542. [Google Scholar] [CrossRef]
- Zhu, Y.; Pan, H.; Gao, M.; Ma, J.; Lei, Y.; Wang, Q. Electrochemical studies on the Ti–Zr–V–Mn–Cr–Ni hydrogen storage electrode alloys. Int. J. Hydrogen Energy 2003, 28, 311–316. [Google Scholar] [CrossRef]
- Pan, H.; Zhu, Y.; Gao, M.; Liu, Y.; Li, R.; Lei, Y.; Wang, Q. A study on the cycling stability of the Ti–V-based hydrogen storage electrode alloys. J. Alloys Compd. 2004, 364, 271–279. [Google Scholar] [CrossRef]
- Yasuoka, S.; Magari, Y.; Murata, T.; Tanaka, T.; Ishida, J.; Nakamura, H.; Nohma, T.; Kihara, M.; Baba, Y.; Teraoka, H. Development of high-capacity nickel-metal hydride batteries using superlattice hydrogen-absorbing alloys. J. Power Sources 2006, 156, 662–666. [Google Scholar] [CrossRef]
- Young, K.; Fetcenko, M.A. Low Cost, High Power, High Energy, Solid-State, Bipolar Metal Hydride Batteries. U.S. Patent 8,974,948, 10 March 2015. [Google Scholar]
- Young, K.; Wong, D.; Nei, J.; Reichman, B.; Chao, B.; Mays, W. Shared Electrode Hybrid Battery-Fuel Cell System. U.S. Patent Appl. 20,150,295,290, 15 October 2015. [Google Scholar]
- Zelinsky, M.A.; Koch, J.M. Nickel/metal hydride batteries in stationary applications. Batteries 2017. submitted. [Google Scholar]
- Wikipedia. Start-Stop System. Available online: https://en.wikipedia.org/wiki/Start-stop_system (accessed on 31 July 2017).
- Yan, S.; Meng, T.; Young, K.; Nei, J. Nickel/metal hydride battery in a pouch design. Batteries 2017. in preparation. [Google Scholar]
- Young, K.; Nei, J.; Meng, T. Alkaline and Non-Aqueous Proton-Conducting Pouch-Cell Batteries. U.S. Patent Appl. 20,160,233,461, 11 August 2016. [Google Scholar]
- Meng, T.; Young, K.; Wong, D.F.; Nei, J. Ionic liquid-based non-aqueous electrolytes for nickel/metal hydride batteries. Batteries 2017, 2, 4. [Google Scholar] [CrossRef]



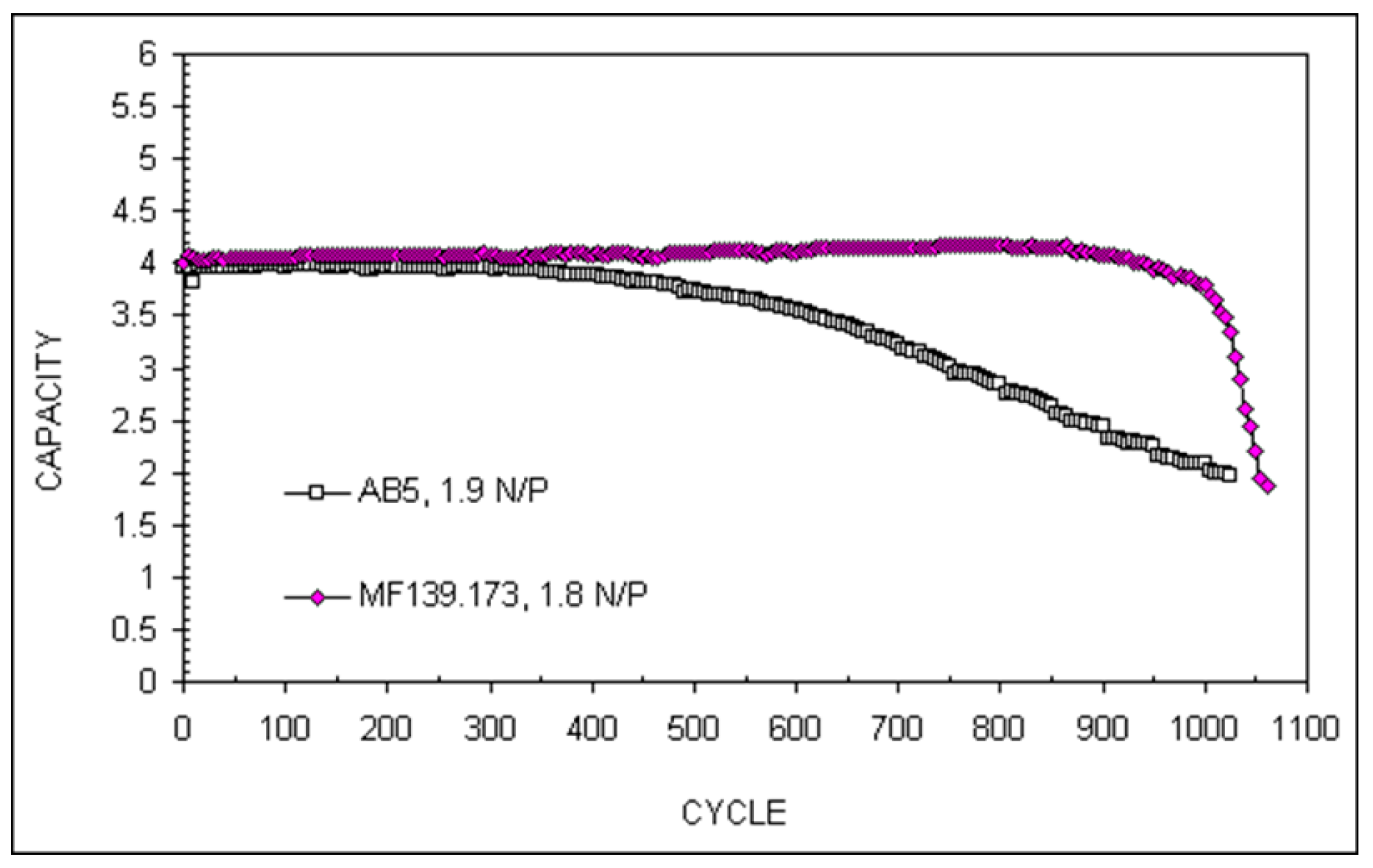
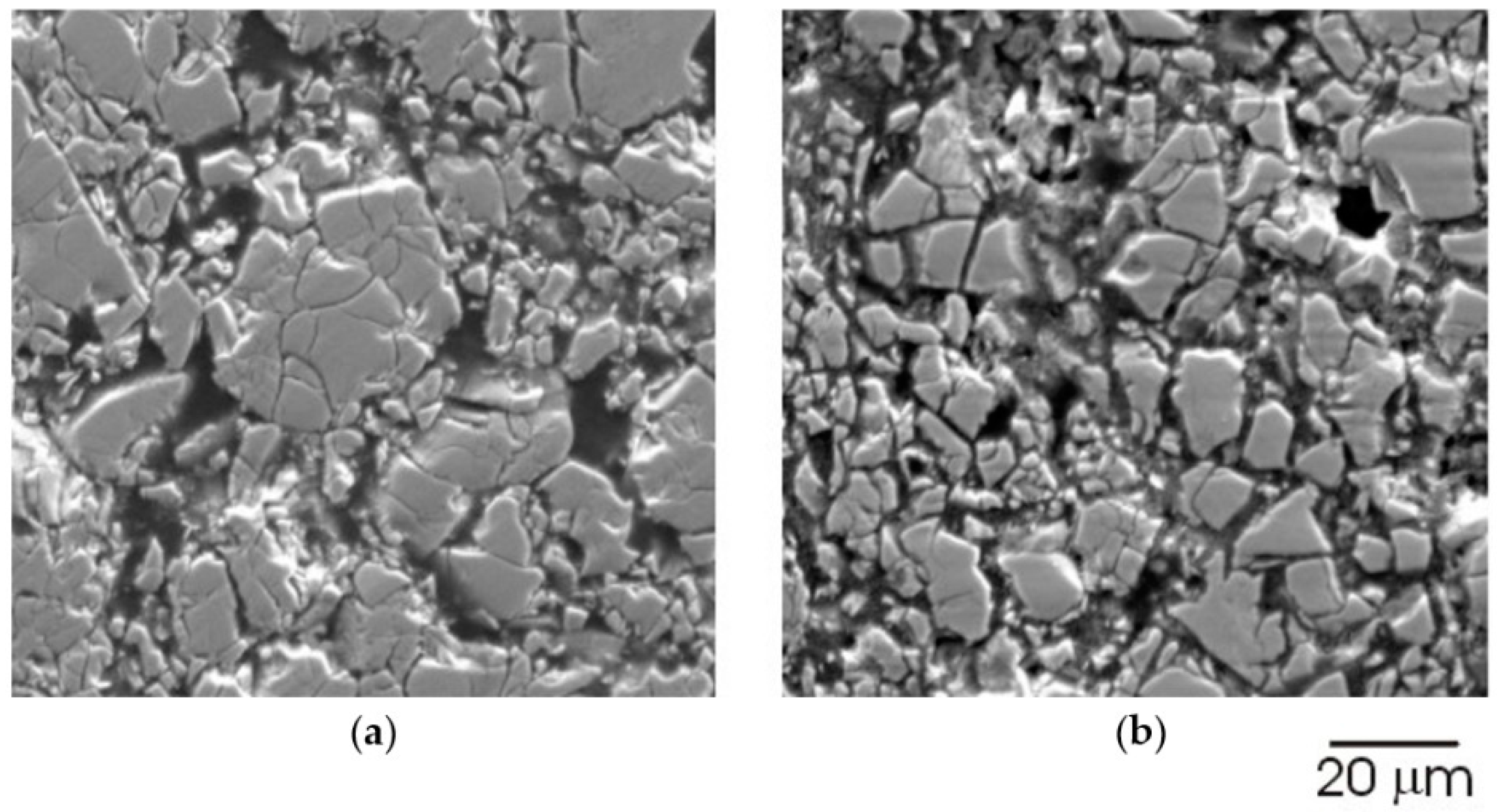
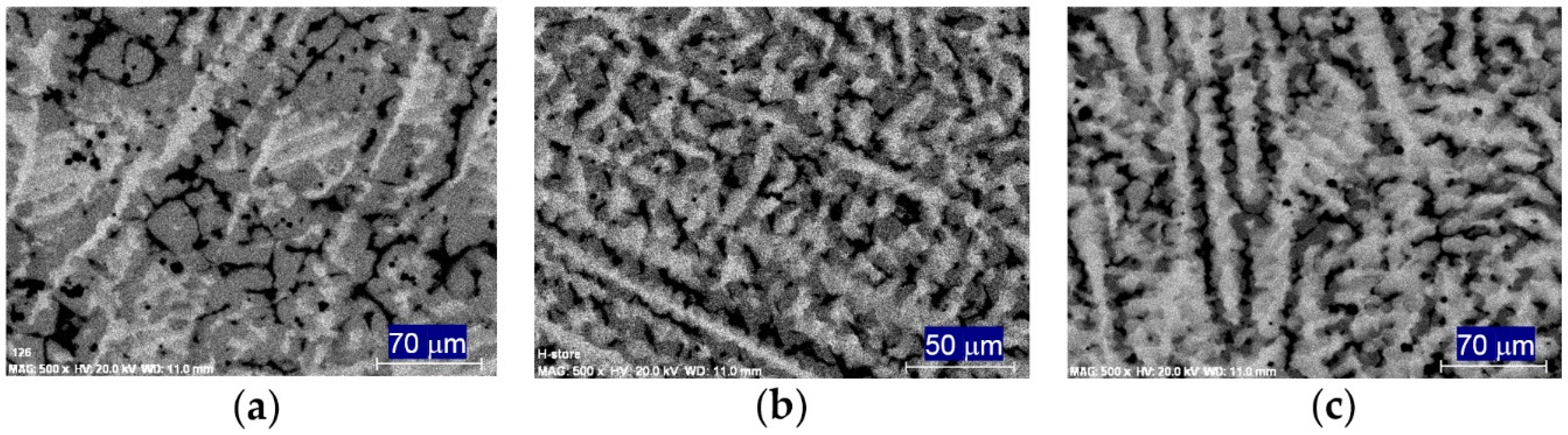
| Properties | AB2 (2) | AB3 (3) | A2B7 (3.5) | A5B19 (3.8) | AB5 (5) |
|---|---|---|---|---|---|
| AB2 number of units | 1 | 1 | 1 | 1 | 0 |
| AB5 number of units | 0 | 1 | 2 | 3 | 1 |
| Electrochemical capacity/weight | ++ | 0 | + | 0 | − |
| Electrochemical capacity/volume | 0 | 0 | + | 0 | − |
| Pulverization of alloy, oxidation (corrosion) | + | + | + | − | − |
| Reversibility of hydrogen absorption/release | − | 0 | + | 0~+ | ++ |
| Battery Life | − | 0 | ++ | 0 | + |
| Properties | VIM | CC | MS | GA | MA | AM | PS |
|---|---|---|---|---|---|---|---|
| Purpose | Production | Research/Production | Research/Production | Research/Production | Research | Research | Research |
| Batch Size | 1–1000 kg | 1–1000 kg | 1–200 kg | 1–1000 kg | 1–1000 g | 5–200 g | 5–100 g |
| Equipment Cost | Medium | High | High | High | Low | Low | High |
| Production Cost | $3 kg−1 | $4 kg−1 | $4 kg−1 | $5 kg−1 | $1000 kg−1 | $1000–$5000 kg−1 | $2000 kg−1 |
| Cooling Rate | 100 °C s−1 | 1 × 103 °C s−1 | 1 × 106 °C s−1 | 1 × 104 °C s−1 | >1 × 106 °C s−1 | 500 °C s−1 | 1 × 104 °C s−1 |
| Micro-structure | Large crystallites | Medium crystallites | Nano-crystallites | Micro-crystallites | Amorphous | Large crystallites | Micro-crystallites |
| Alloy Discharge Capacity | Normal | Normal | Low | Low | High | Normal | Low |
| Alloy High-Rate Discharge-ability | Normal | Normal | High | Low | High | Normal | High |
| Alloy Cycle Stability (Anti-pulverization) | Normal | Better | Excellent | Excellent | Excellent | Normal | Excellent |
| Alloy Requirements | High-Energy (EV) | High-Power (HEV) | Stationary General Purpose | Stationary at High Temperature | Stationary at Low Temperature |
|---|---|---|---|---|---|
| H-storage Capacity | ++ | 0 | + | + | + |
| H-diffusibility | + | ++ | + | + | ++ |
| Surface Catalysis | + | ++ | + | ++ | ++ |
| Anti-corrosion | + | 0 | + | ++ | + |
| Equilibrium Pressure | 0 | ++ | 0 | × | ++ |
| Pulverization | ++ | + | 0 | + | + |
| Cost | ++ | + | ++ | + | + |
| Element | Ti | Zr | V | Al | Mn |
|---|---|---|---|---|---|
| Amount in alloy (at %) | 12.0 | 21.5 | 9.5 | 0.4 | 13.6 |
| Concentration in solution after 1 h etching (ppm) | 0.6 | 15.6 | 11.4 | 7.8 | 0.4 |
| Concentration in solution after 4 h etching (ppm) | 1.0 | 48.9 | 33.8 | 28.1 | 0.5 |
| Leaching rate after 1 h etching (ppm/at %) | 0.05 | 0.73 | 1.2 | 19.5 | 0.03 |
| Leaching rate after 4 h etching (ppm/at %) | 0.08 | 2.27 | 3.6 | 70.2 | 0.04 |
| Properties | Ti | Zr | Hf | Nb | Pd |
|---|---|---|---|---|---|
| Atomic Number | 22 | 40 | 72 | 41 | 46 |
| Atomic Radius in AB2 | 1.614 | 1.771 | 1.743 | 1.625 | 1.521 |
| Electronegativity | 1.54 | 1.33 | 1.30 | 1.60 | 2.20 |
| Earth Crust Abundance (%) | 0.66 | 0.013 | 3.3 × 10−4 | 1.7 × 10−3 | 6 × 10−7 |
| Melting Temperature (°C) | 1668 | 1855 | 2150 | 2468 | 1555 |
| ΔHh (kJ·mol H2−1) | −136 | −164 | −161 | −83 | −41 |
| Number of IMCs with Ni | 3 | 8 | 8 | 3 | 0 |
| Properties | V | Cr | Mn | Fe | Co | Ni | Cu | Zn |
|---|---|---|---|---|---|---|---|---|
| Atomic Number | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 |
| Atomic Radius in AB2 | 1.491 | 1.423 | 1.428 | 1.411 | 1.385 | 1.377 | 1.413 | 1.538 |
| Electronegativity | 1.63 | 1.66 | 1.55 | 1.83 | 1.88 | 1.91 | 1.90 | 1.65 |
| Earth Crust Abundance (%) | 0.019 | 0.014 | 0.11 | 6.3 | 0.003 | 0.009 | 0.007 | 0.008 |
| Melting Temperature (°C) | 1890 | 1857 | 1245 | 1535 | 1495 | 1453 | 1083 | 420 |
| ΔHh (kJ·mol H2‒1) | −35 | −8 | −8 | 10 | 15 | −3 | 20 | 8 |
| Number of IMCs with Ti | 0 | 1 | 1 | 2 | 4 | 3 | 5 | 7 |
| Properties | Y | La | Ce | Pr | Nd | Sm | Gd | Yb |
|---|---|---|---|---|---|---|---|---|
| Atomic Number | 39 | 57 | 58 | 59 | 60 | 61 | 55 | 70 |
| Price (US$/kg) [249] | 35 | 7 | 7 | 85 | 60 | 7 | 55 | 95 |
| Ionic Radius in Laves (Å) [148] | 1.990 | 3.335 | 2.017 | 2.013 | 2.013 | 1.990 | 1.992 | 1.990 |
| Electronegativity | 1.22 | 1.10 | 1.12 | 1.13 | 1.14 | 1.17 | 1.20 | 1.24 |
| Melting Temperature (°C) | 1522 | 918 | 798 | 931 | 1021 | 1072 | 1313 | 819 |
| Oxidation potential (V) | −2.372 | −2.379 | −2.335 | −2.353 | −2.323 | −2.304 | −2.279 | −2.19 |
| Heat of Hydride Formation (kJ·mol H2−1) [250] | −114 | −97 | −103 | −104 | −106 | −100 | −98 | −91 |
| Alloy Requirements | Ti | Zr | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Al | Si | La |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H-storage Capacity | − | ++ | + | 0 | 0/+ | 0 | + | 0 | 0 | 0 | + | 0 | 0 |
| H-diffusibility | + | − | − | 0 | 0 | − | 0 | 0 | − | + | + | + | + |
| Surface Catalysis | − | + | + | − | − | − | 0 | ++ | − | + | + | + | ++ |
| Anti-corrosion | + | − | − | ++ | − | 0 | 0 | ++ | 0 | 0 | 0 | 0 | 0 |
| Equilibrium Pressure | ↑↑ | ↓↓↓ | ↓↓ | ↓ | ↓ | 0 | ↑ | ↑ | 0 | 0 | ↑ | 0 | 0 |
| Anti-pulverization | + | − | 0 | − | 0 | 0 | 0 | 0 | − | − | + | − | 0 |
| Cost | 0 | 0 | − | + | ++ | ++ | − | 0 | ++ | ++ | ++ | ++ | + |
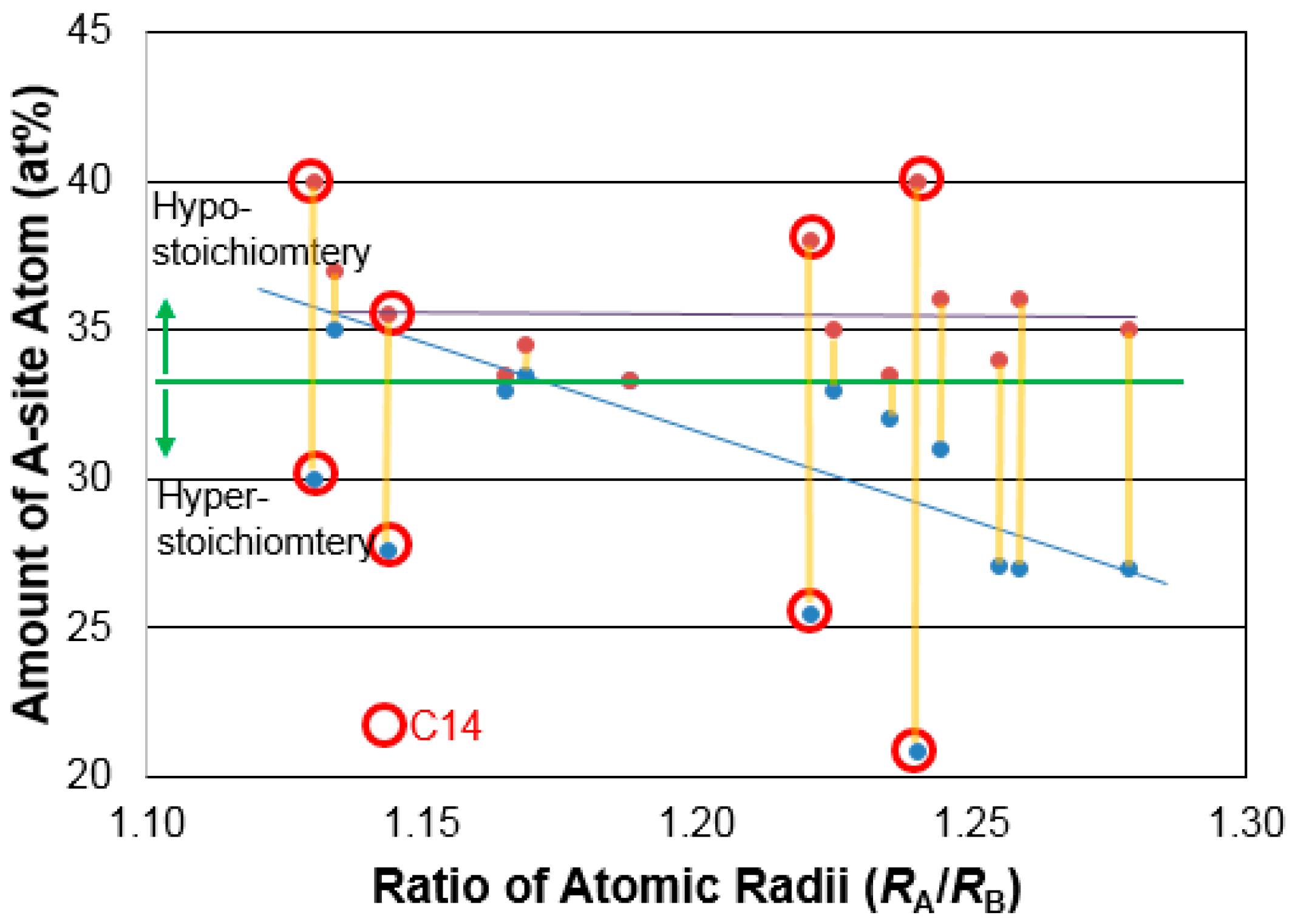
| IMC | Structure | RA/RB | Solubility (at%) |
|---|---|---|---|
| ZrV2 | C15 | 1.19 | 33.3 |
| HfV2 | C15 | 1.17 | 33.5–34.5 |
| TiCr2 | C15 | 1.13 | 35–37 |
| ZrCr2 | C15 | 1.24 | 31–36 |
| HfCr2 | C15 | 1.22 | 33–35 |
| TiMn2 | C14 | 1.13 | 30–40 |
| ZrMn2 | C14 | 1.24 | 20.8–40 |
| HfMn2 | C14 | 1.22 | 25.5–38 |
| TiMn2 | C14 | 1.14 | 27.5–35.5 |
| ZrMn2 | C15 | 1.26 | 27.1–34 |
| HfMn2 | C15 | 1.24 | 32–33.5 |
| TiCo2 | C15 | 1.17 | 33–33.5 |
| ZrCo2 | C15 | 1.28 | 27–35 |
| HfCo2 | C15 | 1.26 | 27–36 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Young, K.-H.; Chang, S.; Lin, X. C14 Laves Phase Metal Hydride Alloys for Ni/MH Batteries Applications. Batteries 2017, 3, 27. https://doi.org/10.3390/batteries3030027
Young K-H, Chang S, Lin X. C14 Laves Phase Metal Hydride Alloys for Ni/MH Batteries Applications. Batteries. 2017; 3(3):27. https://doi.org/10.3390/batteries3030027
Chicago/Turabian StyleYoung, Kwo-Hsiung, Shiuan Chang, and Xinting Lin. 2017. "C14 Laves Phase Metal Hydride Alloys for Ni/MH Batteries Applications" Batteries 3, no. 3: 27. https://doi.org/10.3390/batteries3030027
APA StyleYoung, K.-H., Chang, S., & Lin, X. (2017). C14 Laves Phase Metal Hydride Alloys for Ni/MH Batteries Applications. Batteries, 3(3), 27. https://doi.org/10.3390/batteries3030027






