The Contribution of Exercise in Telemedicine Monitoring in Reducing the Modifiable Factors of Hypertension—A Multidisciplinary Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strategy and Elegibility Criteria
2.2. Information Sources
2.3. Data Extraction
2.4. Methodological Quality and Level of Evidence
3. Results
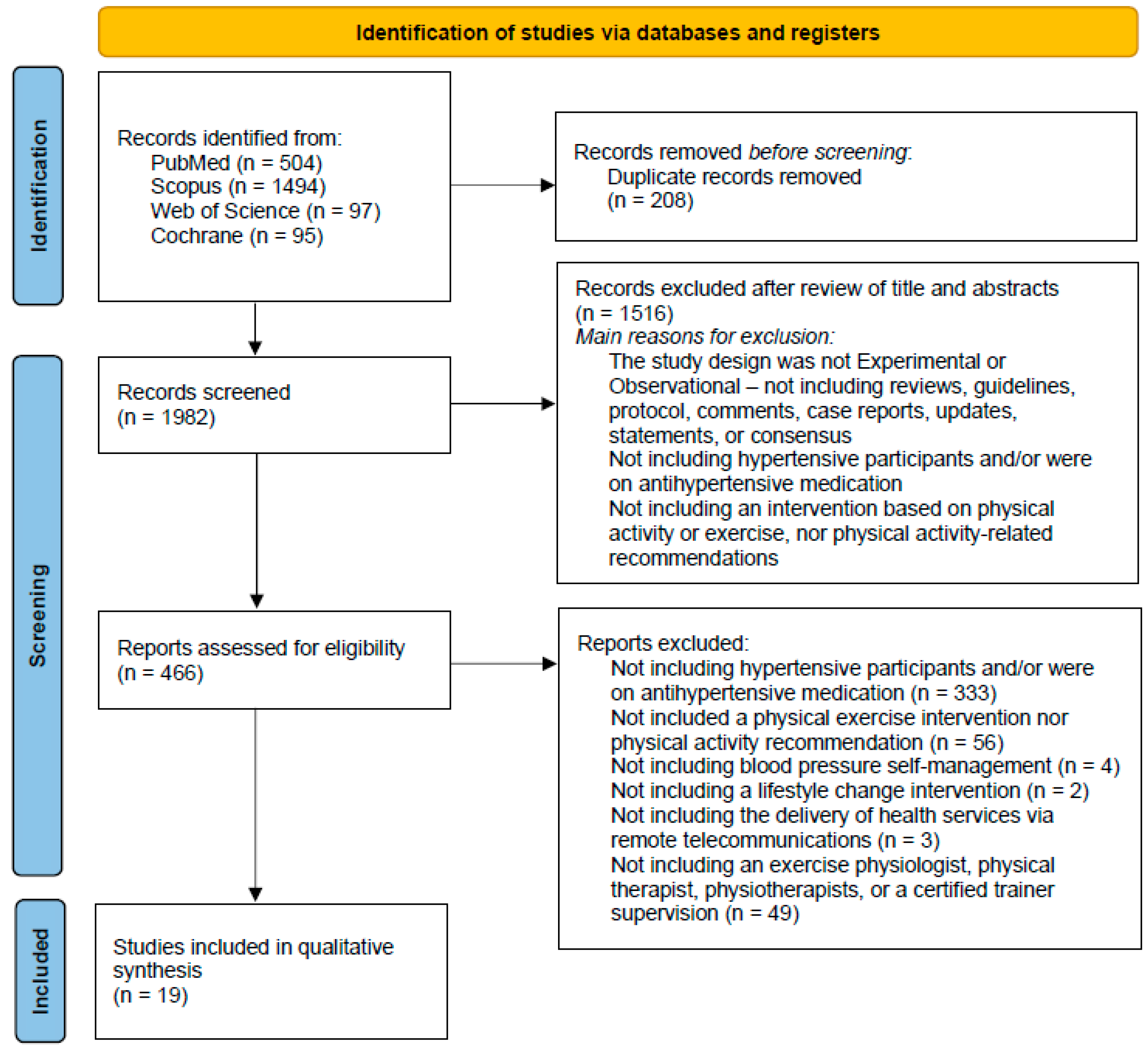
3.1. Identification and Study Selection
3.2. Methodological Quality
3.3. Studies Characteristics
3.4. Exercise Monitoring and Prescription
3.5. Outcomes and Results Regarding Hypertension and Blood Pressure Management
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.L.; Aravkin, A.Y.; Zheng, P.; Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- WHO. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Zhou, B.; Carrillo-Larco, R.M.; Danaei, G.; Riley, L.M.; Paciorek, C.J.; Stevens, G.A.; Gregg, E.W.; Bennett, J.E.; Solomon, B.; Singleton, R.K.; et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25, 1–72. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, e13–e115. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, P.L.; Carrera-Bastos, P.; Galvez, B.G.; Ruiz-Hurtado, G.; Ordovas, J.M.; Ruilope, L.M.; Lucia, A. Lifestyle interventions for the prevention and treatment of hypertension. Nat. Rev. Cardiol. 2021, 18, 251–275. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef]
- Fletcher, G.F.; Landolfo, C.; Niebauer, J.; Ozemek, C.; Arena, R.; Lavie, C.J. Promoting Physical Activity and Exercise: JACC Health Promotion Series. J. Am. Coll. Cardiol. 2018, 72, 1622–1639. [Google Scholar] [CrossRef]
- Brickwood, K.-J.; Watson, G.; O’Brien, J.; Williams, A.D. Consumer-Based Wearable Activity Trackers Increase Physical Activity Participation: Systematic Review and Meta-Analysis. JMIR Mhealth Uhealth 2019, 7, e11819. [Google Scholar] [CrossRef]
- Pellegrini, D.; Torlasco, C.; Ochoa, J.E.; Parati, G. Contribution of telemedicine and information technology to hypertension control. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2020, 43, 621–628. [Google Scholar] [CrossRef]
- Hanley, J.; Pinnock, H.; Paterson, M.; McKinstry, B. Implementing telemonitoring in primary care: Learning from a large qualitative dataset gathered during a series of studies. BMC Fam. Pract. 2018, 19, 118. [Google Scholar] [CrossRef] [PubMed]
- Hoffer-Hawlik, M.; Moran, A.; Zerihun, L.; Usseglio, J.; Cohn, J.; Gupta, R. Telemedicine interventions for hypertension management in low- and middle-income countries: A scoping review. PLoS ONE 2021, 16, e0254222. [Google Scholar] [CrossRef]
- Omboni, S. Connected Health in Hypertension Management. Front. Cardiovasc. Med. 2019, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Bmj 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Gonzalez, M.; Perez-Reviriego, A.A.; Castellano-Martinez, A.; Cascales-Poyatos, H.M. The Assessment of Myocardial Strain by Cardiac Imaging in Healthy Infants with Acute Bronchiolitis: A Systematic Review and Meta-Analysis. Diagnostics 2020, 10, 382. [Google Scholar] [CrossRef] [PubMed]
- Hooper, P.; Jutai, J.W.; Strong, G.; Russell-Minda, E. Age-related macular degeneration and low-vision rehabilitation: A systematic review. Can. J. Ophthalmol. J. Can. D’ophtalmologie 2008, 43, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Deeks, J.J.; Dinnes, J.; D’Amico, R.; Sowden, A.J.; Sakarovitch, C.; Song, F.; Petticrew, M.; Altman, D.G.; International Stroke Trial Collaborative, G.; European Carotid Surgery Trial Collaborative, G. Evaluating non-randomised intervention studies. Health Technol. Assess. 2003, 7, iii-173. [Google Scholar] [CrossRef]
- Kraal, J.J.; Peek, N.; Van den Akker-Van Marle, M.E.; Kemps, H.M.C. Effects of home-based training with telemonitoring guidance in low to moderate risk patients entering cardiac rehabilitation: Short-term results of the FIT@Home study. Eur. J. Prev. Cardiol. 2014, 21, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Piotrowicz, E.; Zieliński, T.; Bodalski, R.; Rywik, T.; Dobraszkiewicz-Wasilewska, B.; Sobieszczańska-Małek, M.; Stepnowska, M.; Przybylski, A.; Browarek, A.; Szumowski, Ł.; et al. Home-based telemonitored Nordic walking training is well accepted, safe, effective and has high adherence among heart failure patients, including those with cardiovascular implantable electronic devices: A randomised controlled study. Eur. J. Prev. Cardiol. 2014, 22, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Piotrowicz, E.; Stepnowska, M.; Leszczyńska-Iwanicka, K.; Piotrowska, D.; Kowalska, M.; Tylka, J.; Piotrowski, W.; Piotrowicz, R. Quality of life in heart failure patients undergoing home-based telerehabilitation versus outpatient rehabilitation—A randomized controlled study. Eur. J. Cardiovasc. Nurs. 2014, 14, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Okura, T.; Enomoto, D.; Miyoshi, K.-i.; Nagao, T.; Kukida, M.; Tanino, A.; Pei, Z.; Higaki, J.; Uemura, H. The Importance of Walking for Control of Blood Pressure: Proof Using a Telemedicine System. Telemed. E-Health 2016, 22, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Hwang, R.; Bruning, J.; Morris, N.R.; Mandrusiak, A.; Russell, T. Home-based telerehabilitation is not inferior to a centre-based program in patients with chronic heart failure: A randomised trial. J. Physiother. 2017, 63, 101–107. [Google Scholar] [CrossRef]
- Kruk, P.J.; Nowicki, M. Effect of the physical activity program on the treatment of resistant hypertension in primary care. Prim. Health Care Res. Dev. 2018, 19, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Brooks, D.; Thomas, S.G.; Eysenbach, G.; Nolan, R.P. Effectiveness of User- and Expert-Driven Web-based Hypertension Programs: An RCT. Am. J. Prev. Med. 2018, 54, 576–583. [Google Scholar] [CrossRef]
- Duscha, B.D.; Piner, L.W.; Patel, M.P.; Craig, K.P.; Brady, M.; McGarrah, R.W.; Chen, C.; Kraus, W.E. Effects of a 12-week mHealth program on peak VO2 and physical activity patterns after completing cardiac rehabilitation: A randomized controlled trial. Am. Heart J. 2018, 199, 105–114. [Google Scholar] [CrossRef]
- Rawstorn, J.C.; Gant, N.; Rolleston, A.; Whittaker, R.; Stewart, R.; Benatar, J.; Warren, I.; Meads, A.; Jiang, Y.; Maddison, R. End Users Want Alternative Intervention Delivery Models: Usability and Acceptability of the REMOTE-CR Exercise-Based Cardiac Telerehabilitation Program. Arch. Phys. Med. Rehabil. 2018, 99, 2373–2377. [Google Scholar] [CrossRef] [PubMed]
- Maddison, R.; Rawstorn, J.C.; Stewart, R.A.H.; Benatar, J.; Whittaker, R.; Rolleston, A.; Jiang, Y.; Gao, L.; Moodie, M.; Warren, I.; et al. Effects and costs of real-time cardiac telerehabilitation: Randomised controlled non-inferiority trial. Heart 2019, 105, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Nolan, R.P.; Feldman, R.; Dawes, M.; Kaczorowski, J.; Lynn, H.; Barr, S.I.; MacPhail, C.; Thomas, S.; Goodman, J.; Eysenbach, G.; et al. Randomized Controlled Trial of E-Counseling for Hypertension. Circ. Cardiovasc. Qual. Outcomes 2018, 11, e004420. [Google Scholar] [CrossRef]
- Fang, J.; Huang, B.; Xu, D.; Li, J.; Au, W.W. Innovative Application of a Home-Based and Remote Sensing Cardiac Rehabilitation Protocol in Chinese Patients After Percutaneous Coronary Intervention. Telemed. E-Health 2019, 25, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Avila, A.; Claes, J.; Buys, R.; Azzawi, M.; Vanhees, L.; Cornelissen, V. Home-based exercise with telemonitoring guidance in patients with coronary artery disease: Does it improve long-term physical fitness? Eur. J. Prev. Cardiol. 2019, 27, 367–377. [Google Scholar] [CrossRef]
- Lisón, J.F.; Palomar, G.; Mensorio, M.S.; Baños, R.M.; Cebolla-Martí, A.; Botella, C.; Benavent-Caballer, V.; Rodilla, E. Impact of a Web-Based Exercise and Nutritional Education Intervention in Patients Who Are Obese With Hypertension: Randomized Wait-List Controlled Trial. J. Med. Internet Res. 2020, 22, e14196. [Google Scholar] [CrossRef] [PubMed]
- Lunde, P.; Bye, A.; Bergland, A.; Grimsmo, J.; Jarstad, E.; Nilsson, B.B. Long-term follow-up with a smartphone application improves exercise capacity post cardiac rehabilitation: A randomized controlled trial. Eur. J. Prev. Cardiol. 2020, 27, 1782–1792. [Google Scholar] [CrossRef] [PubMed]
- Laustsen, S.; Oestergaard, L.G.; van Tulder, M.; Hjortdal, V.E.; Petersen, A.K. Telemonitored exercise-based cardiac rehabilitation improves physical capacity and health-related quality of life. J. Telemed. Telecare 2018, 26, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Szalewska, D.; Główczyńska, R.; Piotrowicz, R.; Kowalik, I.; Pencina, M.J.; Opolski, G.; Zaręba, W.; Banach, M.; Orzechowski, P.; Pluta, S.; et al. An aetiology-based subanalysis of the Telerehabilitation in Heart Failure Patients (TELEREH-HF) trial. ESC Heart Fail. 2021, 8, 1263–1273. [Google Scholar] [CrossRef]
- Myers, J.; Chan, K.; Chen, Y.; Lit, Y.; Patti, A.; Massaband, P.; Kiratli, B.J.; Tamura, M.; Chertow, G.M.; Rabkin, R. Effect of a Home-Based Exercise Program on Indices of Physical Function and Quality of Life in Elderly Maintenance Hemodialysis Patients. Kidney Blood Press. Res. 2021, 46, 196–206. [Google Scholar] [CrossRef]
- Hong, E.; Jakacic, A.N.; Sahoo, A.; Breyman, E.; Ukegbu, G.; Tabacof, L.; Sachs, D.; Migliaccio, J.; Phipps, C.; Schwartz, J.; et al. Use of Fitbit Technology Does Not Impact Health Biometrics in a Community of Older Adults. Telemed. E-Health 2021, 27, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, H.; Boardman, H.; Deiseroth, A.; Moholdt, T.; Simonenko, M.; Krankel, N.; Niebauer, J.; Tiberi, M.; Abreu, A.; Solberg, E.E.; et al. Personalized exercise prescription in the prevention and treatment of arterial hypertension: A Consensus Document from the European Association of Preventive Cardiology (EAPC) and the ESC Council on Hypertension. Eur. J. Prev. Cardiol. 2021, 29, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Pescatello, L.S.; Buchner, D.M.; Jakicic, J.M.; Powell, K.E.; Kraus, W.E.; Bloodgood, B.; Campbell, W.W.; Dietz, S.; Dipietro, L.; George, S.M.; et al. Physical Activity to Prevent and Treat Hypertension: A Systematic Review. Med. Sci. Sports Exerc. 2019, 51, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Li, X.; Yan, P.; Wang, X.; Li, M.; Li, R.; Shi, X.; Liu, X.; Yang, K. The effectiveness of aerobic exercise for hypertensive population: A systematic review and meta-analysis. J. Clin. Hypertens. 2019, 21, 868–876. [Google Scholar] [CrossRef]
- Saco-Ledo, G.; Valenzuela, P.L.; Ruiz-Hurtado, G.; Ruilope, L.M.; Lucia, A. Exercise Reduces Ambulatory Blood Pressure in Patients With Hypertension: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2020, 9, e018487. [Google Scholar] [CrossRef] [PubMed]
- Farinatti, P.; Monteiro, W.D.; Oliveira, R.B. Long Term Home-Based Exercise is Effective to Reduce Blood Pressure in Low Income Brazilian Hypertensive Patients: A Controlled Trial. High Blood Press. Cardiovasc. Prev. Off. J. Ital. Soc. Hypertens. 2016, 23, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Ruberti, O.M.; Yugar-Toledo, J.C.; Moreno, H.; Rodrigues, B. Hypertension telemonitoring and home-based physical training programs. Blood Press. 2021, 30, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Omboni, S.; Caserini, M.; Coronetti, C. Telemedicine and M-Health in Hypertension Management: Technologies, Applications and Clinical Evidence. High Blood Press. Cardiovasc. Prev. Off. J. Ital. Soc. Hypertens. 2016, 23, 187–196. [Google Scholar] [CrossRef]
- Wang, J.G.; Li, Y.; Chia, Y.C.; Cheng, H.M.; Minh, H.V.; Siddique, S.; Sogunuru, G.P.; Tay, J.C.; Teo, B.W.; Tsoi, K.; et al. Telemedicine in the management of hypertension: Evolving technological platforms for blood pressure telemonitoring. J. Clin. Hypertens. 2021, 23, 435–439. [Google Scholar] [CrossRef]
- Suchomel, T.J.; Nimphius, S.; Bellon, C.R.; Hornsby, W.G.; Stone, M.H. Training for Muscular Strength: Methods for Monitoring and Adjusting Training Intensity. Sports Med. 2021, 51, 2051–2066. [Google Scholar] [CrossRef]
- Omboni, S.; McManus, R.J.; Bosworth, H.B.; Chappell, L.C.; Green, B.B.; Kario, K.; Logan, A.G.; Magid, D.J.; McKinstry, B.; Margolis, K.L.; et al. Evidence and Recommendations on the Use of Telemedicine for the Management of Arterial Hypertension: An International Expert Position Paper. Hypertension 2020, 76, 1368–1383. [Google Scholar] [CrossRef] [PubMed]
| PICOS Components | Details |
|---|---|
| Population | Individuals with hypertension or high blood pressure |
| Intervention | Exercise or physical activity |
| Comparison or Control | Telemonitored vs. non-monitored |
| Outcome | All outcomes |
| Study Design | Experimental (RCT and quasi-experimental) and observational (cohort, case-control, cross-sectional) |
| Inclusion | Exclusion |
|---|---|
| Full article available | Not including participants that were hypertensive and/or were on antihypertensive medication |
| Articles published in English language | Not including an intervention based on physical activity or exercise, nor physical activity-related recommendations |
| The study design was experimental or observational—not including reviews, guidelines, protocol, comments, case reports, updates, statements, or consensus | Not including blood pressure self-management |
| Human participants | Not including a lifestyle change intervention |
| Not including the delivery of health services via remote telecommunications | |
| Not including an exercise physiologist, physical therapist, physiotherapists or a certified trainer supervision |
| Article | Reporting | External Validity | Internal Validity—Bias | Internal Validity—Confounding (Selection Bias) | Power | Total |
|---|---|---|---|---|---|---|
| Kraal et al. (2014) [20] | 8/11 | 1/3 | 5/7 | 4/6 | 0/1 | 18 |
| Piotrowicz et al. (2014) [21] | 9/11 | 2/3 | 5/7 | 4/6 | 0/1 | 20 |
| Piotrowicz et al. (2014) [22] | 9/11 | 2/3 | 5/7 | 4/6 | 0/1 | 20 |
| Okura et al. (2016) [23] | 6/11 | 1/3 | 4/7 | 3/6 | 0/1 | 14 |
| Hwang et al. (2017) [24] | 11/11 | 2/3 | 6/7 | 5/6 | 0/1 | 24 |
| Kruk and Nowicki (2018) [25] | 8/11 | 1/3 | 4/7 | 3/6 | 0/1 | 16 |
| Liu et al. (2018) [26] | 10/11 | 1/3 | 7/7 | 6/6 | 0/1 | 24 |
| Duscha et al. (2018) [27] | 9/11 | 1/3 | 6/7 | 5/6 | 0/1 | 21 |
| Rawstorn et al. (2018) [28] | 9/11 | 2/3 | 6/7 | 4/6 | 1/1 | 22 |
| Maddison et al. (2019) [29] | 11/11 | 2/3 | 6/7 | 4/6 | 0/1 | 23 |
| Nolan et al. (2018) [30] | 8/11 | 1/3 | 7/7 | 5/6 | 0/1 | 21 |
| Fang et al. (2019) [31] | 10/11 | 1/3 | 5/7 | 5/6 | 1/1 | 22 |
| Avila et al. (2019) [32] | 9/11 | 2/3 | 5/7 | 6/6 | 0/1 | 22 |
| Lisón et al. (2020) [33] | 8/11 | 1/3 | 5/7 | 4/6 | 1/1 | 19 |
| Lunde et al. (2020) [34] | 9/11 | 1/3 | 6/7 | 6/6 | 0/1 | 22 |
| Laustsen et al. (2018) [35] | 10/11 | 0/3 | 5/7 | 3/6 | 0/1 | 18 |
| Szalewska et al. (2021) [36] | 11/11 | 1/3 | 5/7 | 5/6 | 0/1 | 22 |
| Myers et al. (2021) [37] | 9/11 | 1/3 | 6/7 | 4/6 | 0/1 | 20 |
| Hong et al. (2021) [38] | 5/11 | 1/3 | 5/7 | 3/6 | 0/1 | 14 |
| Author/Country | Sample Group(s) | Intervention | Timing | Outcomes Assessed | Main Results |
|---|---|---|---|---|---|
| Kraal, Peek, Van den Akker-Van Marle and Kemps [20]/Netherlands | Centre-based training (CT) (n = 25) Home-based training (HT) (n = 25) | The CT group performed group-based training sessions on a treadmill or cycle ergometer, supervised by physical therapists and exercise specialists. Patients in the HT group received three initial supervised training sessions with instructions on how to use a wearable heart rate monitor and a web application. They received telemonitoring guidance from the physical therapist once a week via telephone. | At least two training sessions per week, during 12-weeks (20.5 supervised training sessions, on average attended by the cardiac rehabilitation (CR) patients, and 24.0 by the HT patients). | Exercise capacity defined as the average peak oxygen uptake (peak VO2) during the final 30 s of exercise. Health-related quality of life and training adherence. | Both groups showed a significant improvement in peak VO2 (10% and 14%, respectively) and quality of life, without significant between-group differences. The average training intensity of the HT group was 73.2 ± 3.5% of HRmax. Training adherence was similar between groups. |
| Piotrowicz, Zieliński, Bodalski, Rywik, Dobraszkiewicz-Wasilewska, Sobieszczańska-Małek, Stepnowska, Przybylski, Browarek, Szumowski, Piotrowski and Piotrowicz [21]/Poland | Training group (TG) (n = 75) Control group (CG) (n = 32) | All participants with heart failure. Training group: home-based telemonitored Nordic walking (NW). The control group received usual care and were not provided with a formal exercise training prescription and did not perform supervised rehabilitation. All patients received recommendations for suitable lifestyle changes and self-management according to the European Society of Cardiology (ESC) guidelines. | Patients underwent an eight-week home-based telerehabilitation program and trained five times a week. | Functional capacity assessed by VO2peak. Workload duration (t) in cardiopulmonary exercise test (CPET), six-minute walking test (6-MWT) distance and quality of life (QoL) Medical Outcome Survey Short Form 36 (SF-36); safety; adherence to and acceptance of NW. | There was a significant improvement in functional capacity assessed by VO2 peak only in the TG (16.1 ± 4.0 vs. 18.4 ± 4.1 (mL/kg/min), p = 0.0001), t (471 ± 141 vs. 577 ± 158 (s), p = 0.0001), 6-MWT (428 ± 93 vs. 480 ± 87 (m), p = 0.0001) and QoL (79.0 ± 31.3 vs. 70.8 ± 30.3 (score), p = 0.0001). In the CG, favorable effects were not observed. The differences between the TG and CG were significant in ∆ VO2 peak, ∆t, and in ∆6 MWT. All participants in the TG completed rehabilitation and accepted it well. |
| Piotrowicz, Stepnowska, Leszczyńska-Iwanicka, Piotrowska, Kowalska, Tylka, Piotrowski and Piotrowicz [22]/Poland | Home-based telemonitored cardiac rehabilitation (HTCR) (n = 75) Outpatient-based standard cardiac rehabilitation (SCR) (n = 56) | HTCR patients received remote equipment for telemonitoring, and supervised exercise training based on walking training. SCR patients participating in traditional outpatient-based rehabilitation (cycloergometer training). The training session in both groups consisted of three parts: a warm-up lasting 5–10 min (breathing and light resistance exercises, calisthenics); basic aerobic endurance training for 10–30 min; and five minutes cooling down. | Three times a week for eight weeks. | Quality of life (Medical Outcome Survey Short Form 36—SF-36). | After rehabilitation, both groups achieved a significant QoL improvement, both physically and mentally. HTCR group patients improved in QoL physical categories in one subscale (physical function), and in two subscales in the mental categories (mental health, vitality). In the SCR group, three physical subscales improved (physical function, role limitation caused by physical problems, bodily pain). In the mental categories, three subscales improved (social function, mental health, vitality). |
| Okura, Enomoto, Miyoshi, Nagao, Kukida, Tanino, Pei, Higaki and Uemura [23]/Japan | Low daily walking (n = 35) High daily walking (n = 34) | The Yawatahama General Hospital provided an electronic sphygmomanometer, a weight scale with a body fat scale, and a pedometer to patients who attended lecture for hypertensive patients to educate them about hypertension control by exercise and diet. Daily parameters were transmitted through the Internet. | The mean follow-up period was 378 ± 132 days (range 134–580 days). | Blood pressure (BP), body mass index (BMI), body weight (BW) and percent body fat (%BF), daily walking steps (DWS). | Systolic BP, BW, %BF and BMI were significantly reduced in the high daily walking group, but only systolic BP was significantly reduced in the low daily walking group. |
| Hwang, Bruning, Morris, Mandrusiak and Russell [24]/Australia | Control group (n = 26) Experimental group (n = 23) | The control group received a center-based rehabilitation program based on current recommended guidelines encompassing education, aerobic and strength training exercise. The experimental group received a real-time exercise and education intervention delivered into the participant’s home twice weekly, using online videoconferencing software, and the physiotherapist guided participants through an exercise program like the control group. | Immediately after completion of the rehabilitation program (two sessions per week of 60 min long, during a 12-week period) and at follow-up 12 weeks later. | Distance completed in the Six-Minute Walking Test (6-MWT); balance tests, a 10-m walk test, grip strength, quadriceps strength, urinary incontinence, quality of life, patient satisfaction, program attendance and adverse events. | No significant between-group differences on the 6-MWT distance gains, with a mean difference of 15 m (95% CI, –28 to 59) at Week 12. At Week 24, this difference was non-significant at 2 m (95% CI, −36 to 41), again in favor of the telerehabilitation group. No between-group differences were observed in the other outcomes. Mixed-model analyses showed that both intervention groups experienced significant improvements in their quality of life from pre-program to post-program, and improvements were sustained at follow-up. Significantly higher attendance rates were observed in the telerehabilitation group. |
| Kruk and Nowicki [25]/Poland | Resistant hypertension (RH) (n = 27) Well-controlled hypertension (WCH) (n = 26) | All participants received the recommendations concerning their diet and healthy lifestyle including physical activity in cardiovascular diseases. At each stage of the study the patients from RH group received also verbal instructions on how to intensify physical activity tailored to their needs and comorbidities. Additionally, a meeting with a physical therapist was provided to these patients. In addition, the patients received text messages to their cell phones with reminders about the benefits of regular physical activity three times a week. | Six months. | Ambulatory and office systolic blood pressure (SBP) and diastolic blood pressure (DBP), pulse pressure (PP), physical activity profile, energy expenditure and body composition. | Physical activity in RH increased significantly after six months compared with control subjects (p = 0.001). Office SBP and DBP in the RH group decreased significantly after three months, but after six months only office DBP remained significantly lower. After three months, 24-h SBP decreased by 3.1 ± 11 mmHg (p = 0.08) and DBP by 2.0 ± 6 mmHg (p = 0.17) in RH, whereas in WCH respective changes were +1.2 ± 10 and −0.3 ± 6 mmHg. After six months, 24-h BP changes were similar. |
| Liu, Brooks, Thomas, Eysenbach and Nolan [26]/Canada | Control (n = 39) User-driven (n = 37) Expert-driven (n = 39) | The control group received a weekly e-mail containing a brief newsletter article regarding BP management through lifestyle changes. The user-driven e-counseling group received weekly e-mails that enabled participants to select their intervention goals using text and video web links embedded in the e-mail. Participants in the expert-driven group received the same hypertension management recommendations for lifestyle change as the user driven group; however, the weekly e-mails consisted of predetermined exercise and dietary goals. | Four months. | Primary outcome was SBP measured at baseline and four-month follow-up. Secondary outcomes included DBP, PP, total cholesterol, 10-year Framingham cardiovascular risk, daily steps, and daily fruit and vegetable consumption. | Expert-driven groups showed a greater SBP decrease than controls at follow-up (expert-driven vs. control: −7.5 mmHg, 95% CI, −12.5, −2.6, p = 0.01), with no significant changes between user- and expert-driven groups. Expert-driven compared with controls also showed a significant improvement in pulse pressure, cholesterol, and Framingham risk score. The expert-driven intervention was significantly more effective than both user-driven and control groups in increasing daily steps and fruit intake (p < 0.01). |
| Duscha, Piner, Patel, Craig, Brady, McGarrah, Chen and Kraus [27]/USA | Mobile health (mHealth) (n = 16) Usual care (n = 9) | Usual care participants followed standard care as ordered by their treating physician. Patients receiving mHealth continued to wear their Fitbit activity trackers and were given an exercise prescription by daily step count based on their last two weeks of CR. In addition, mHealth patients received health coaching for the duration of the study though the Vida health app. | 12 weeks. | Peak VO2, physical activity patterns—steps per day, amount of moderate-low and moderate-high activity (minutes per week). | The combination of a 4.7 ± 13.8% increase in the mHealth and an 8.5 ± 11.5% decrease in the usual care group resulted in a difference between groups (p ≤ 0.05) for absolute peak VO2. Usual care decreased the amount of moderate-low physical activity minutes per week (117 ± 78 vs. 50 ± 53; p < 0.05) as well as moderate–high (111 ± 87 vs. 65 ± 64; p < 0.05). mHealth increased moderate-high physical activity (138 ± 113 vs. 159 ± 156; ns). Contradictory changes between mHealth and usual care in moderate-high physical activity minutes/week resulted in a difference between groups (21 ± 103 vs. 46 ± 36; p < 0.05). |
| Rawstorn, Gant, Rolleston, Whittaker, Stewart, Benatar, Warren, Meads, Jiang and Maddison [28]/New Zeeland | Remote-CR (n = 67) | Remote-CR comprised 12 weeks of individualized exercise prescription, real-time physiological monitoring, coaching, and behavioral support, delivered via a bespoke telerehabilitation platform (wearable sensor, mobile and web applications [apps], middleware). | Two to three supervised CR sessions of 30–60 min of aerobic exercise per week for 12 weeks | Participants completed dichotomous, categorical, and open-ended questions regarding usability and acceptability of the telerehabilitation platform. | Components of usability and acceptability were positively evaluated by most participants (44–66 of 67, 66–99%); 58 of 67 (87%) would choose Remote-CR if it was available as a usual care service, primarily because it provides convenient and flexible access to real-time individualized support from exercise specialists. Technology challenges were rare and had little effect on user experiences or demand for Remote-CR. |
| Maddison, Rawstorn, Stewart, Benatar, Whittaker, Rolleston, Jiang, Gao, Moodie, Warren, Meads and Gant [29]/Australia | Centre-based (n = 80) Remote-CR (n = 82) | Centre-based exercise cardiac rehabilitation (exCR) comprised 12 weeks of supervised exercise delivered by clinical exercise physiologists in cardiac rehabilitation clinics. Remote-CR consisted of individualized exercise prescription, exercise monitoring and coaching plus theory-based behavioral strategies to promote exercise and habitual physical activity, delivered via a customized telerehabilitation platform. | Remote-CR comprised three exercise sessions per week over 12 weeks, with follow-up assessment at 24 weeks. | Between-groupdifference in VO2max at 12 weeks; fasted blood lipid (total, high-density and low-density lipoprotein cholesterol; triglyceride) and glucose concentrations, anthropometry (height, weight, body mass index (BMI), waist/hip circumference), blood pressure (systolic/diastolic), physical activity (accelerometry), exercise-related motivation (self-efficacy, intention, confidence, locus of causality), exercise adherence, adverse events (any self-reported change in health state) and health-related quality of life (HRQoL). | VO2max was comparable in both groups at 12 weeks and the 95% CI indicated Remote-CR was non-inferior to centre-based exCR. A sensitivity analysis of complete cases supported this finding (adjusted mean difference = 0.46 (95% CI, −0.92 to 1.84) mL/kg/min, p = 0.51), suggesting it was not sensitive to attrition. Small between-group differences in waist and hip circumferences favored centre-based exCR at 12 but not 24 weeks, while a small difference in sedentary time favored Remote-CR at 24 weeks. Remaining outcomes were comparable in both groups. |
| Nolan, Feldman, Dawes, Kaczorowski, Lynn, Barr, MacPhail, Thomas, Goodman, Eysenbach, Liu, Tanaka and Surikova [30]/USA | Control + usual care (n = 97) e-Counselling + usual care (n = 100) | Intervention in both groups was organized by sessions that included a URL that linked participants to their session content. For controls, each session included content from the resource section of the Blood Pressure Action Plan of the Heart and Stroke Foundation of Canada. The e-counseling intervention was based on a combined protocol (REACH) of motivational interviewing and cognitive behavioral therapy in keeping with guidelines to promote adherence to self-care behaviors. | During the 12-month intervention, the e-program proactively contacted participants by e-mail weekly for months 1 to 4, biweekly for months 5 to 8, and monthly for months 9 to 12. | SBP, DBP, pulse pressure (PP), non-high-density lipoprotein cholesterol (non-HDL-C), total lipoprotein cholesterol (TC), low-density lipoprotein cholesterol, TC/HDL-C ratio, and the Framingham 10-year absolute risk index for cardiovascular disease (FRI). | Both control and e-counseling groups significantly decreased SBP and DBP from baseline at four and twelve months. The magnitude of SBP reduction did not differ between groups at four months, but there was significantly greater reduction for e-counseling at 12 months. The PP reduction from baseline was significant for e-counseling and control at four and twelve months. However, PP decreased to a greater degree for e-counseling at both end points. At 4 and 12 months, lipoprotein cholesterol (non-HDL-C, TC, low-density lipoprotein cholesterol, and TC/HDL-C ratio) did not deviate significantly from the non-elevated values at baseline for e-counseling and control. Nevertheless, significantly lower non-HDL-C and a trend toward significantly lower TC at four months was observed for e-counseling vs. control. No other significant group differences in lipoprotein cholesterol were observed at four or twelve months. FRI reduction was significantly greater for e-counseling vs. control at both four and twelve months. |
| Fang, Huang, Xu, Li and Au [31]/China | Usual care (n = 34) Home-based cardiac telerehabilitation (HBCTR) (n = 33) | Usual care (UC) group received a standard after percutaneous coronary intervention protocol, involving a paper-based and self-study CHD booklet and a biweekly outpatient review by assigned clinicians. The HBCTR group were also provided with the same CHD booklet to manage their lifestyle and risk factors. Additionally, they were instructed to complete outdoor walking or jogging with real-time physiological monitoring no less than thrice/week for six weeks and received two home visits by a physical therapist during a six-week interval to enhance their training in HBCTR programs, performed inside and/or outside of their homes. | Six weeks. | Exercise capacity determined by the Six-Minute Walking Test (6-MWT), SBP and DBP, anxiety and depression (CDS score), risk factors (FTND score), quality of life (SF-36 PCS and SF-36 MCS). | After the six-week intervention, the 6-MWT (distance), SF36 (PCS, MCS), FTND, and CDS in both groups had statistically improved compared with baseline data. In addition, no significant changes in blood pressure were observed in either group at six-week follow-up compared to those for baseline. After the six-week intervention, the improvement in SF36, FTND scores, and 6-MWT was significantly greater for the HBCTR than those in the UC groups (p < 0.05). However, there was no significant difference between the two groups for improvement in SBP and DBP, and CDS scores between the baseline and six-week follow-up. |
| Avila, Claes, Buys, Azzawi, Vanhees and Cornelissen [32]/Belgium | Home-based (n = 30) Centre-based (n = 30) Control group (n = 30) | HB group received an individualized exercise prescription recommending them to exercise for at least 150 min a week at a target heart rate of 70–80% of heart rate reserve (HRR). Patients randomly assigned to CB continued their training on an ambulatory basis, including three weekly sessions, consisting of approximately 45 min of endurance training at 70–80% of HRR followed by relaxation. The CG was advised to maintain a physically active lifestyle. | Three-month intervention and one-year follow-up. | Cardiorespiratory fitness or exercise capacity determined as peak VO2 assessed by a maximal graded test on a bicycle; Borg scale; physical activity levels. muscle strength: hand grip strength (kg), isometric quadriceps extension (Nm), isokinetic total work (J), sit and rising test, cardiovascular risk factors and anthropometrics. Health-related quality of life (HRQoL). | Overall, peak VO2 (mL/min/kg) and the maximal test duration remained stable over time whatever the group. Difference in responses between groups did not reach statistical significance (Pinteraction ≥ 0.05 for all). There were no differences across groups. At one year of follow-up, the number of patients fulfilling the guidelines for physical activity had decreased from 96.6% to 85% (Ptime < 0.05). No interaction effect was found for physical activity. Improvement in isometric quadriceps extension, isokinetic total work and hand grip strength reached statistical significance (Ptime ≤ 0.001) without significant differences among groups (Pinteraction ≥ 0.05). Body weight (Ptime = 0.14) increased over time with no change in other measures of body composition. SBP remained stable (Ptime = 0.36), although a significant increase was observed for diastolic blood pressure from baseline to follow-up (Ptime = 0.05). Other cardiovascular risk factors did not change significantly at one year of follow-up. All groups maintained high scores for all HRQoL parameters at one year of follow-up. |
| Lisón, Palomar, Mensorio, Baños, Cebolla-Martí, Botella, Benavent-Caballer and Rodilla [33]/Spain | Internet-based intervention group (IBI) (n = 55) Wait-list control group (WLC) (n = 50) | The intervention participants, in addition to usual medical care, received a three-month multimedia, interactive, and self-administered online intervention program, aiming to progressively establish healthy eating habits and increase the patient’s physical activity levels, as recommended by the World Health Organization’s guidelines. | Three months. | BMI and secondary outcomes (body fat mass (BFM), SBP and DBP, plasma glucose, insulin, habitual level of physical activity, and functional capacity for aerobic exercise) were measured. | The results of the two-way mixed ANCOVA showed a significant decrease in BMI, BFM, and blood glucose after three months in the IBI group, with a moderate to large effect size for BMI and BFM; the analysis also highlighted a borderline significant trend (p = 0.05) for DBP and insulin. In contrast, a significant increase in BMI and insulin among the WLC group was noted. Additionally, intragroup analysis revealed a statistically significant increase in the functional capacity for aerobic exercise both in the IBI and the WLC groups; however, no between-group differences were found. No changes were observed in either group for the level of physical activity measured with accelerometers. |
| Lunde, Bye, Bergland, Grimsmo, Jarstad and Nilsson [34]/Norway | Control group (n = 54) Intervention group (n = 48) | The intervention group (IG) received individualized follow-up enabled with an app for one year, while the control group (CG) received usual care. | 12 months. | Difference in VO2 peak; exercise performance, evaluated as time to exhaustion, peak incline (%) and peak velocity (km/h), in addition to body weight, resting BP, blood samples (lipid profile and triglycerides), exercise habits, HRQL, health status and self-perceived goal achievement. | There was a statistically significant difference in both relative and absolute VO2 peak between IG and CG from baseline to one-year follow-up, with a mean difference of 2.2 mL/kg/min, 95% confidence interval (CI) 0.9–3.5 (p = 0.001) and 0.17 L/min, 95% CI, 0.06–0.28 (p = 0.002), respectively. Statistically significant differences between groups emerged in three of the secondary outcomes: exercise performance, exercise habits and self-perceived goal achievement. |
| Laustsen, Oestergaard, van Tulder, Hjortdal and Petersen [35]/Denmark | Patients completing a telemonitored exercise-based cardiac rehabilitation program (n = 34) | Participants received basic information on how exercise impacts on their disease and health, set their own goals and choose their own exercise mode. Individual weekly feedback on exercise training intensity was given by e-mail, Skype, phone or short message service (SMS) according to patient preferences. Participants were also provided with a smartphone, an application and a heart rate monitor. | Exercise training three times weekly for 12 weeks. | VO2 peak, muscle endurance, muscle power, muscle strength, HRQoL physical and mental component. | Significant increase in VO2 peak of 10%, in muscle endurance of 17%, in muscle power of 7%, and in muscle strength of 10% after the TCR program. HRQoL was significantly improved by 19% in the physical and 17% in the mental component scores. |
| Szalewska, Główczyńska, Piotrowicz, Kowalik, Pencina, Opolski, Zaręba, Banach, Orzechowski, Pluta, Irzmański, Kalarus and Piotrowicz [36]/Poland | Ischaemic (IS) aetiology group (n = 555) IS-HCTR n = 281 IS-UC n = 274 Non-ischaemic (NIS) aetiology group (n = 295) NIS-HCTR n = 144 NIS-UC n = 151 | Hybrid comprehensive telerehabilitation (HCTR) program in heart failure (HF). Patients with both aetiologies (IS and NIS) underwent a HCTR program, which comprised two stages: an initial stage (one week) conducted in a hospital and a basic, home-based stage (eight weeks) in which HCTR was performed five times weekly. | A nine-week HCTR program, comprising an initial stage (one week) conducted in a hospital and a basic, followed by a home-based stage (eight weeks) in which HCTR was performed five times weekly. | All-cause and CV mortalities, as well as for all-cause, CV, and heart failure hospitalizations. Functional test: six-min walking test. | For all-cause and CV mortalities, as well as for all-cause, CV, and HF hospitalizations, differences were not statistically significant for either aetiology and between aetiologies; HCTR improved functional status alone in patients with IS HF aetiology; however, the magnitude of the changes in the clinical and functional statuses of HF patients did not differ between the IS and NIS groups. |
| Myers, Chan, Chen, Lit, Patti, Massaband, Kiratli, Tamura, Chertow and Rabkin [37]/USA | Exercise group (n = 13) Usual care group (n = 15) | Exercise group performed a home-based combination of aerobic and resistance exercise for a minimum of 45 min with hand-held weights, Thera-bands, and portable cycle ergometers. Intensity: 70–80% of HR reserve and 12–14 on the Borg scale. | Daily activity logs were used during 12-weeks. | Functional tests: one-min sit-to-stand test, sit-to-stand duration of five repetitions, six-min walk test. Strength: hand grip, upper body strength, lower body strength. Pulmonary function: forced vital capacity, forced expiratory volume. Body composition: total leg mass kg, total body mass kg, total body fat %. Cardiopulmonary exercise test responses at the ventilatory threshold; cardiopulmonary exercise test responses at peak exercise. | The exercise group generally improved their performance on functional and strength evaluations and the usual care group was generally unchanged, the differences between groups were not significant. Body composition indices were not different between groups. Both FEV1 and FVC tended to improve in the exercise group after the training period (by 20 and 28%; p = 0.53 and 0.07 between groups, respectively). |
| Hong, Jakacic, Sahoo, Breyman, Ukegbu, Tabacof, Sachs, Migliaccio, Phipps, Schwartz, Capasso, Carpenter and Putrino [38]/USA | All participants (n = 94) Non-Fitbit users (n = 64) Fitbit users (n = 30) | Evaluate the Fitbit technology impact in health biometrics of older adults. | 3–6 months (short term); >6 months (long term). | Difference between pre- and post-values in SBP and DBP, heart rate, weight, and blood oxygen saturation. | No differences were found between Fitbit users and non-users; SBP was on average 6.5 mmHg lower (p < 0.004) in all participants, regardless of Fitbit usage. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viana, S.; Salvador, R.; Morouço, P.; Rebelo-Gonçalves, R. The Contribution of Exercise in Telemedicine Monitoring in Reducing the Modifiable Factors of Hypertension—A Multidisciplinary Approach. Eur. J. Investig. Health Psychol. Educ. 2022, 12, 363-386. https://doi.org/10.3390/ejihpe12040027
Viana S, Salvador R, Morouço P, Rebelo-Gonçalves R. The Contribution of Exercise in Telemedicine Monitoring in Reducing the Modifiable Factors of Hypertension—A Multidisciplinary Approach. European Journal of Investigation in Health, Psychology and Education. 2022; 12(4):363-386. https://doi.org/10.3390/ejihpe12040027
Chicago/Turabian StyleViana, Silvane, Rogério Salvador, Pedro Morouço, and Ricardo Rebelo-Gonçalves. 2022. "The Contribution of Exercise in Telemedicine Monitoring in Reducing the Modifiable Factors of Hypertension—A Multidisciplinary Approach" European Journal of Investigation in Health, Psychology and Education 12, no. 4: 363-386. https://doi.org/10.3390/ejihpe12040027
APA StyleViana, S., Salvador, R., Morouço, P., & Rebelo-Gonçalves, R. (2022). The Contribution of Exercise in Telemedicine Monitoring in Reducing the Modifiable Factors of Hypertension—A Multidisciplinary Approach. European Journal of Investigation in Health, Psychology and Education, 12(4), 363-386. https://doi.org/10.3390/ejihpe12040027










