Timing of Interleukin-4 Stimulation of Macrophages Determines Their Anti-Microbial Activity during Infection with Salmonella enterica Serovar Typhimurium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of Bone-Marrow-Derived Macrophages
2.2. Preparation of Salmonella Typhimurium
2.3. Infection of Bone-Marrow-Derived Macrophages with Salmonella Enterica Serovar Typhimurium
2.4. Arginine Free Media
2.5. Bacterial Growth Assay
2.6. Analysis of Colony-Forming Units
2.7. The Multiplicity of Infection Test
2.8. RNA Isolation, Reverse Transcription and TaqMan Quantitative Real-Time PCR (qRT-PCR)
2.9. Griess Reaction
2.10. Western Blot Analysis
2.11. FACS Analysis
2.12. CellROX Measurement
2.13. AlamarBlue Assay
2.14. LDH Assay
2.15. Metabolomics
2.16. Statistical Analysis
3. Results
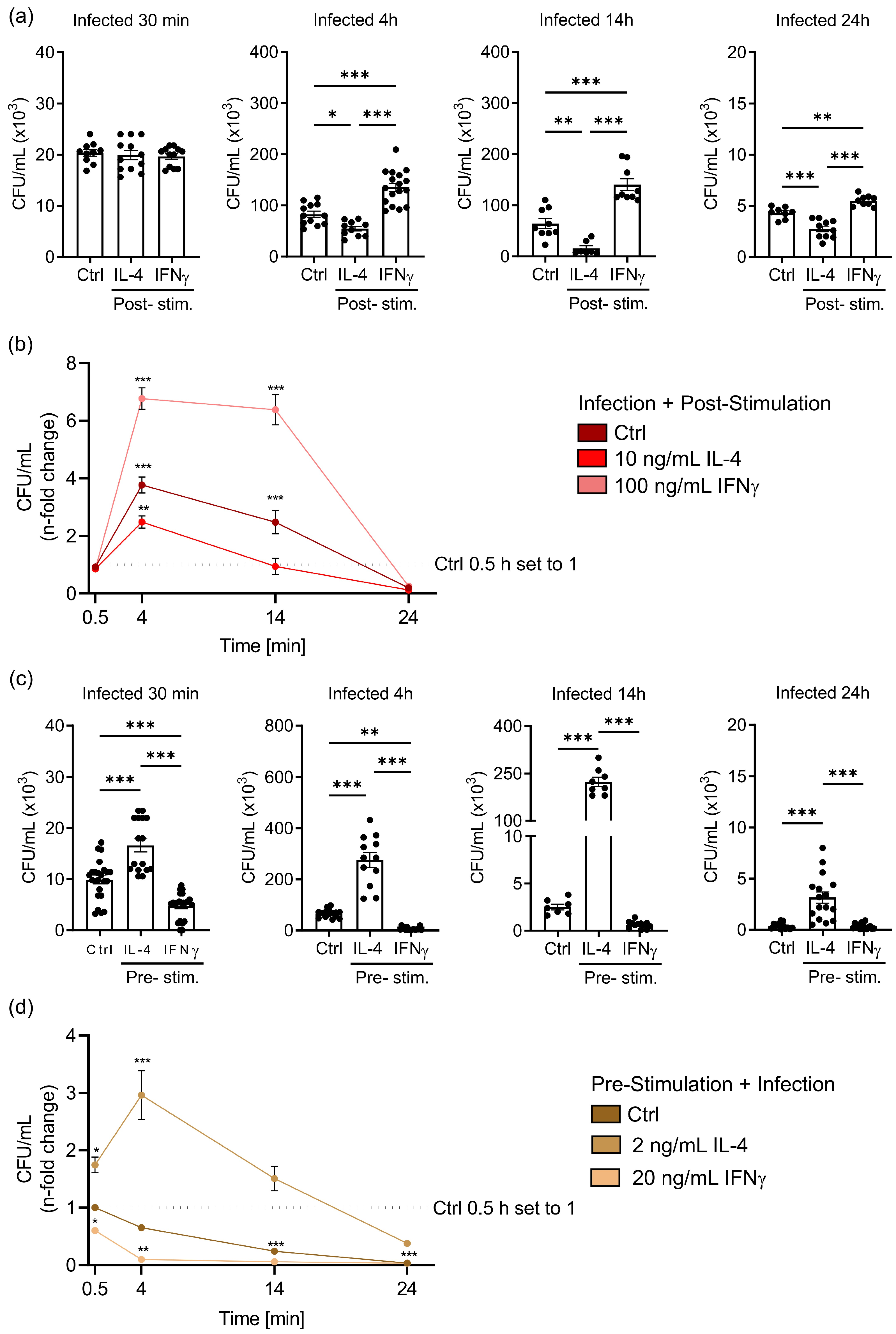
3.1. IL-4 Stimulation of Macrophages before or after Salmonella Infection Differently Affects Bacterial Multiplication
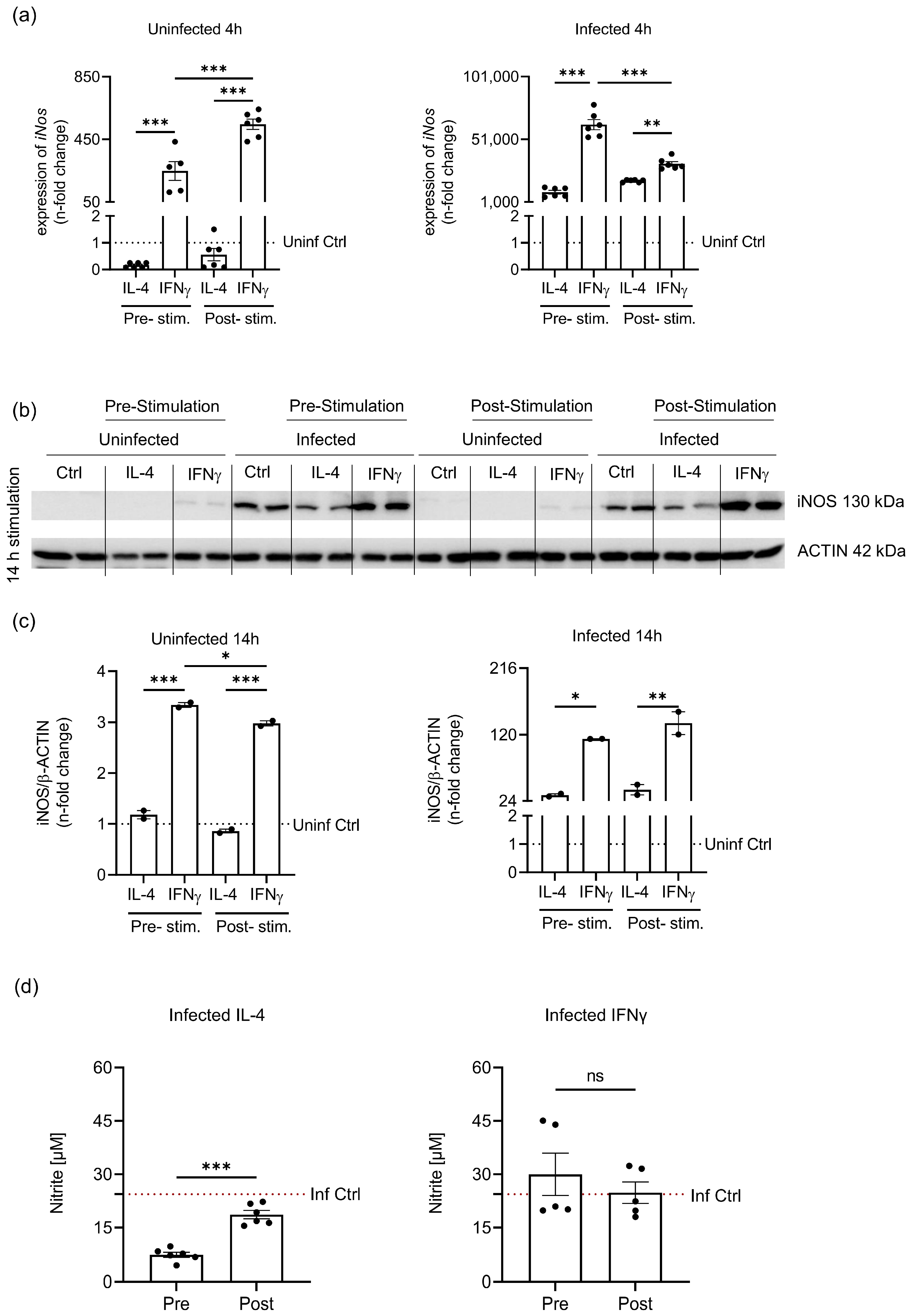
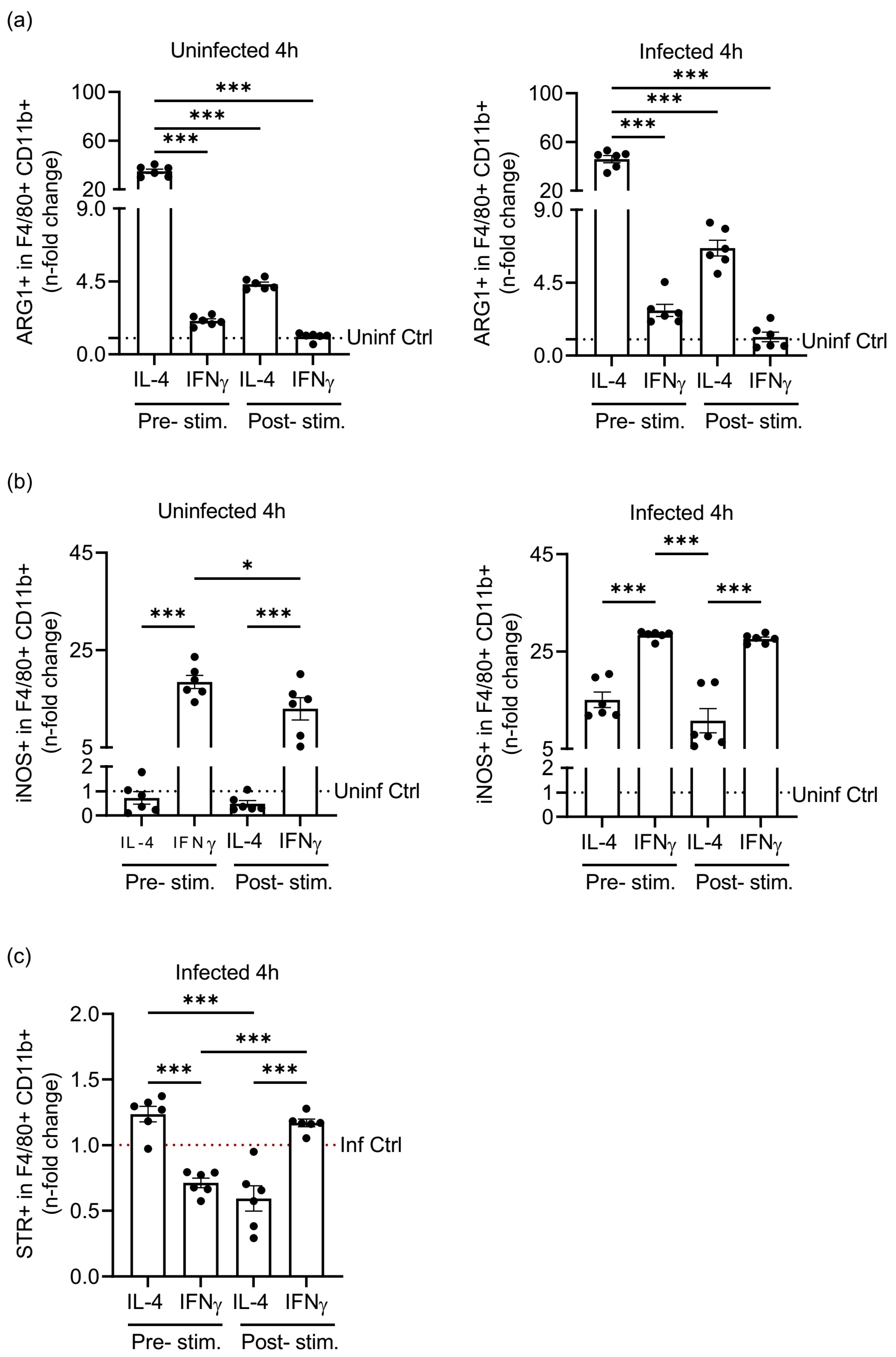
3.2. Priming of Macrophages before S.tm Infection Shows Differences in Arginase1 (ARG1) Regulation
3.3. Pre- or Post-Stimulation of Macrophages with IL-4 and IFNγ Impacts the Amount of S.tm Phagocytosed by F4/80+CD11b+ Macrophages
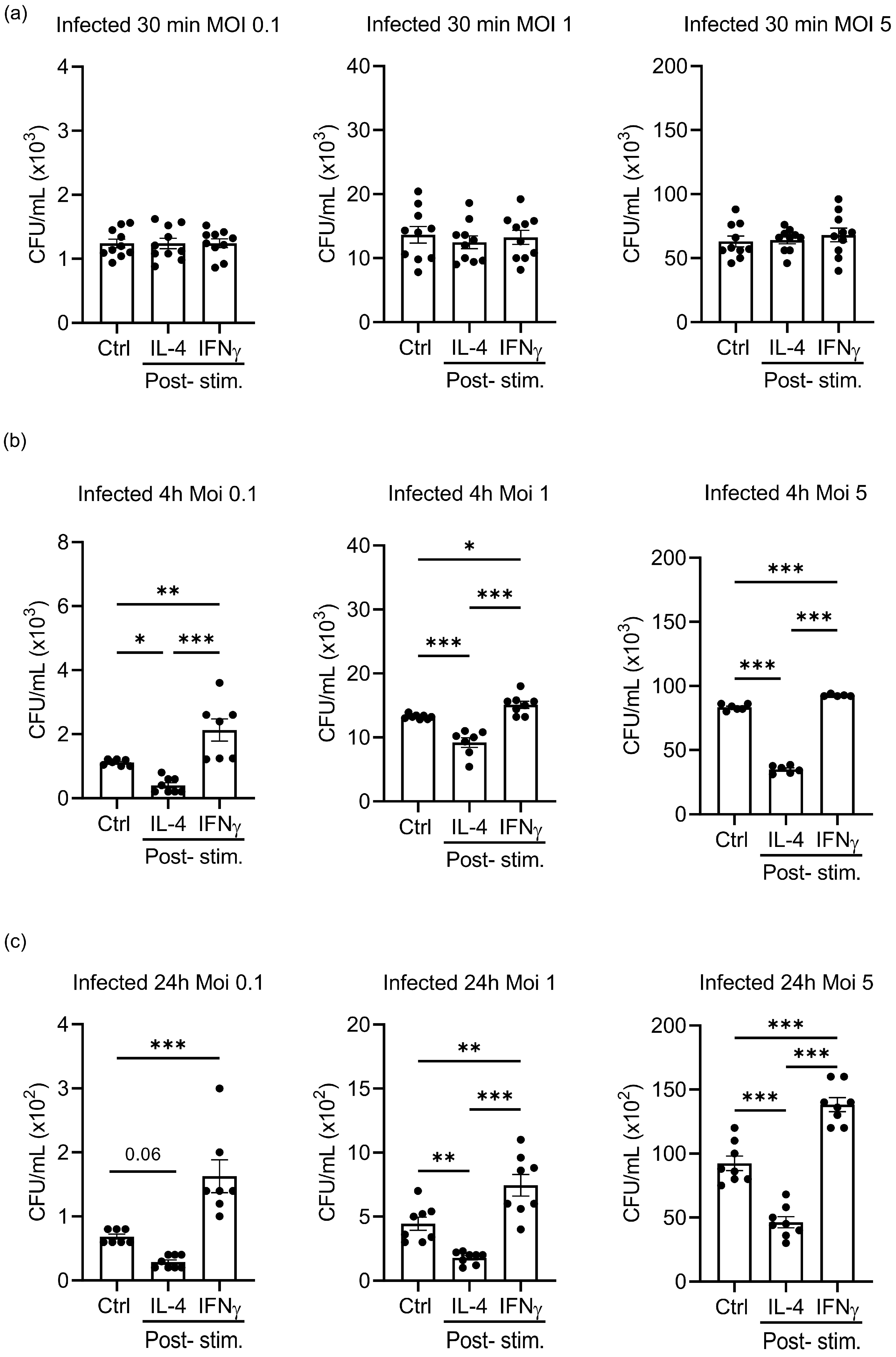
3.4. Different Multiplicities of Infection Do Not Alter the Effects of IL-4 and IFNγ Stimulation on the Growth of S.tm in Infected Macrophages
3.5. IL-4 Stimulation of Unpolarised S.tm-Infected BMDM Does Not Affect Cellular Viability, Radical Formation and Expression of Radical Detoxifying Enzymes
3.6. IL-4 Treatment of S.tm.-Infected BMDM Affects L-arginine-Driven Metabolic Pathways Causing Accumulaiton of Putrescine, Spermidine, and Spermine
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bosurgi, L.; Cao, Y.G.; Cabeza-Cabrerizo, M.; Tucci, A.; Hughes, L.D.; Kong, Y.; Weinstein, J.S.; Licona-Limon, P.; Schmid, E.T.; Pelorosso, F.; et al. Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science 2017, 356, 1072–1076. [Google Scholar] [CrossRef] [PubMed]
- Nairz, M.; Theurl, I.; Swirski, F.K.; Weiss, G. “Pumping iron”-how macrophages handle iron at the systemic, microenvironmental, and cellular levels. Pflug. Arch. Eur. J. Physiol. 2017, 469, 397–418. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Martinez-Pomares, L. Physiological roles of macrophages. Pflug. Arch. Eur. J. Physiol. 2017, 469, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Hannemann, N.; Cao, S.; Eriksson, D.; Schnelzer, A.; Jordan, J.; Eberhardt, M.; Schleicher, U.; Rech, J.; Ramming, A.; Uebe, S.; et al. Transcription factor Fra-1 targets arginase-1 to enhance macrophage-mediated inflammation in arthritis. J. Clin. Investig. 2019, 129, 2669–2684. [Google Scholar] [CrossRef]
- Weiss, G.; Schaible, U.E. Macrophage defense mechanisms against intracellular bacteria. Immunol. Rev. 2015, 264, 182–203. [Google Scholar] [CrossRef]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Luzina, I.G.; Keegan, A.D.; Heller, N.M.; Rook, G.A.; Shea-Donohue, T.; Atamas, S.P. Regulation of inflammation by interleukin-4: A review of “alternatives”. J. Leukoc. Biol. 2012, 92, 753–764. [Google Scholar] [CrossRef]
- Gray, M.J.; Poljakovic, M.; Kepka-Lenhart, D.; Morris, S.M., Jr. Induction of arginase I transcription by IL-4 requires a composite DNA response element for STAT6 and C/EBPbeta. Gene 2005, 353, 98–106. [Google Scholar] [CrossRef]
- Satriano, J. Arginine pathways and the inflammatory response: Interregulation of nitric oxide and polyamines: Review article. Amino Acids 2004, 26, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Naglak, E.K.; Morrison, S.G.; Morrison, R.P. IFNgamma is Required for Optimal Antibody-Mediated Immunity against Genital Chlamydia Infection. Infect. Immun. 2016, 84, 3232–3242. [Google Scholar] [CrossRef] [PubMed]
- Green, A.M.; Difazio, R.; Flynn, J.L. IFN-gamma from CD4 T cells is essential for host survival and enhances CD8 T cell function during Mycobacterium tuberculosis infection. J. Immunol. 2013, 190, 270–277. [Google Scholar] [CrossRef]
- Frucht, D.M.; Fukao, T.; Bogdan, C.; Schindler, H.; O’Shea, J.J.; Koyasu, S. IFN-gamma production by antigen-presenting cells: Mechanisms emerge. Trends Immunol. 2001, 22, 556–560. [Google Scholar] [CrossRef]
- Brigo, N.; Pfeifhofer-Obermair, C.; Tymoszuk, P.; Demetz, E.; Engl, S.; Barros-Pinkelnig, M.; Dichtl, S.; Fischer, C.; Valente De Souza, L.; Petzer, V.; et al. Cytokine-Mediated Regulation of ARG1 in Macrophages and Its Impact on the Control of Salmonella enterica Serovar Typhimurium Infection. Cells 2021, 10, 1823. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Koskenkorva-Frank, T.S.; Weiss, G.; Koppenol, W.H.; Burckhardt, S. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: Insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free. Radic. Biol. Med. 2013, 65, 1174–1194. [Google Scholar] [CrossRef]
- Wong Fok Lung, T.; Charytonowicz, D.; Beaumont, K.G.; Shah, S.S.; Sridhar, S.H.; Gorrie, C.L.; Mu, A.; Hofstaedter, C.E.; Varisco, D.; McConville, T.H.; et al. Klebsiella pneumoniae induces host metabolic stress that promotes tolerance to pulmonary infection. Cell Metab. 2022, 34, 761–774.e769. [Google Scholar] [CrossRef]
- Sedivy-Haley, K.; Blimkie, T.; Falsafi, R.; Lee, A.H.; Hancock, R.E.W. A transcriptomic analysis of the effects of macrophage polarization and endotoxin tolerance on the response to Salmonella. PLoS ONE 2022, 17, e0276010. [Google Scholar] [CrossRef]
- Schleicher, U.; Paduch, K.; Debus, A.; Obermeyer, S.; Konig, T.; Kling, J.C.; Ribechini, E.; Dudziak, D.; Mougiakakos, D.; Murray, P.J.; et al. TNF-Mediated Restriction of Arginase 1 Expression in Myeloid Cells Triggers Type 2 NO Synthase Activity at the Site of Infection. Cell Rep. 2016, 15, 1062–1075. [Google Scholar] [CrossRef]
- De Muylder, G.; Daulouede, S.; Lecordier, L.; Uzureau, P.; Morias, Y.; Van Den Abbeele, J.; Caljon, G.; Herin, M.; Holzmuller, P.; Semballa, S.; et al. A Trypanosoma brucei kinesin heavy chain promotes parasite growth by triggering host arginase activity. PLoS Pathog. 2013, 9, e1003731. [Google Scholar] [CrossRef]
- Iniesta, V.; Gomez-Nieto, L.C.; Molano, I.; Mohedano, A.; Carcelen, J.; Miron, C.; Alonso, C.; Corraliza, I. Arginase I induction in macrophages, triggered by Th2-type cytokines, supports the growth of intracellular Leishmania parasites. Parasite Immunol. 2002, 24, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Knippenberg, S.; Brumshagen, C.; Aschenbrenner, F.; Welte, T.; Maus, U.A. Arginase 1 activity worsens lung-protective immunity against Streptococcus pneumoniae infection. Eur. J. Immunol. 2015, 45, 1716–1726. [Google Scholar] [CrossRef] [PubMed]
- Lardone, R.D.; Chan, A.A.; Lee, A.F.; Foshag, L.J.; Faries, M.B.; Sieling, P.A.; Lee, D.J. Mycobacterium bovis Bacillus Calmette-Guerin Alters Melanoma Microenvironment Favoring Antitumor T Cell Responses and Improving M2 Macrophage Function. Front. Immunol. 2017, 8, 965. [Google Scholar] [CrossRef] [PubMed]
- Mily, A.; Kalsum, S.; Loreti, M.G.; Rekha, R.S.; Muvva, J.R.; Lourda, M.; Brighenti, S. Polarization of M1 and M2 Human Monocyte-Derived Cells and Analysis with Flow Cytometry upon Mycobacterium tuberculosis Infection. J. Vis. Exp. 2020, 163, e61807. [Google Scholar] [CrossRef]
- Liu, J.; Huang, S.; Lu, F. Galectin-3 and Galectin-9 May Differently Regulate the Expressions of Microglial M1/M2 Markers and T Helper 1/Th2 Cytokines in the Brains of Genetically Susceptible C57BL/6 and Resistant BALB/c Mice Following Peroral Infection With Toxoplasma gondii. Front. Immunol. 2018, 9, 1648. [Google Scholar] [CrossRef]
- Bhutta, Z.A.; Gaffey, M.F.; Crump, J.A.; Steele, D.; Breiman, R.F.; Mintz, E.D.; Black, R.E.; Luby, S.P.; Levine, M.M. Typhoid Fever: Way Forward. Am. J. Trop. Med. Hyg. 2018, 99, 89–96. [Google Scholar] [CrossRef]
- Baumler, A.; Fang, F.C. Host specificity of bacterial pathogens. Cold Spring Harb. Perspect. Med. 2013, 3, a010041. [Google Scholar] [CrossRef]
- Herb, M.; Schramm, M. Functions of ROS in Macrophages and Antimicrobial Immunity. Antioxidants 2021, 10, 313. [Google Scholar] [CrossRef]
- Malik-Kale, P.; Jolly, C.E.; Lathrop, S.; Winfree, S.; Luterbach, C.; Steele-Mortimer, O. Salmonella—At home in the host cell. Front. Microbiol. 2011, 2, 125. [Google Scholar] [CrossRef]
- Eisele, N.A.; Ruby, T.; Jacobson, A.; Manzanillo, P.S.; Cox, J.S.; Lam, L.; Mukundan, L.; Chawla, A.; Monack, D.M. Salmonella require the fatty acid regulator PPARdelta for the establishment of a metabolic environment essential for long-term persistence. Cell Host Microbe 2013, 14, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.H.M.; Brewer, S.M.; Thurston, T.; Massis, L.M.; Honeycutt, J.; Lugo, K.; Jacobson, A.R.; Vilches-Moure, J.G.; Hamblin, M.; Helaine, S.; et al. Salmonella-Driven Polarization of Granuloma Macrophages Antagonizes TNF-Mediated Pathogen Restriction during Persistent Infection. Cell Host Microbe 2020, 27, 54–67.e5. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.J.; Winter, S.E. Salmonella finds a way: Metabolic versatility of Salmonella enterica serovar Typhimurium in diverse host environments. PLoS Pathog. 2020, 16, e1008540. [Google Scholar] [CrossRef] [PubMed]
- Brigo, N.; Pfeifhofer-Obermair, C.; Demetz, E.; Tymoszuk, P.; Weiss, G. Flow Cytometric Characterization of Macrophages Infected in vitro with Salmonella enterica Serovar Typhimurium Expressing Red Fluorescent Protein. Bio-Protocol 2022, 12, e4440. [Google Scholar] [CrossRef]
- Pfeifhofer-Obermair, C.; Brigo, N.; Tymoszuk, P.; Weiss, A.G. A Mouse Infection Model with a Wildtype Salmonella enterica Serovar Typhimurium Strain for the Analysis of Inflammatory Innate Immune Cells. Bio-Protocol 2022, 12, e4378. [Google Scholar] [CrossRef]
- Dichtl, S.; Demetz, E.; Haschka, D.; Tymoszuk, P.; Petzer, V.; Nairz, M.; Seifert, M.; Hoffmann, A.; Brigo, N.; Wurzner, R.; et al. Dopamine Is a Siderophore-Like Iron Chelator That Promotes Salmonella enterica Serovar Typhimurium Virulence in Mice. mBio 2019, 10, e02624-18. [Google Scholar] [CrossRef]
- Nairz, M.; Theurl, I.; Schroll, A.; Theurl, M.; Fritsche, G.; Lindner, E.; Seifert, M.; Crouch, M.L.; Hantke, K.; Akira, S.; et al. Absence of functional Hfe protects mice from invasive Salmonella enterica serovar Typhimurium infection via induction of lipocalin-2. Blood 2009, 114, 3642–3651. [Google Scholar] [CrossRef]
- Ludwiczek, S.; Theurl, I.; Muckenthaler, M.U.; Jakab, M.; Mair, S.M.; Theurl, M.; Kiss, J.; Paulmichl, M.; Hentze, M.W.; Ritter, M.; et al. Ca2+ channel blockers reverse iron overload by a new mechanism via divalent metal transporter-1. Nat. Med. 2007, 13, 448–454. [Google Scholar] [CrossRef]
- Grubwieser, P.; Hoffmann, A.; Hilbe, R.; Seifert, M.; Sonnweber, T.; Bock, N.; Theurl, I.; Weiss, G.; Nairz, M. Airway Epithelial Cells Differentially Adapt Their Iron Metabolism to Infection With Klebsiella pneumoniae and Escherichia coli In Vitro. Front. Cell Infect. Microbiol. 2022, 12, 875543. [Google Scholar] [CrossRef]
- Mastroeni, P.; Vazquez-Torres, A.; Fang, F.C.; Xu, Y.; Khan, S.; Hormaeche, C.E.; Dougan, G. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. II. Effects on microbial proliferation and host survival in vivo. J. Exp. Med. 2000, 192, 237–248. [Google Scholar] [CrossRef]
- Il’in, D.A.; Shkurupy, V.A. The In Vitro M1/M2 Polarization of Macrophages of BCG-Infected Mice. Bull. Exp. Biol. Med. 2020, 169, 467–469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Y.; He, Y.; Wang, X.; Fang, Q. Lipopolysaccharide mediates time-dependent macrophage M1/M2 polarization through the Tim-3/Galectin-9 signalling pathway. Exp. Cell. Res. 2019, 376, 124–132. [Google Scholar] [CrossRef]
- Major, J.; Fletcher, J.E.; Hamilton, T.A. IL-4 pretreatment selectively enhances cytokine and chemokine production in lipopolysaccharide-stimulated mouse peritoneal macrophages. J. Immunol. 2002, 168, 2456–2463. [Google Scholar] [CrossRef] [PubMed]
- Gladue, R.P.; Laquerre, A.M.; Magna, H.A.; Carroll, L.A.; O’Donnell, M.; Changelian, P.S.; Franke, A.E. In vivo augmentation of IFN-gamma with a rIL-12 human/mouse chimera: Pleiotropic effects against infectious agents in mice and rats. Cytokine 1994, 6, 318–328. [Google Scholar] [CrossRef]
- Liu, B.; Bao, L.; Wang, L.; Li, F.; Wen, M.; Li, H.; Deng, W.; Zhang, X.; Cao, B. Anti-IFN-gamma therapy alleviates acute lung injury induced by severe influenza A (H1N1) pdm09 infection in mice. J. Microbiol. Immunol. Infect. 2021, 54, 396–403. [Google Scholar] [CrossRef]
- Gadotti, A.C.; de Castro Deus, M.; Telles, J.P.; Wind, R.; Goes, M.; Garcia Charello Ossoski, R.; de Padua, A.M.; de Noronha, L.; Moreno-Amaral, A.; Baena, C.P.; et al. IFN-gamma is an independent risk factor associated with mortality in patients with moderate and severe COVID-19 infection. Virus Res. 2020, 289, 198171. [Google Scholar] [CrossRef]
- Rath, M.; Muller, I.; Kropf, P.; Closs, E.I.; Munder, M. Metabolism via Arginase or Nitric Oxide Synthase: Two Competing Arginine Pathways in Macrophages. Front. Immunol. 2014, 5, 532. [Google Scholar] [CrossRef] [PubMed]
- Haider, P.; Kral-Pointner, J.B.; Salzmann, M.; Moik, F.; Bleichert, S.; Schrottmaier, W.C.; Kaun, C.; Brekalo, M.; Fischer, M.B.; Speidl, W.S.; et al. Interleukin-4 receptor alpha signaling regulates monocyte homeostasis. FASEB J. 2022, 36, e22532. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, S.; Wang, K.; Wang, N.; Liu, Q.; Sun, Z.; Wang, L.; Wang, L.; Liu, Q.; Song, C.; et al. Spermine enhances antiviral and anticancer responses by stabilizing DNA binding with the DNA sensor cGAS. Immunity 2023, 56, 272–288 e277. [Google Scholar] [CrossRef]
- Casero, R.A., Jr.; Murray Stewart, T.; Pegg, A.E. Polyamine metabolism and cancer: Treatments, challenges and opportunities. Nat. Rev. Cancer 2018, 18, 681–695. [Google Scholar] [CrossRef]
- Das, P.; Lahiri, A.; Lahiri, A.; Chakravortty, D. Modulation of the arginase pathway in the context of microbial pathogenesis: A metabolic enzyme moonlighting as an immune modulator. PLoS Pathog. 2010, 6, e1000899. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, M.; Datey, A.; Wilson, K.T.; Chakravortty, D. Dual role of arginine metabolism in establishing pathogenesis. Curr. Opin. Microbiol. 2016, 29, 43–48. [Google Scholar] [CrossRef] [PubMed]





| Murine Gene | Forward Primer 5′-3′ | Reverse Primer 5′-3′ | Probe |
|---|---|---|---|
| Hprt | GACCGGTCCCGTCATGC | TCATAACCTGGTTCATCATCGC | ACCCGCAGTCCCAGCGTCGTC |
| Arg1 | AACACGGCAGTGGCTTTAAC | GAGGAGAAGGCGTTTGCTTA | TGGCTTATGGTTACCCTCCCGTTG |
| iNos | CAGCTGGGCTGTACAAACCTT | CATTGGAAGTGAAGCGTTTCG | CGGGCAGCCTGTGAGACCTTTGA |
| Phoxp47 | GAGGCGGAGGATCCGG | TCTTCAACAGCAGCGTACGC | CAACTACGCAGGTGAACCGTATGTAACCATCA |
| Detected Protein | Antibody Dilution | Clone Number | Commercial Source | Catalogue Number |
|---|---|---|---|---|
| ARG1 | 1:2000 | Polyclonal | Novusbio | NBP1-32731 |
| iNOS | 1:1000 | Polyclonal | Abcam | ab15323 |
| SOD1 | 1:1000 | Polyclonal | Enzo | ADI-SOD-100 |
| NRF2 | 1:1000 | D1Z9C | CellSignalling | 12721 |
| β-ACTIN | 1:500 | Polyclonal | Sigma-Aldrich | A 2066 |
| Antibody against | Conjugated Fluorochrome | Clone Number | Commercial Source | Catalogue Number |
|---|---|---|---|---|
| Surface: | ||||
| CD3 | FITC | 17.A2 | ImmunoTools | 100204 |
| CD19 | FITC | PeCa1 | ImmunoTools | 22220193S |
| CD49b | FITC | HMa2 | BD Biosciences | 103503 |
| CD11b | BV650 | M1/70 | BioLegend | 101239 |
| CD45 | APC-R700 | 30-F11 | BD Horizon | 565478 |
| F4/80 | BV421 | T45-2342 | BD Horizon | 565478 |
| Ly6G | Percp-eflour 710 | 1AB-Ly6g | Invitrogen | 46-9668-82 |
| Intracellular: | ||||
| iNOS | PeCy7 | CXNFT | Invitrogen | 25-5920-80 |
| ARG1 | APC | A1exF5 | Invitrogen | 17-3697-82 |
| Antibody against | Conjugated Fluorochrome | Clone Number | Commercial Source | Catalogue Number |
|---|---|---|---|---|
| Surface: | ||||
| CD11b | BV650 | M1/70 | BioLegend | 101239 |
| F4/80 | Pe/Cy7 | BM8 | BioLegend | 123114 |
| CD80 | FITC | 16-10A1 | BioLegend | 104705 |
| CD206 | BV421 | C068C2 | BioLegend | 141717 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brigo, N.; Neumaier, E.; Pfeifhofer-Obermair, C.; Grubwieser, P.; Engl, S.; Berger, S.; Seifert, M.; Reinstadler, V.; Oberacher, H.; Weiss, G. Timing of Interleukin-4 Stimulation of Macrophages Determines Their Anti-Microbial Activity during Infection with Salmonella enterica Serovar Typhimurium. Cells 2023, 12, 1164. https://doi.org/10.3390/cells12081164
Brigo N, Neumaier E, Pfeifhofer-Obermair C, Grubwieser P, Engl S, Berger S, Seifert M, Reinstadler V, Oberacher H, Weiss G. Timing of Interleukin-4 Stimulation of Macrophages Determines Their Anti-Microbial Activity during Infection with Salmonella enterica Serovar Typhimurium. Cells. 2023; 12(8):1164. https://doi.org/10.3390/cells12081164
Chicago/Turabian StyleBrigo, Natascha, Emely Neumaier, Christa Pfeifhofer-Obermair, Philipp Grubwieser, Sabine Engl, Sylvia Berger, Markus Seifert, Vera Reinstadler, Herbert Oberacher, and Günter Weiss. 2023. "Timing of Interleukin-4 Stimulation of Macrophages Determines Their Anti-Microbial Activity during Infection with Salmonella enterica Serovar Typhimurium" Cells 12, no. 8: 1164. https://doi.org/10.3390/cells12081164
APA StyleBrigo, N., Neumaier, E., Pfeifhofer-Obermair, C., Grubwieser, P., Engl, S., Berger, S., Seifert, M., Reinstadler, V., Oberacher, H., & Weiss, G. (2023). Timing of Interleukin-4 Stimulation of Macrophages Determines Their Anti-Microbial Activity during Infection with Salmonella enterica Serovar Typhimurium. Cells, 12(8), 1164. https://doi.org/10.3390/cells12081164









