Muscular and Prefrontal Cortex Activity during Dual-Task Performing in Young Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Tasks Protocol
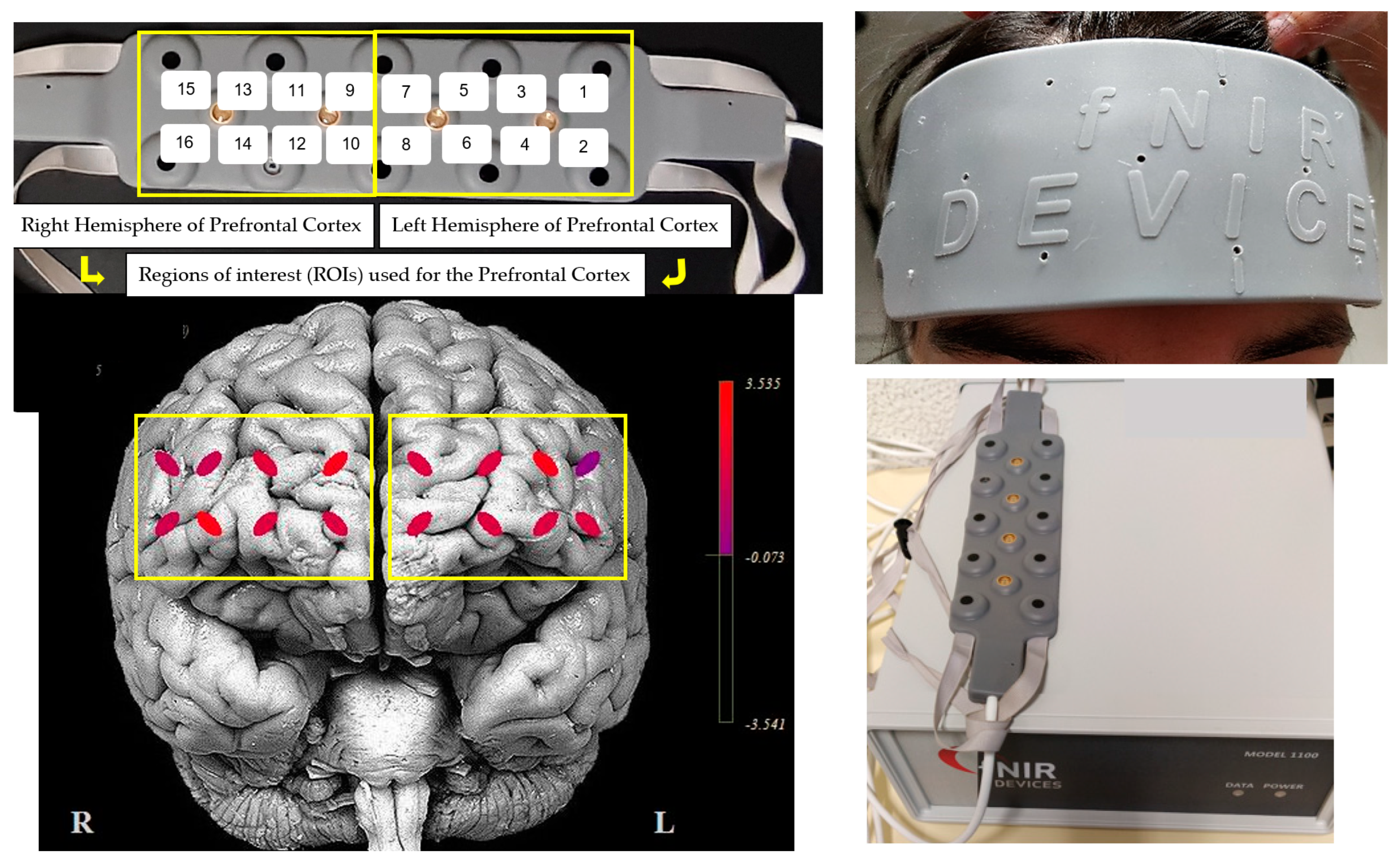
2.3. Prefrontal Cortex Acquisition and Analysis
2.4. Muscular Activity Assessment
2.5. Statistical Analysis
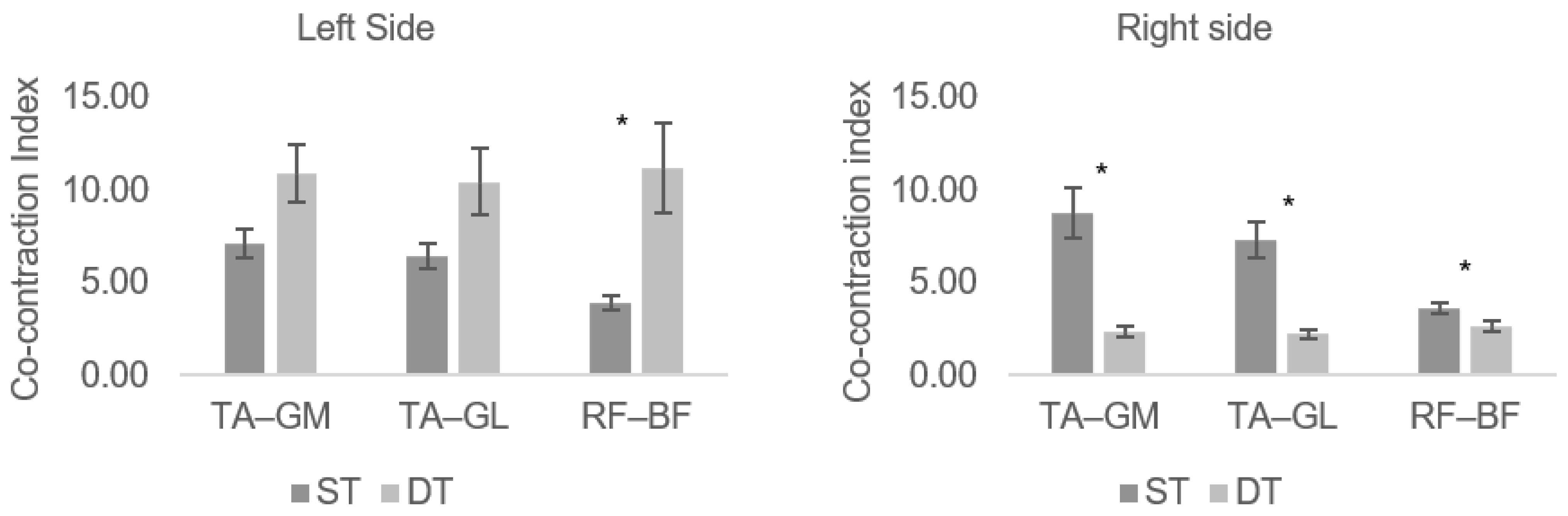
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plummer, P.; Eskes, G.; Wallace, S.; Giuffrida, C.; Fraas, M.; Campbell, G.; Clifton, K.-L.; Skidmore, E.R. Cognitive-motor interference during functional mobility after stroke: State of the science and implications for future research. Arch. Phys. Med. Rehabil. 2013, 94, 2565–2574.e6. [Google Scholar] [CrossRef] [Green Version]
- Reilly, D.S.; Woollacott, M.H.; van Donkelaar, P.; Saavedra, S. The interaction between executive attention and postural control in dual-task conditions: Children with cerebral palsy. Arch. Phys. Med. Rehabil. 2008, 89, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Behrangrad, S.; Zoghi, M.; Mansouri, F.; Jaberzadeh, S. How increased cognitive load affects the dual-task cost in healthy? A randomized, double-blind sham-controlled study. bioRxiv 2021. [Google Scholar] [CrossRef]
- Woollacott, M.; Shumway-Cook, A. Attention and the control of posture and gait: A review of an emerging area of research. Gait Posture 2002, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rankin, J.K.; Woollacott, M.H.; Shumway-Cook, A.; Brown, L.A. Cognitive influence on postural stability: A neuromuscular analysis in young and older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, M112–M119. [Google Scholar] [CrossRef] [PubMed]
- Ghai, S.; Ghai, I.; Effenberg, A.O. Effects of dual tasks and dual-task training on postural stability: A systematic review and meta-analysis. Clin. Interv. Aging 2017, 12, 557–577. [Google Scholar] [CrossRef] [Green Version]
- Horak, F.B. Postural orientation and equilibrium: What do we need to know about neural control of balance to prevent falls? Age Ageing 2006, 35 (Suppl. 2), ii7–ii11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baudry, S.; Lecoeuvre, G.; Duchateau, J. Age-related changes in the behavior of the muscle-tendon unit of the gastrocnemius medialis during upright stance. J. Appl. Physiol. 2012, 112, 296–304. [Google Scholar] [CrossRef]
- Iwamoto, Y.; Takahashi, M.; Shinkoda, K. Differences of muscle co-contraction of the ankle joint between young and elderly adults during dynamic postural control at different speeds. J. Physiol. Anthropol. 2017, 36, 32. [Google Scholar] [CrossRef] [Green Version]
- Ozdemir, R.A.; Contreras-Vidal, J.L.; Lee, B.-C.; Paloski, W.H. Cortical activity modulations underlying age-related performance differences during posture–cognition dual tasking. Exp. Brain Res. 2016, 234, 3321–3334. [Google Scholar] [CrossRef]
- Lo, J.; Lo, O.Y.; Olson, E.A.; Habtemariam, D.; Iloputaife, I.; Gagnon, M.M.; Manor, B.; Lipsitz, L.A. Functional implications of muscle co-contraction during gait in advanced age. Gait Posture 2017, 53, 110–114. [Google Scholar] [CrossRef] [Green Version]
- Makizako, H.; Furuna, T.; Ihira, H.; Shimada, H. Age-related Differences in the Influence of Cognitive Task Performance on Postural Control Under Unstable Balance Conditions. Int. J. Gerontol. 2013, 7, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Hermens, H.J. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Drost, G.; Stegeman, D.F.; van Engelen, B.G.M.; Zwarts, M.J. Clinical applications of high-density surface EMG: A systematic review. J. Electromyogr. Kinesiol. 2006, 6, 586–602. [Google Scholar] [CrossRef]
- Fujita, N.; Kuminuse, S.; Okakada, S. Contribution of the dorsolateral prefrontal cortex activation, ankle muscle activities, and coactivation during dual-tasks to postural steadiness: A pilot study. J. Phys. Ther. Sci. 2020, 32, 467–472. [Google Scholar] [CrossRef]
- Leff, D.R.; Orihuela-Espina, F.; Elwell, C.E.; Athanasiou, T.; Delpy, D.T.; Darzi, A.W.; Yang, G.Z. Assessment of the cerebral cortex during motor task behaviours in adults: A systematic review of functional near infrared spectroscopy (fNIRS) studies. NeuroImage 2011, 54, 2922–2936. [Google Scholar] [CrossRef] [PubMed]
- Herold, F.; Orlowski, K.; Börmel, S.; Müller, N.G. Cortical activation during balancing on a balance board. Hum. Mov. Sci. 2017, 51, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Nieuwhof, F.; Reelick, M.F.; Maidan, I.; Mirelman, A.; Hausdorff, J.M.; Rikkert, M.G.O.; Bloem, B.R.; Muthalib, M.; Claassen, J.A. Measuring prefrontal cortical activity during dual task walking in patients with Parkinson’s disease: Feasibility of using a new portable fNIRS device. Pilot Feasibility Stud. 2016, 2, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holtzer, R.; Mahoney, J.R.; Izzetoglu, M.; Izzetoglu, K.; Onaral, B.; Verghese, J. fNIRS Study of Walking and Walking While Talking in Young and Old Individuals. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 879–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirelman, A.; Maidan, I.; Bernad-Elazari, H.; Nieuwhof, F.; Reelick, M.; Giladi, N.; Hausdorff, J.M. Increased frontal brain activation during walking while dual tasking: An fNIRS study in healthy young adults. J. Neuroeng. Rehabil. 2014, 11, 85. [Google Scholar] [CrossRef] [Green Version]
- Callicott, J.; Mattay, V.S.; Bertolino, A.; Finn, K.; Coppola, R.; Frank, J.A.; Goldberg, T.E.; Weinberger, D.R. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb. Cortex 1999, 9, 20–26. [Google Scholar] [CrossRef]
- Beurskens, R.; Helmich, I.; Rein, R.; Bock, O. Age-related changes in prefrontal activity during walking in dual-task situations: A fNIRS study. Int. J. Psychophysiol. 2014, 92, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-J.; Mercer, V.S. Dual-Task Methodology: Applications in studies of cognitive and motor performance in adults and chldren. Pediatr. Phys. Ther. 2001, 13, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Gabbett, T.; Wake, M.; Abernethy, B. Use of dual-task methodology for skill assessment and development: Examples from rugby league. J. Sport. Sci. 2011, 29, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Gabbett, T.J.; Abernethy, B. Dual-task assessment of a sporting skill: Influence of task complexity and relationship with competitive performances. J. Sport. Sci. 2012, 30, 1735–1745. [Google Scholar] [CrossRef] [PubMed]
- Beauchet, O.; Annweiler, C.; Dubost, V.; Allali, G.; Kressig, R.W.; Bridenbaugh, S.; Berrut, G.; Assal, F.; Herrmann, F. Stops walking when talking: A predictor of falls in older adults? Eur. J. Neurol. 2009, 16, 786–795. [Google Scholar] [CrossRef]
- Gillain, S.; Boutaayamou, M.; Schwartz, C.; Dardenne, N.; Bruyère, O.; Brüls, O.; Croisier, J.-L.; Salmon, E.; Reginster, J.-Y.; Garraux, G.; et al. Gait symmetry in the dual task condition as a predictor of future falls among independent older adults: A 2-year longitudinal study. Aging Clin. Exp. Res. 2019, 31, 1057–1067. [Google Scholar] [CrossRef] [Green Version]
- Herold, F.; Wiegel, P.; Scholkmann, F.; Thiers, A.; Hamacher, D.; Schega, L. Functional near-infrared spectroscopy in movement science: A systematic review on cortical activity in postural and walking tasks. Neurophotonics 2017, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Onofrei, R.R.; Amaricai, E.; Suciu, O.; David, V.L.; Rata, A.L.; Hogea, E. Smartphone Use and Postural Balance in Healthy Young Adults. Int. J. Environ. Res. Public Health 2020, 17, 3307. [Google Scholar] [CrossRef]
- Bayot, M.; Dujardin, K.; Tard, C.; Defebvre, L.; Bonnet, C.T.; Allart, E.; Delval, A. The interaction between cognition and motor control: A theoretical framework for dual-task interference effects on posture, gait initiation, gait and turning. Neurophysiol. Clin. 2018, 48, 361–375. [Google Scholar] [CrossRef]
- Ayaz, H.; Izzetoglu, M.; Shewokis, P.A.; Onaral, B. Sliding-window motion artifact rejection for Functional Near-Infrared Spectroscopy. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 6567–6570. [Google Scholar]
- Izzetoglu, M.; Chitrapu, P.; Bunce, S.; Onaral, B. Motion artifact cancellation in NIR spectroscopy using discrete Kalman filtering. Biomed. Eng. Online 2010, 9, 16. [Google Scholar] [CrossRef] [Green Version]
- Dans, P.W.; Foglia, S.D.; Nelson, A.J. Data Processing in Functional Near-Infrared Spectroscopy (fNIRS) Motor Control Research. Brain Sci. 2021, 11, 606. [Google Scholar] [CrossRef]
- Liang, L.-Y.; Shewokis, P.A.; Getchell, N. Brain Activation in the Prefrontal Cortex during Motor and Cognitive Tasks in Adults. J. Behav. Brain Sci. 2016, 6, 463–474. [Google Scholar] [CrossRef] [Green Version]
- Hermens, H.J.; Freriks, B.; Merletti, R.; Stegeman, D.; Blok, J.; Rau, G.; Disselhorst-Klug, C.; Hägg, G. Seniam: European Recommendations for Surface Electromyography; Roessingh Research and Development: Enschede, The Netherlands, 1999. [Google Scholar]
- Konrad, P. The ABC of EMG: A Pratical Introduction to Kinesiological Electromyography, 1st ed.; Noraxon U.S.A., Inc.: Scottsdale, AZ, USA, 2006; Volume 100. [Google Scholar]
- Rudolph, K.S.; Axe, M.J.; Snyder-Mackler, L. Dynamic stability after ACL injury: Who can hop? Knee Surg. Sport. Traumatol. Arthrosc. 2000, 8, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Plummer, P.; Eskes, G. Measuring treatment effects on dual-task performance: A framework for research and clinical practice. Front. Hum. Neurosci. 2015, 9, 225. [Google Scholar] [CrossRef] [PubMed]
- Fuster, J.M. The Prefrontal Cortex—An Update: Time is of the Essence. Neuron 2001, 30, 319–333. [Google Scholar] [CrossRef] [Green Version]
- Rowe, J.B.; Stephan, K.E.; Friston, K.; Frackowiak, R.S.J.; Passingham, R.E. The Prefrontal Cortex shows Context- specific Changes in Effective Connectivity to Motor or Visual Cortex during the Selection of Action or Colour. Cereb. Cortex 2005, 15, 85–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowe, J.; Friston, K.; Frackowiak, R.; Passingham, R. Attention to Action: Specific Modulation of Corticocortical Interactions in Humans. Neuroimage 2002, 17, 988–998. [Google Scholar] [CrossRef]
- Hortobágyi, T.; Devita, P. Muscle pre- and coactivity during downward stepping are associated with leg stiffness in aging. J. Electromyogr. Kinesiol. 2000, 10, 117–126. [Google Scholar] [CrossRef]
- Melzer, I.; Benjuya, N.; Kaplanski, J. Age-Related Changes of Postural Control: Effect of Cognitive Tasks. Gerontology 2001, 47, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Gabrieli, J.; Poldrack, R.; Desmond, J. The role of left prefrontal cortex in language and memory. Proc. Natl. Acad. Sci. USA 1998, 95, 906–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, J.A.; Zielinski, B.A.; Ferguson, M.A.; Lainhart, J.E.; Anderson, J.S. An Evaluation of the Left-Brain vs. Right-Brain Hypothesis with Resting State Functional Connectivity Magnetic Resonance Imaging. PLoS ONE 2013, 8, e71275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqui, S.V.; Chatterjee, U.; Kumar, D.; Siddiqui, A.; Goyal, N. Neuropsychology of prefrontal cortex. Indian J. Psychiatry 2008, 50, 202–208. [Google Scholar] [PubMed]
- Nashner, L.M. The organisation of human postural movements: A formal basis and experimental synthesis. Behav. Brain Sci. 1985, 8, 135–172. [Google Scholar] [CrossRef]
- Bray, S.R.; Graham, J.D.; Martin Ginis, K.A.; Hicks, A.L. Cognitive task performance causes impaired maximum force production in human hand flexor muscles. Biol. Psychol. 2012, 89, 195–200. [Google Scholar] [CrossRef]
- Ozdemir, R.A.; Contreras-Vidal, J.L.; Paloski, W.H. Cortical control of upright stance in elderly. Mech. Ageing Dev. 2018, 169, 19–31. [Google Scholar] [CrossRef]
- Perrey, S.; Ferrari, M. Muscle Oximetry in Sports Science: A Systematic Review. Sport. Med. 2018, 48, 597–616. [Google Scholar] [CrossRef]
- Burcal, C.J.; Needle, A.R.; Custer, L.; Rosen, A.B. The Effects of Cognitive Loading on Motor Behavior in Injured Individuals: A Systematic Review. Sport. Med. 2019, 49, 1233–1253. [Google Scholar] [CrossRef]
- Yang, Z.-Q.; Wei, M.-F.; Chen, L.; Xi, J.-N. Research progress in the application of motor-cognitive dual-task training in rehabilitation of walking function in stroke patients. J. Neurorestoratol. 2023, 11, 100028. [Google Scholar] [CrossRef]


| Single-Task | Dual-Task | p-Value 1 | ||
|---|---|---|---|---|
| [oxy-Hb] | PFC | 0.419 (−0.099–0.660) | 0.812 (0.025–1.297) | 0.029 |
| LPFC | 0.393 (−0.132–0.755) | 0.689 (−0.193–1.489) | 0.033 | |
| RPFC | 0.302 (−0.107–0.677) | 0.525 (0.154–1.437) | 0.035 | |
| [deoxy-Hb] | PFC | −1.864 (−2.916–(−1.239)) | −0.897 (−2.347–0.530) | 0.001 |
| LPFC | −1.614 (−2.903–(−0.984)) | −0.909 (−2.601–(−0.289)) | 0.008 | |
| RPFC | −1.974 (−2.891–(−1.166)) | −0.883 (−2.634–0.938) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saraiva, M.; Castro, M.A.; Vilas-Boas, J.P. Muscular and Prefrontal Cortex Activity during Dual-Task Performing in Young Adults. Eur. J. Investig. Health Psychol. Educ. 2023, 13, 736-747. https://doi.org/10.3390/ejihpe13040055
Saraiva M, Castro MA, Vilas-Boas JP. Muscular and Prefrontal Cortex Activity during Dual-Task Performing in Young Adults. European Journal of Investigation in Health, Psychology and Education. 2023; 13(4):736-747. https://doi.org/10.3390/ejihpe13040055
Chicago/Turabian StyleSaraiva, Marina, Maria António Castro, and João Paulo Vilas-Boas. 2023. "Muscular and Prefrontal Cortex Activity during Dual-Task Performing in Young Adults" European Journal of Investigation in Health, Psychology and Education 13, no. 4: 736-747. https://doi.org/10.3390/ejihpe13040055
APA StyleSaraiva, M., Castro, M. A., & Vilas-Boas, J. P. (2023). Muscular and Prefrontal Cortex Activity during Dual-Task Performing in Young Adults. European Journal of Investigation in Health, Psychology and Education, 13(4), 736-747. https://doi.org/10.3390/ejihpe13040055












