Acute Relaxation Response Induced by Tibetan Singing Bowl Sounds: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
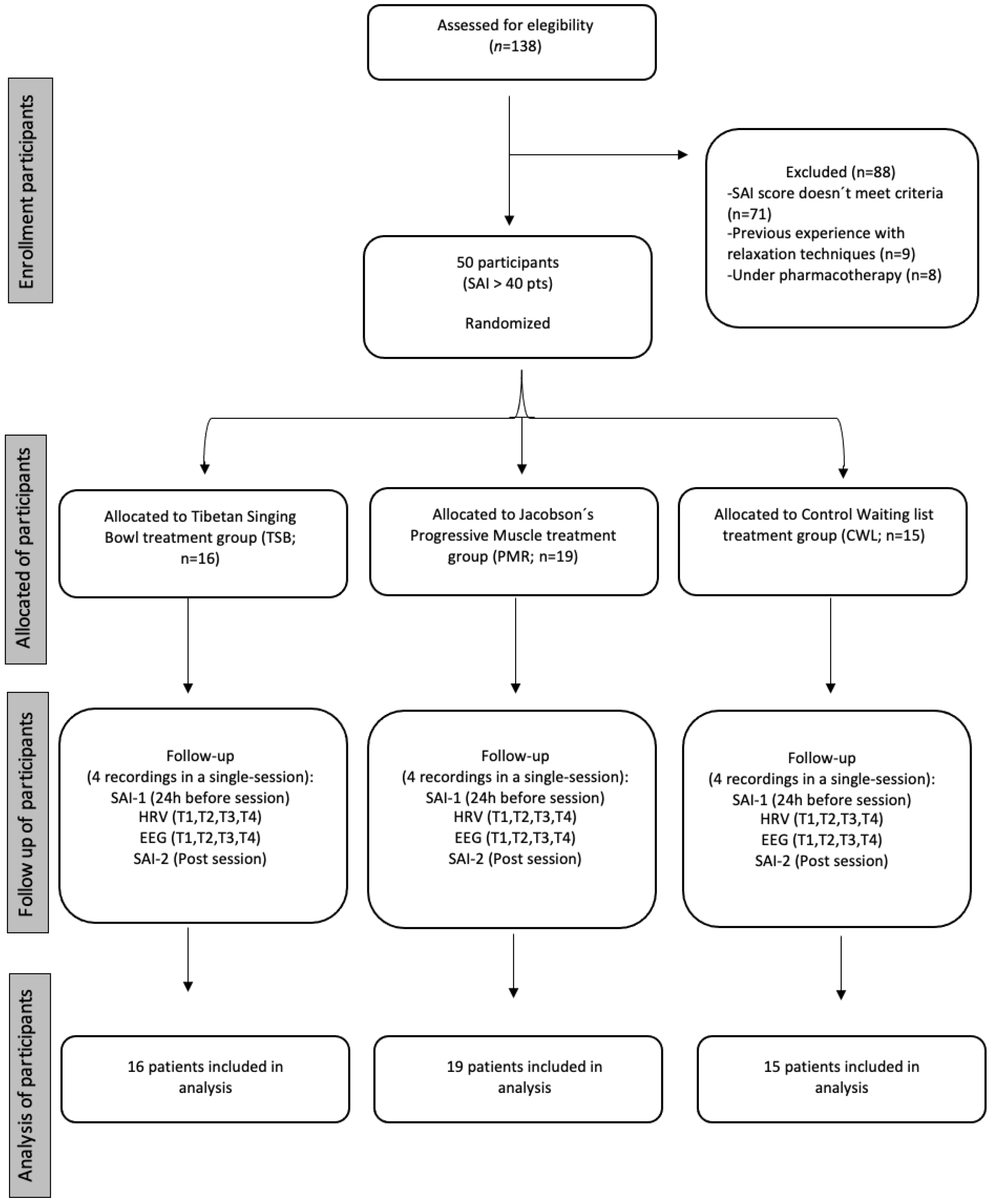
2.1. Design and Participants
2.2. Outcome Measures
2.2.1. Heart Rate Variability (HRV)
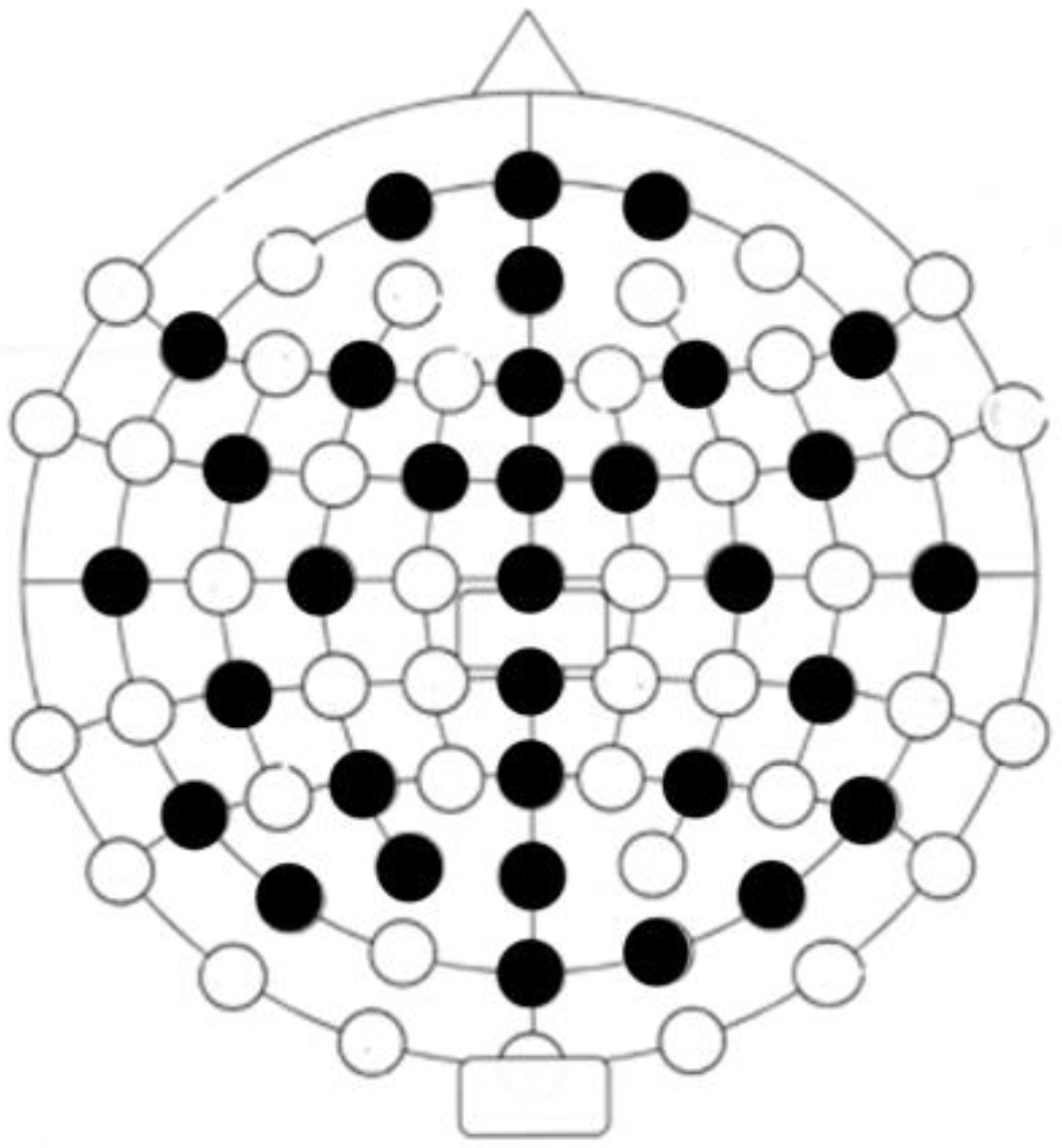
2.2.2. Electroencephalography (EEG)
2.2.3. Spielberg’s State Anxiety Inventory (SAI)
2.3. Interventions
2.3.1. Tibetan Singing Bowl (TSB)
2.3.2. Jacobson’s Progressive Muscle Relaxation (PMR)
2.3.3. Control Waiting List (CWL)
2.4. Procedure
2.5. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calhoon, G.G.; Tye, K.M. Resolving the neural circuits of anxiety. Nat. Neurosci. 2015, 18, 1394–1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, J.A. The Neuropsychology of Anxiety: An Enquiry into the Functions into the Septo-Hippocampal System; Cambridge University Press: Cambridge, UK, 1982. [Google Scholar]
- Gray, J.A.; McNaughton, N. The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septo-Hippocampal System, 2nd ed.; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- LeDoux, J.E. Emotion circuits in the brain. Ann. Rev. Neurosci. 2000, 23, 155–184. [Google Scholar] [CrossRef]
- Baxter, A.J.; Scott, K.M.; Vos, T.; Whiteford, H.A. Global prevalence of anxiety disorders: A systematic review and meta-regression. Psychol. Med. 2013, 43, 897–910. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zhou, F.; Hou, W.; Silver, Z.; Wong, C.Y.; Chang, C.; Huang, E.; Zuo, Q.K. The prevalence of depression, anxiety, and sleep disturbances in COVID-19 patients: A meta-analysis. Ann. N. Y. Acad. Sci. 2021, 1486, 90–111. [Google Scholar] [CrossRef] [PubMed]
- Santabárbara, J.; Lasheras, I.; Lipnicki, D.M.; Bueno-Notivol, J.; Pérez-Moreno, M.; López-Antón, R.; De la Cámara, C.; Lobo, A.; Gracia-García, P. Prevalence of anxiety in the COVID-19 pandemic: An updated meta-analysis of community-based studies. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 109, 110207. [Google Scholar] [CrossRef] [PubMed]
- LeDoux, J.; Daw, N.D. Surviving threats: Neural circuit and computational implications of a new taxonomy of defensive behaviour. Nat. Rev. Neurosci. 2018, 19, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Niles, B.L.; Mori, D.L.; Polizzi, C.; Kaiser, A.P.; Weinstein, E.S.; Gershkovich, M.; Wang, C. A systematic review of randomized trials of mind-body interventions for PTSD. J. Clin. Psychol. 2018, 74, 1485–1508. [Google Scholar] [CrossRef]
- Ravindran, L.N.; Stein, M.B. The pharmacologic treatment of anxiety disorders: A review of progress. J. Clin. Psychiatry 2010, 71, 14308. [Google Scholar] [CrossRef]
- Treit, D.; Engin, E.; McEown, K. Animal Models of Anxiety and Anxiolytic Drug Action. Behav. Neurobiol. Anxiety Its Treat. 2009, 2, 121–160. [Google Scholar] [CrossRef]
- Benson, H.; Greenwood, M.M.; Klemchuk, H. The Relaxation Response: Psychophysiologic Aspects and Clinical Applications. Int. J. Psychiatry Med. 1975, 6, 87–98. [Google Scholar] [CrossRef]
- Benson, H. The Relaxation Response: History, Physiological Basis and Clinical Usefulness. Acta Med. Scand. 2009, 211, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, G.D.; Friedman, R. EEG Spectral Analysis of Relaxation Techniques. Appl. Psychophysiol. Biofeedback 2004, 29, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, G.M.; Pagnini, F.; Castelnuovo, G.; Molinari, E. Relaxation training for anxiety: A ten-years systematic review with meta-analysis. BMC Psychiatry 2008, 8, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esch, T.; Fricchione, G.L.; Stefano, G.B. The therapeutic use of the relaxation response in stress-related diseases. Med. Sci. Monit. 2003, 9, RA23–RA34. [Google Scholar] [PubMed]
- Cotoia, A.; Dibello, F.; Moscatelli, F.; Sciusco, A.; Polito, P.; Modolo, A.; Gallo, C.; Cibelli, G.; Cinnella, G. Effects of Tibetan Music on Neuroendocrine and Autonomic Functions in Patients Waiting for Surgery: A Randomized, Controlled Study. Anesthesiol. Res. Pract. 2018, 2018, 9683780. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, E.J. Effects of relaxation therapy on anxiety disorders: A systematic review and meta-analysis. Arch. Psychiatr. Nurs. 2018, 32, 278–284. [Google Scholar] [CrossRef]
- Knyazev, G.G.; Savostyanov, A.N.; Levin, E.A. Alpha oscillations as a correlate of trait anxiety. Int. J. Psychophysiol. 2004, 53, 147–160. [Google Scholar] [CrossRef]
- Knyazev, G.G.; Savostyanov, A.N.; Levin, E.A. Anxiety and synchrony of alpha oscillations. Int. J. Psychophysiol. 2005, 57, 175–180. [Google Scholar] [CrossRef]
- Conrad, A.; Roth, W.T. Muscle relaxation therapy for anxiety disorders: It works but how? J. Anxiety Disord. 2007, 21, 243–264. [Google Scholar] [CrossRef]
- Chalmers, J.A.; Quintana, D.S.; Abbott, M.J.-A.; Kemp, A.H. Anxiety Disorders are Associated with Reduced Heart Rate Variability: A Meta-Analysis. Front. Psychiatry 2014, 5, 80. [Google Scholar] [CrossRef]
- Pagnini, F.; Molinari, E. Efficacy of relaxation techniques in different clinical situations. Riv. Psichiatr. 2013, 48, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, L.; Nguyen, Q.A.; Roettger, C.; Dixon, K.; Offenbächer, M.; Kohls, N.; Hirsch, J.; Sirois, F. Effectiveness of Progressive Muscle Relaxation, Deep Breathing, and Guided Imagery in Promoting Psychological and Physiological States of Relaxation. Evid.-Based Complement. Altern. Med. 2021, 2021, 5924040. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.R.; Hoyle, R.H. Efficacy of abbreviated progressive muscle relaxation training: A quantitative review of behavioral medicine research. J. Consult. Clin. Psychol. 1993, 61, 1059. [Google Scholar] [CrossRef] [PubMed]
- Carrington, P.; Collings, G.H.; Benson, H.; Robinson, H.; Wood, L.W.; Lehrer, P.M.; WoolFolk, R.L.; Cole, J.W. The use of meditation—Relaxation techniques for the management of stress in a working population. J. Occup. Med. 1980, 22, 221–231. [Google Scholar] [PubMed]
- Chellew, K.; Evans, P.; Fornes-Vives, J.; Pérez, G.; Garcia-Banda, G. The effect of progressive muscle relaxation on daily cortisol secretion. Stress 2015, 18, 538–544. [Google Scholar] [CrossRef]
- Pawlow, L.A.; Jones, G.E. The impact of abbreviated progressive muscle relaxation on salivary cortisol. Biol. Psychol. 2002, 60, 1–16. [Google Scholar] [CrossRef]
- Pawlow, L.A.; Jones, G.E. The Impact of Abbreviated Progressive Muscle Relaxation on Salivary Cortisol and Salivary Immunoglobulin A (sIgA). Appl. Psychophysiol. Biofeedback 2005, 30, 375–387. [Google Scholar] [CrossRef]
- Korkut, S.; Ülker, T.; Çidem, A.; Şahin, S. The effect of progressive muscle relaxation and nature sounds on blood pressure measurement skills, anxiety levels, and vital signs in nursing students. Perspect. Psychiatr. Care 2021, 57, 1782–1790. [Google Scholar] [CrossRef]
- Lee, E.-J.; Bhattacharya, J.; Sohn, C.; Verres, R. Monochord sounds and progressive muscle relaxation reduce anxiety and improve relaxation during chemotherapy: A pilot EEG study. Complement. Ther. Med. 2012, 20, 409–416. [Google Scholar] [CrossRef]
- Goldsby, T.; Goldsby, M.E.; McWalters, M.; Mills, P.J. Effects of Singing Bowl Sound Meditation on Mood, Tension, and Well-being: An Observational Study. J. Evid. Based Complement. Altern. Med. 2016, 22, 401–406. [Google Scholar] [CrossRef]
- Perry, F. Himalayan Sound Revelations: The Complete Singing Bowl Book, 2nd ed.; Polair Publishing: London, UK, 2014. [Google Scholar]
- Stanhope, J.; Weinstein, P. The human health effects of singing bowls: A systematic review. Complement. Ther. Med. 2020, 51, 102412. [Google Scholar] [CrossRef] [PubMed]
- Terwagne, D.; Bush, J.W.M. Tibetan singing bowls. Nonlinearity 2011, 24, R51–R66. [Google Scholar] [CrossRef]
- Kesmodel, U.S. Cross-sectional studies-What are they good for? Acta Obstet. Gynecol. Scand. 2018, 7, 388–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Cheng, Z. Cross-Sectional Studies: Strengths, Weaknesses, and Recommendations. Chest 2020, 158, S65–S71. [Google Scholar] [CrossRef] [PubMed]
- Tola, Y.O.; Chow, K.M.; Liang, W. Effects of non-pharmacological interventions on preoperative anxiety and postoperative pain in patients undergoing breast cancer surgery: A systematic review. J. Clin. Nurs. 2021, 30, 3369–3384. [Google Scholar] [CrossRef]
- Zhou, K.; Li, X.; Li, J.; Liu, M.; Dang, S.; Wang, D.; Xin, X. A clinical randomized controlled trial of music therapy and progressive muscle relaxation training in female breast cancer patients after radical mastectomy: Results on depression, anxiety and length of hospital stay. Eur. J. Oncol. Nurs. 2014, 19, 54–59. [Google Scholar] [CrossRef]
- Tarvainen, M.P.; Niskanen, J.-P.; Lipponen, J.A.; Ranta-Aho, P.O.; Karjalainen, P.A. Kubios HRV–Heart rate variability analysis software. Comput. Methods Progr. Biomed. 2014, 113, 210–220. [Google Scholar] [CrossRef]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart Rate Variability and Cardiac Vagal Tone in Psychophysiological Research—Recommendations for Experiment Planning, Data Analysis, and Data Reporting. Front. Psychol. 2017, 8, 213. [Google Scholar] [CrossRef] [Green Version]
- Oostenveld, R.; Fries, P.; Maris, E.; Schoffelen, J.-M. FieldTrip: Open Source Software for Advanced Analysis of MEG, EEG, and Invasive Electrophysiological Data. Comput. Intell. Neurosci. 2011, 156869. [Google Scholar] [CrossRef]
- Spielberger, C.D. State-trait anxiety inventory. In The Corsini Encyclopedia of Psychology; American Cancer Society: Atlanta, GA, USA, 2010; p. 1. [Google Scholar]
- Jacobson, E. Progressive Relaxation. Am. J. Psychol. 1987, 100, 522. [Google Scholar] [CrossRef]
- Porges, S.W. Vagal tone: An autonomic mediator of affect. In The Development of Emotion Regulation and Dysregulation; Garber, J., Dodge, K.A., Eds.; Cambridge University Press: Cambridge, UK, 1991; pp. 111–128. [Google Scholar] [CrossRef]
- Porges, S. Autonomic Regulation and Attention; Campbell, B.A., Hayne, H., Richardson, R., Eds.; Psychology Press: East Sussex, UK, 1992; pp. 201–223. [Google Scholar]
- Friedman, B.H.; Thayer, J.F. Autonomic balance revisited: Panic anxiety and heart rate variability. J. Psychosom. Res. 1998, 44, 133–151. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Jeong, H.C.; Yim, J.E.; Jeon, M.Y. Effects of Music Therapy on the Cardiovascular and Autonomic Nervous System in Stress-Induced University Students: A Randomized Controlled Trial. J. Altern. Complement. Med. 2016, 22, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Cygankiewicz, I.; Zareba, W. Heart rate variability. Handb. Clin. Neurol. 2013, 117, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Su, M.; Liu, C.; Huang, Y.; Huang, W. Heart rate variability in patients with anxiety disorders: A systematic review and meta-analysis. Psychiatry Clin. Neurosci. 2022, 76, 292–302. [Google Scholar] [CrossRef]
- Groß, D.; Kohlmann, C.-W. Increasing Heart Rate Variability through Progressive Muscle Relaxation and Breathing: A 77-Day Pilot Study with Daily Ambulatory Assessment. Int. J. Environ. Res. Public Health 2021, 18, 11357. [Google Scholar] [CrossRef]
- Zou, L.; Sasaki, J.E.; Wei, G.-X.; Huang, T.; Yeung, A.S.; Neto, O.B.; Chen, K.W.; Hui, S.S.-C. Effects of Mind–Body Exercises (Tai Chi/Yoga) on Heart Rate Variability Parameters and Perceived Stress: A Systematic Review with Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2018, 7, 404. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, G.Y.; Saboo, B. A Comparative Study of the Impact of Himalayan Singing Bowls and Supine Silence on Stress Index and Heart Rate Variability. J. Behav. Ther. Ment. Health 2018, 2, 40–50. [Google Scholar] [CrossRef] [Green Version]
- Panchal, S.; Irani, F.; Trivedi, G.Y. Impact of Himalayan Singing Bowls Meditation Session on Mood and Heart Rate Variability—An Observational Study. Int. J. Psychother. Pr. Res. 2019, 1, 20–30. [Google Scholar] [CrossRef] [Green Version]
- Cahn, B.R.; Polich, J. Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychol. Bull. 2006, 132, 180–211. [Google Scholar] [CrossRef]
- Inanaga, K. Frontal midline theta rhythm and mental activity. Psychiatry Clin. Neurosci. 1998, 52, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Sato, W.; Toichi, M.; Murai, T.; Okada, T.; Hayashi, A.; Sengoku, A. Frontal midline theta rhythm is correlated with cardiac autonomic activities during the performance of an attention demanding meditation procedure. Cogn. Brain Res. 2001, 11, 281–287. [Google Scholar] [CrossRef]
- Suetsugi, M.; Mizuki, Y.; Ushijima, I.; Yamada, M.; Imaizumi, J. Anxiolytic effects of low-dose clomipramine in highly anxious healthy volunteers assessed by frontal midline theta activity. Prog. Neuro. Psychopharmacol. Biol. Psychiatry 1998, 22, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Suetsugi, M.; Mizuki, Y.; Ushijima, I.; Kobayashi, T.; Tsuchiya, K.; Aoki, T.; Watanabe, Y. Appearance of Frontal Midline Theta Activity in Patients with Generalized Anxiety Disorder. Neuropsychobiology 2000, 41, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Bell, I.R.; Schwartz, G.E.; Hardin, E.E.; Baldwin, C.; Kline, J.P. Differential Resting Quantitative Electroencephalographic Alpha Patterns in Women with Environmental Chemical Intolerance, Depressives, and Normals. Biol. Psychiatry 1998, 43, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, W.M.; Winterer, G. Electroencephalography in psychiatry—Current status and outlook. Der Nervenarzt 1996, 67, 348–359. [Google Scholar]
- Knyazev, G.G.; Savostyanov, A.N.; Levin, E.A. Uncertainty, anxiety, and brain oscillations. Neurosci. Lett. 2005, 387, 121–125. [Google Scholar] [CrossRef]
- Knyazev, G.G.; Slobodskaya, H.R. Personality trait of behavioral inhibition is associated with oscillatory systems reciprocal relationships. Int. J. Psychophysiol. 2003, 48, 247–261. [Google Scholar] [CrossRef]
- Barrós-Loscertales, A.; Meseguer, V.; Sanjuán, A.; Belloch, V.; Parcet, M.; Torrubia, R.; Ávila, C. Behavioral Inhibition System activity is associated with increased amygdala and hippocampal gray matter volume: A voxel-based morphometry study. Neuroimage 2006, 33, 1011–1015. [Google Scholar] [CrossRef]
- Knyazev, G.G.; Savostyanov, A.N.; Levin, E.A. Alpha synchronization and anxiety: Implications for inhibition vs. alertness hypotheses. Int. J. Psychophysiol. 2006, 59, 151–158. [Google Scholar] [CrossRef]
- Nyklícek, I.; Vingerhoets, A.J.; van Heck, G.L.; Kamphuis, P.L.; van Poppel, J.W.; van Limpt, M.C. Blood pressure, self-reported symptoms and job-related problems in schoolteachers. J. Psychosom. Res. 1997, 42, 287–296. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. A model of neurovisceral integration in emotion regulation and dysregulation. J. Affect. Disord. 2000, 61, 201–216. [Google Scholar] [CrossRef] [PubMed]

| Tibetan Singing Bowl nº-(Diameter) | Tone Frequency (Hz) | 1º Overtone (Hz) | 2º Overtone (Hz) | 3º Overtone (Hz) | 4º Overtone (Hz) |
|---|---|---|---|---|---|
| 1-(23 cm) | 99 | 263 | 465 | 737 | 971 |
| 2-(15 cm) | 219 | 603 | 1115 | 1713 | 2515 |
| 3-(12 cm) | 465 | 1185 | 2224 | 3471 | 4865 |
| 4-(8 cm) | 927 | 1879 | 2401 | 4437 | - |
| Mean ± (SEM) | ANOVA Repeated Measures | One-Way ANOVA | |||
|---|---|---|---|---|---|
| Dependent variable | TSB (n = 16) | PMR (n = 19) | CWL (n = 15) | “Effect” (F) * p | (F) * p |
| RMSSD (T1) | 35.2 (3.55) | 36.19 (3.04) | 34.81 (3.63) | “Treatment” (3.6) * 0.04 “Time × treatment” (4.2) * 0.001 Figure 3A | |
| RMSSD (T2) | 36.54 (5.17) | 32 (2.20) | 30.5 (2.50) | ||
| RMSSD (T3) | 48.4 (5.81) | 34.07 (2.04) | 29.4 (2.30) | (8.0) * 0.001 | |
| RMSSD (T4) | 42.02 (2.78) | 37.12 (2.76) | 28.3 (2.65) | (6.5) * 0.003 | |
| pNN50 (T1) | 14.7 (2.69) | 12.05 (2.33) | 19.90 (3.4) | “Time × treatment” (5.2) * 0.001 | |
| pNN50 (T2) | 16.5 (3.7) | 8.84 (2.30) | 10.8 (2.2) | ||
| pNN50 (T3) | 29.05 (5.55) | 10.2 (2.73) | 16.74 (4.77) | ||
| pNN50 (T4) | 16.94 (2.06) | 16.16 (3.04) | 14.62 (4.4) | ||
| HRV-HF(log) (T1) | 6.3 (0.11) | 6.21 (0.07) | 6.14 (0.15) | “Treatment” (7.4) * 0.002 Figure 3B | |
| HRV-HF(log) (T2) | 6.51 (0.3) | 6.28 (0.47) | 6.07 (0.08) | ||
| HRV-HF(log) (T3) | 6.87 (0.23) | 6.16 (0.22) | 6.08 (0.14) | (8.5) * 0.001 | |
| HRV-HF(log) (T4) | 6.76 (0.22) | 6.33 (0.15) | 6.14 (0.12) | (8.3) * 0.001 | |
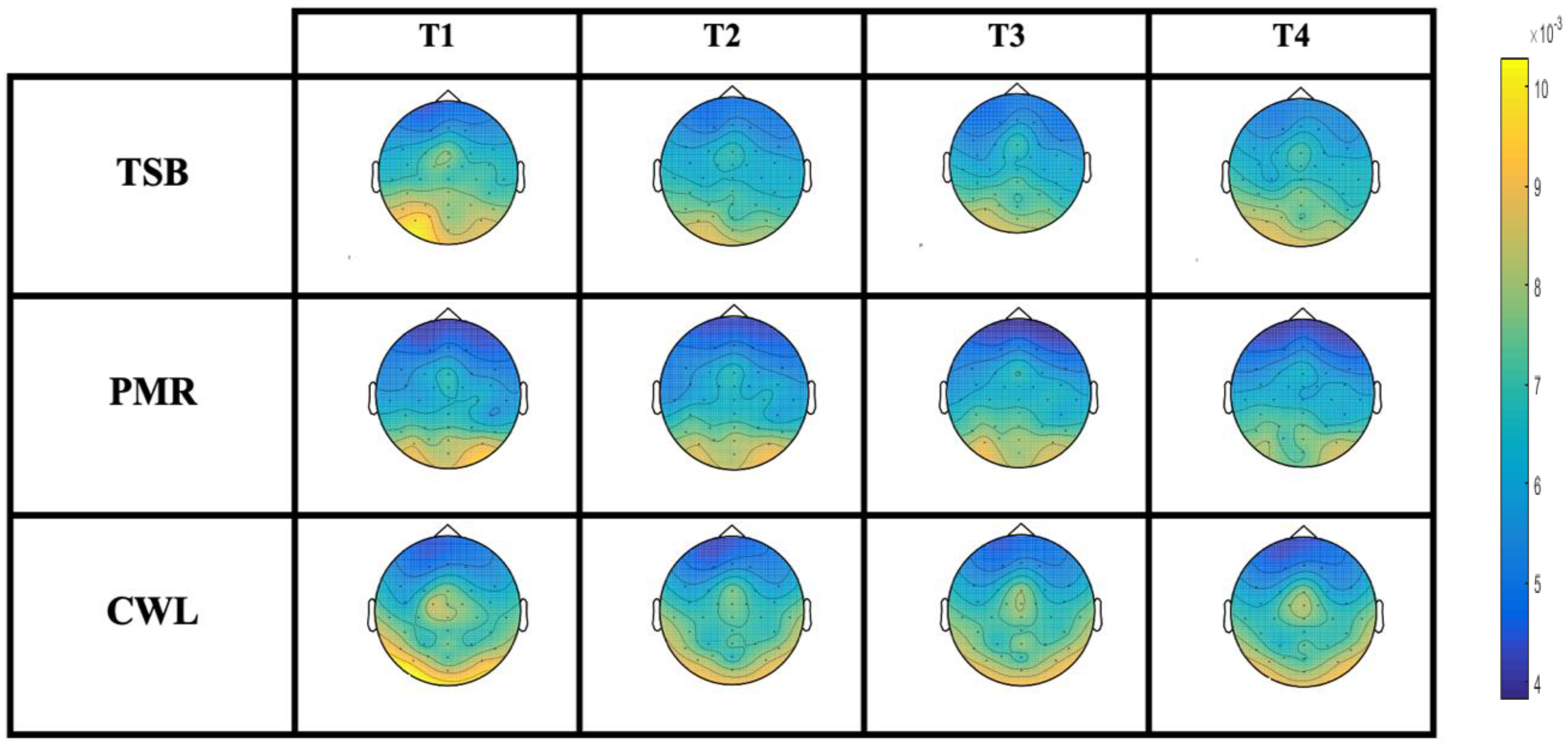
| Alpha (T1) | 0.01 (0.0013) | 0.009 (0.0011) | 0.007 (0.0012) | “Time × treatment” (4.9) * 0.001 Figure 3C | |
| Alpha (T2) | 0.007 (0.0013) | 0.008 (0.0013) | 0.008 (0.0014) | ||
| Alpha (T3) | 0.006 (0.001) | 0.007 (0.0011) | 0.008 (0.0016) | ||
| Alpha (T3) | 0.006 (0.001) | 0.007 (0.0011) | 0.008 (0.0012) | ||
| Alpha (T1–T2) | 0.0028 (0.0005) | 0.0011 (0.0007) | 0.0013 (0.0008) | (3.9) * 0.03 | |
| Alpha (T1–T3) | 0.0039 (0.0006) | 0.002 (0.0006) | 0.0003 (0.0006) | (8.0) * 0.001 | |
| Alpha (T1–T4) | 0.0034 (0.0005) | 0.0019 (0.0006) | 0.0001 (0.0005) | (8.4) * 0.001 Figure 3D | |
| SAI-1 | 44.94 (1.1) | 47.3 (1.7) | 46.3 (0.82) | “Treatment” (9.2) * 0.001 “Time × treatment” (11.61) * 0.001 Figure 3E | |
| SAI-2 | 35.37 (0.27) | 40.0 (0.78) | 44.8 (0.80) | (45.9) * 0.001 | |
| SAI-R | 9.56 (1.1) | 7.68 (1.3) | 1.73 (0.85) | (11.6) * 0.001 Figure 3F | |
| Factor 1 | Factor 2 | |
|---|---|---|
| SAI-1 | 0.53 | 0.42 |
| SAI-2 | −0.47 | 0.81 |
| Alpha (T1) | - | - |
| Alpha reduction (T1–T3) | - | −0.82 |
| RMSSD (T1) | 0.83 | - |
| RMSSD (T3) | 0.86 | - |
| % of variance (cumulative) | 37.18 | 59.57 |
| Correlation | −0.13 | |
| N sample = | 27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rio-Alamos, C.; Montefusco-Siegmund, R.; Cañete, T.; Sotomayor, J.; Fernandez-Teruel, A. Acute Relaxation Response Induced by Tibetan Singing Bowl Sounds: A Randomized Controlled Trial. Eur. J. Investig. Health Psychol. Educ. 2023, 13, 317-330. https://doi.org/10.3390/ejihpe13020024
Rio-Alamos C, Montefusco-Siegmund R, Cañete T, Sotomayor J, Fernandez-Teruel A. Acute Relaxation Response Induced by Tibetan Singing Bowl Sounds: A Randomized Controlled Trial. European Journal of Investigation in Health, Psychology and Education. 2023; 13(2):317-330. https://doi.org/10.3390/ejihpe13020024
Chicago/Turabian StyleRio-Alamos, Cristobal, Rodrigo Montefusco-Siegmund, Toni Cañete, Joaquín Sotomayor, and Alberto Fernandez-Teruel. 2023. "Acute Relaxation Response Induced by Tibetan Singing Bowl Sounds: A Randomized Controlled Trial" European Journal of Investigation in Health, Psychology and Education 13, no. 2: 317-330. https://doi.org/10.3390/ejihpe13020024
APA StyleRio-Alamos, C., Montefusco-Siegmund, R., Cañete, T., Sotomayor, J., & Fernandez-Teruel, A. (2023). Acute Relaxation Response Induced by Tibetan Singing Bowl Sounds: A Randomized Controlled Trial. European Journal of Investigation in Health, Psychology and Education, 13(2), 317-330. https://doi.org/10.3390/ejihpe13020024








