Nocturnal Smartphone Use Affects Sleep Quality and Cognitive and Physical Performance in Tunisian School-Age Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
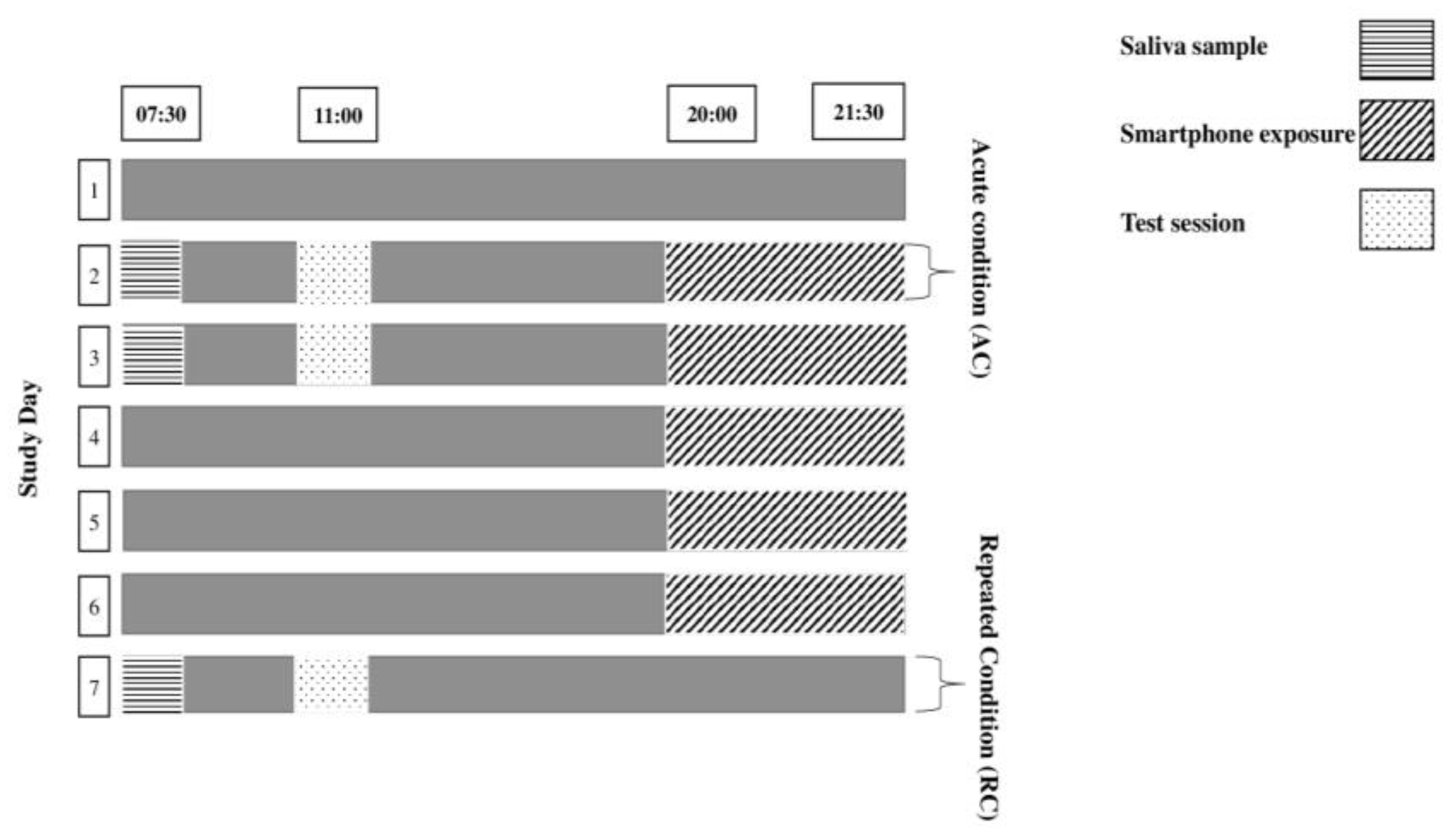
2.2. Experimental Design
2.3. Measurements
2.3.1. Actigraphy
2.3.2. Cortisol Measurements
2.3.3. Cognitive Measures
Stroop Task
Choice Reaction Time
N-Back Task
2.3.4. Physical Measures
Counter-Movement Jump
Sprint Test
2.4. Statistical Analysis
3. Results
3.1. Objective Sleep Measurements
3.2. Salivary Cortisol
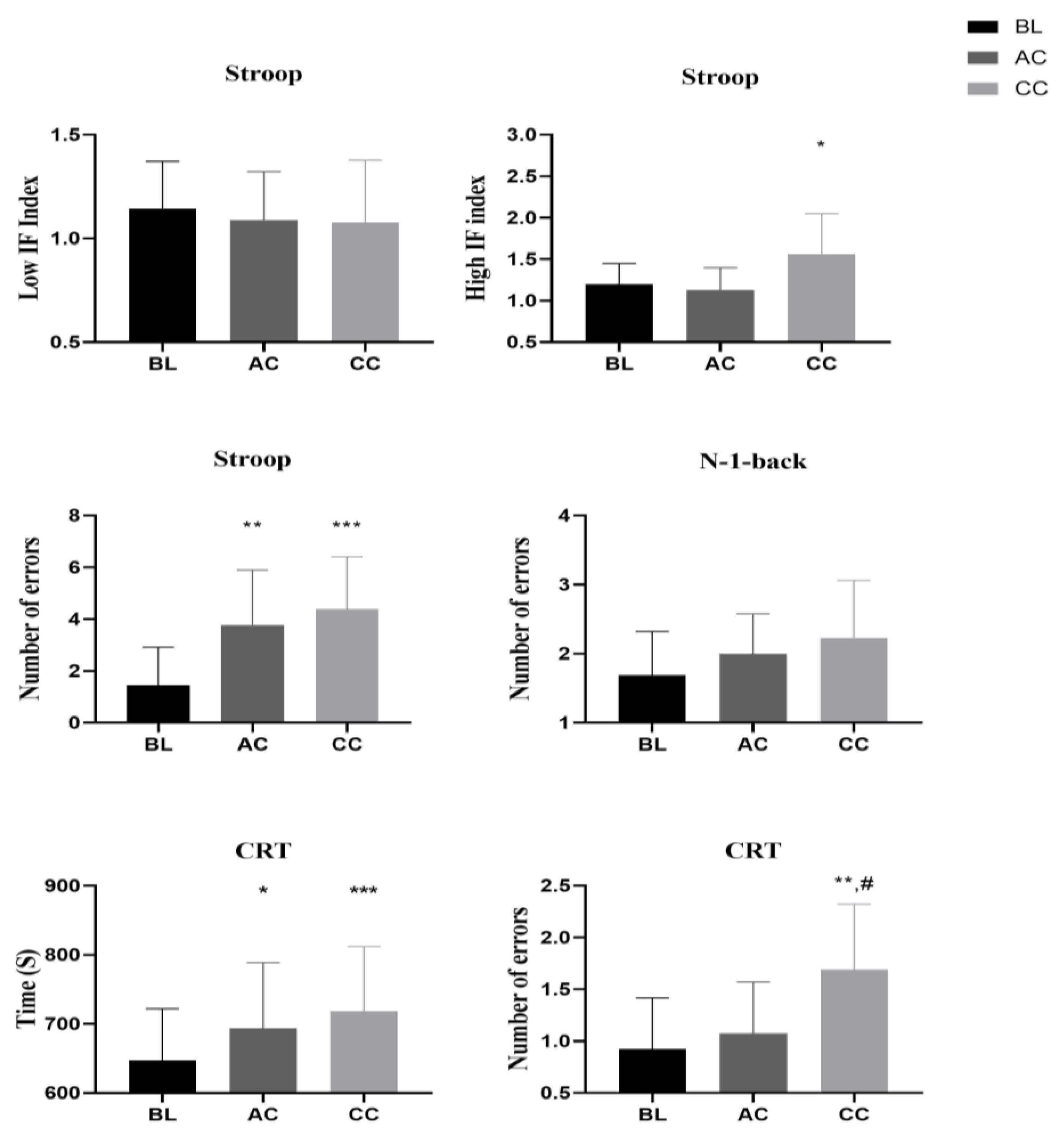
3.3. Cognitive Performance
3.4. Physical Performance
4. Discussion
5. Strengths, Limitations, and Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simmons, N.; Mandal, S.; Paton, B.; Ahmed, I. Are Circadian Rhythms a New Frontier in Athletic Performance? Curr. Sports Med. Rep. 2022, 21, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Lowe, C.J.; Safati, A.; Hall, P.A. The neurocognitive consequences of sleep restriction: A meta-analytic review. Neurosci. Biobehav. Rev. 2017, 80, 586–604. [Google Scholar] [CrossRef] [PubMed]
- Czeisler, C.A.; Shanahan, T.L. Problems associated with use of mobile devices in the sleep environment—Streaming instead of dreaming. JAMA Pediatr. 2016, 170, 1146–1147. [Google Scholar] [CrossRef] [PubMed]
- Prayag, A.S.; Najjar, R.P.; Gronfier, C. Melatonin suppression is exquisitely sensitive to light and primarily driven by melanopsin in humans. J. Pineal Res. 2019, 66, e12562. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.M.; Santhi, N.; St Hilaire, M.; Gronfier, C.; Bradstreet, D.S.; Duffy, J.F.; Lockley, S.W.; Kronauer, R.E.; Czeisler, C.A. Human responses to bright light of different durations. J. Physiol. 2012, 590, 3103–3112. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.-H.; Lee, H.-J.; Yoon, H.-K.; Kang, S.-G.; Bok, K.-N.; Jung, K.-Y.; Kim, L.; Lee, E.-I. Exposure to dim artificial light at night increases REM sleep and awakenings in humans. Chronobiol. Int. 2016, 33, 117–123. [Google Scholar] [CrossRef]
- Cho, Y.; Ryu, S.H.; Lee, B.R.; Kim, K.H.; Lee, E.; Choi, J. Effects of artificial light at night on human health: A literature review of observational and experimental studies applied to exposure assessment. Chronobiol. Int. 2015, 32, 1294–1310. [Google Scholar] [CrossRef] [PubMed]
- Akacem, L.D.; Wright, K.P., Jr.; LeBourgeois, M.K. Sensitivity of the circadian system to evening bright light in preschool-age children. Physiol. Rep. 2018, 6, e13617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Beier, C.; Weil, T.; Hattar, S. The retinal ipRGC-preoptic circuit mediates the acute effect of light on sleep. Nat. Commun. 2021, 12, 5115. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Scheer, F.A. Circadian system and glucose metabolism: Implications for physiology and disease. Trends Endocrinol. Metab. 2016, 27, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Scheiermann, C.; Kunisaki, Y.; Frenette, P.S. Circadian control of the immune system. Nat. Rev. Immunol. 2013, 13, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Dacey, D.M.; Liao, H.W.; Peterson, B.B.; Robinson, F.R.; Smith, V.C.; Pokorny, J.; Yau, K.W.; Gamlin, P.D. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 2005, 433, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Twenge, J.M. Increases in depression, self-harm, and suicide among US adolescents after 2012 and links to technology use: Possible mechanisms. Psychiatr. Res. Clin. Pract. 2020, 2, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Perrault, A.A.; Bayer, L.; Peuvrier, M.; Afyouni, A.; Ghisletta, P.; Brockmann, C.; Spiridon, M.; Hulo Vesely, S.; Haller, D.M.; Pichon, S. Reducing the use of screen electronic devices in the evening is associated with improved sleep and daytime vigilance in adolescents. Sleep 2019, 42, zsz125. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.A.; Wright, K.P., Jr.; Lockley, S.W.; Czeisler, C.A.; Gronfier, C. Characterizing the temporal Dynamics of Melatonin and cortisol changes in Response to nocturnal Light exposure. Sci. Rep. 2019, 9, 19720. [Google Scholar] [CrossRef] [PubMed]
- Hartstein, L.E.; Behn, C.D.; Akacem, L.D.; Stack, N.; Wright, K.P., Jr.; LeBourgeois, M.K. High sensitivity of melatonin suppression response to evening light in preschool-aged children. J. Pineal Res. 2022, 72, e12780. [Google Scholar] [CrossRef] [PubMed]
- Eto, T.; Ohashi, M.; Nagata, K.; Shin, N.; Motomura, Y.; Higuchi, S. Crystalline lens transmittance spectra and pupil sizes as factors affecting light-induced melatonin suppression in children and adults. Ophthalmic Physiol. Opt. 2021, 41, 900–910. [Google Scholar] [CrossRef]
- Rideout, V. The Common Sense census: Media use by kids age zero to eight. San Fr. CA Common Sense Media 2017, 263, 283. [Google Scholar]
- Girela-Serrano, B.M.; Spiers, A.D.; Ruotong, L.; Gangadia, S.; Toledano, M.B.; Di Simplicio, M. Impact of mobile phones and wireless devices use on children and adolescents’ mental health: A systematic review. Eur. Child Adolesc. Psychiatry 2022, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Hale, L.; Guan, S. Screen time and sleep among school-aged children and adolescents: A systematic literature review. Sleep Med. Rev. 2015, 21, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.J.; Willett, W.; Forman, M.R. The association of television viewing in childhood with overweight and obesity throughout the life course. Am. J. Epidemiol. 2019, 188, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Foerster, M.; Henneke, A.; Chetty-Mhlanga, S.; Röösli, M. Impact of adolescents’ screen time and nocturnal mobile phone-related awakenings on sleep and general health symptoms: A prospective cohort study. Int. J. Environ. Res. Public Health 2019, 16, 518. [Google Scholar] [CrossRef] [PubMed]
- Mineshita, Y.; Kim, H.K.; Chijiki, H.; Nanba, T.; Shinto, T.; Furuhashi, S.; Oneda, S.; Kuwahara, M.; Suwama, A.; Shibata, S. Screen time duration and timing: Effects on obesity, physical activity, dry eyes, and learning ability in elementary school children. BMC Public Health 2021, 21, 422. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, S.; Nagafuchi, Y.; Lee, S.I.; Harada, T. Influence of light at night on melatonin suppression in children. J. Clin. Endocrinol. Metab. 2014, 99, 3298–3303. [Google Scholar] [CrossRef]
- Sweetser, P.; Johnson, D.; Ozdowska, A.; Wyeth, P. Active versus passive screen time for young children. Australas. J. Early Child. 2012, 37, 94–98. [Google Scholar] [CrossRef]
- Domingues-Montanari, S. Clinical and psychological effects of excessive screen time on children. J. Paediatr. Child Health 2017, 53, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Christakis, D.A.; Garrison, M.M.; Herrenkohl, T.; Haggerty, K.; Rivara, F.P.; Zhou, C.; Liekweg, K. Modifying media content for preschool children: A randomized controlled trial. Pediatrics 2013, 131, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, M.D.; Barnes, J.D.; Chaput, J.P.; Tremblay, M.S. Screen time and problem behaviors in children: Exploring the mediating role of sleep duration. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 105. [Google Scholar] [CrossRef] [PubMed]
- Hisler, G.; Twenge, J.M.; Krizan, Z. Associations between screen time and short sleep duration among adolescents varies by media type: Evidence from a cohort study. Sleep Med. 2020, 66, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Fullagar, H.H.; Skorski, S.; Duffield, R.; Hammes, D.; Coutts, A.J.; Meyer, T. Sleep and athletic performance: The effects of sleep loss on exercise performance, and physiological and cognitive responses to exercise. Sports Med. 2015, 45, 161–186. [Google Scholar] [CrossRef] [PubMed]
- Killgore, W.D. Effects of sleep deprivation on cognition. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2010; Volume 185, pp. 105–129. [Google Scholar]
- Goel, N.; Basner, M.; Rao, H.; Dinges, D.F. Circadian rhythms, sleep deprivation, and human performance. Prog. Mol. Biol. Transl. Sci. 2013, 119, 155–190. [Google Scholar]
- Craven, J.; McCartney, D.; Desbrow, B.; Sabapathy, S.; Bellinger, P.; Roberts, L.; Irwin, C. Effects of acute sleep loss on physical performance: A systematic and meta-analytical review. Sports Med. 2022, 52, 2669–2690. [Google Scholar] [CrossRef] [PubMed]
- Abid, R.; Ammar, A.; Maaloul, R.; Souissi, N.; Hammouda, O. Effect of COVID-19-related home confinement on sleep quality, screen time and physical activity in Tunisian boys and girls: A survey. Int. J. Environ. Res. Public Health 2021, 18, 3065. [Google Scholar] [CrossRef] [PubMed]
- Dosseville, F.; Laborde, S.; Lericollais, R. Validation of a chronotype questionnaire including an amplitude dimension. Chronobiol. Int. 2013, 30, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Verlaan, P.; Cantin, S.; Boivin, M. L’Échelle de développement pubertaire: Équivalence en langue française. Can. J. Behav. Sci. Rev. Can. Sci. Comport. 2001, 33, 143. [Google Scholar] [CrossRef]
- Lucena, J.M.S.D.; Cheng, L.A.; Cavalcante, T.L.M.; Silva, V.A.D.; Farias Júnior, J.C.D. Prevalence of excessive screen time and associated factors in adolescents. Rev. Paul. Pediatr. 2015, 33, 407–414. [Google Scholar] [CrossRef]
- Moraes, C.; Sobral, D.; Duarte, D.W.; Cavalcanti, G.Z.; Salazar-Gamarra, R.; Dornelles, R. Protocolo Complementar Para Melhor Resolução do Nariz em Fotogrametria 3d. Available online: https://www.researchgate.net/publication/344380790_Protocolo_Complementar_para_Melhor_Resolucao_do_Nariz_em_Fotogrametria_3D (accessed on 30 September 2020).
- Cerqueira, D.; Carvalho, F.; Melo, R.B. Is It Smart to Use smartphones to measure illuminance for occupational Health and Safety Purposes? In Proceedings of the International Conference on Applied Human Factors and Ergonomics, Los Angeles, CA, USA, 17–21 July 2017; pp. 258–268. [Google Scholar]
- Marino, M.; Li, Y.; Rueschman, M.N.; Winkelman, J.W.; Ellenbogen, J.; Solet, J.M.; Dulin, H.; Berkman, L.F.; Buxton, O.M. Measuring sleep: Accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep 2013, 36, 1747–1755. [Google Scholar] [CrossRef]
- Sadeh, A. Assessment of intervention for infant night waking: Parental reports and activity-based home monitoring. J. Consult. Clin. Psychol. 1994, 62, 63. [Google Scholar] [CrossRef] [PubMed]
- Sadeh, A. The role and validity of actigraphy in sleep medicine: An update. Sleep Med. Rev. 2011, 15, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Kryger, M.H.; Roth, T.; Dement, W.C. Principles and Practice of Sleep Medicine; Elsevier Health Sciences: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Dorn, L.D.; Lucke, J.F.; Loucks, T.L.; Berga, S.L. Salivary cortisol reflects serum cortisol: Analysis of circadian profiles. Ann. Clin. Biochem. 2007, 44, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Urfer-Maurer, N.; Ludyga, S.; Stalder, T.; Brand, S.; Holsboer-Trachsler, E.; Gerber, M.; Grob, A.; Weber, P.; Lemola, S. Heart rate variability and salivary cortisol in very preterm children during school age. Psychoneuroendocrinology 2018, 87, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Spreen, O.; Strauss, E. A Compendium of Neuropsychological Tests; Oxford University Press: New York, NY, USA, 1998; pp. 213–218. [Google Scholar]
- Regard, M. Cognitive Rigidity and Flexibility: A Neuropsychological Study. Ph.D. Thesis, University of Victoria, Victoria, BC, Canada, 1983. [Google Scholar]
- Lezak, M.D. Neuropsychological Assessment; Oxford University Press: Cary, NC, USA, 2004. [Google Scholar]
- Mathôt, S.; Schreij, D.; Theeuwes, J. OpenSesame: An open-source, graphical experiment builder for the social sciences. Behav. Res. Methods 2012, 44, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Jarraya, S.; Jarraya, M.; Chtourou, H.; Souissi, N. Diurnal variations on cognitive performances in handball goalkeepers. Biol. Rhythm Res. 2014, 45, 93–101. [Google Scholar] [CrossRef]
- Robinson, B.; Fuller, B. N-Back Test [Computer Software]. 2004. Available online: http://step.psy.cmu.edu/scripts-plus/ (accessed on 21 June 2012).
- Kane, M.J.; Conway, A.R.; Miura, T.K.; Colflesh, G.J. Working memory, attention control, and the N-back task: A question of construct validity. J. Exp. Psychol. Learn. Mem. Cogn. 2007, 33, 615. [Google Scholar] [CrossRef] [PubMed]
- Glatthorn, J.F.; Gouge, S.; Nussbaumer, S.; Stauffacher, S.; Impellizzeri, F.M.; Maffiuletti, N.A. Validity and reliability of Optojump photoelectric cells for estimating vertical jump height. J. Strength Cond. Res. 2011, 25, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Papaiakovou, G.; Giannakos, A.; Michailidis, C.; Patikas, D.; Bassa, E.; Kalopisis, V.; Anthrakidis, N.; Kotzamanidis, C. The effect of chronological age and gender on the development of sprint performance during childhood and puberty. J. Strength Cond. Res. 2009, 23, 2568–2573. [Google Scholar] [CrossRef] [PubMed]
- Schipman, J.; Saulière, G.; Sedeaud, A.; Deschamps, T.; Ovigneur, H.; Maillet, H.; Berthelot, G.; Toussaint, J.-F. Indice de masse corporelle et condition physique chez 49,600 collégiens et lycéens de six régions françaises, 2007–2014. Bull. Epidémiologique Hebd. BEH 2015, 30–31, 552–561. [Google Scholar]
- Christensen, M.A.; Bettencourt, L.; Kaye, L.; Moturu, S.T.; Nguyen, K.T.; Olgin, J.E.; Pletcher, M.J.; Marcus, G.M. Direct measurements of smartphone screen-time: Relationships with demographics and sleep. PLoS ONE 2016, 11, e0165331. [Google Scholar] [CrossRef] [PubMed]
- Green, A.; Cohen-Zion, M.; Haim, A.; Dagan, Y. Comparing the response to acute and chronic exposure to short wavelength lighting emitted from computer screens. Chronobiol. Int. 2018, 35, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H.; Andy, C.Y.; Wu, C.S.; Mak, Y.W.; Lee, U. Temporal association between objectively measured smartphone usage, sleep quality and physical activity among Chinese adolescents and young adult. J. Sleep Res. 2021, 1–20. [Google Scholar] [CrossRef]
- Hartstein, L.E.; Diniz Behn, C.; Wright, K.P., Jr.; Akacem, L.D.; Stowe, S.R.; LeBourgeois, M.K. Evening Light Intensity and Phase Delay of the Circadian Clock in Early Childhood. J. Biol. Rhythm. 2023, 38, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, E.L.; Purcell, K.; Asis, A.; Paech, G.; Metse, A.; Murphy, D.; Robson, A. Preschoolers’ engagement with screen content and associations with sleep and cognitive development. Acta Psychol. 2022, 230, 103762. [Google Scholar] [CrossRef] [PubMed]
- Hale, L.; Kirschen, G.W.; LeBourgeois, M.K.; Gradisar, M.; Garrison, M.M.; Montgomery-Downs, H.; Kirschen, H.; McHale, S.M.; Chang, A.-M.; Buxton, O.M. Youth screen media habits and sleep: Sleep-friendly screen behavior recommendations for clinicians, educators, and parents. Child Adolesc. Psychiatr. Clin. North Am. 2018, 27, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Falbe, J.; Davison, K.K.; Franckle, R.L.; Ganter, C.; Gortmaker, S.L.; Smith, L.; Land, T.; Taveras, E.M. Sleep duration, restfulness, and screens in the sleep environment. Pediatrics 2015, 135, e367–e375. [Google Scholar] [CrossRef] [PubMed]
- King, D.L.; Gradisar, M.; Drummond, A.; Lovato, N.; Wessel, J.; Micic, G.; Douglas, P.; Delfabbro, P. The impact of prolonged violent video-gaming on adolescent sleep: An experimental study. J. Sleep Res. 2013, 22, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Ivarsson, M.; Anderson, M.; Åkerstedt, T.; Lindblad, F. The effect of violent and nonviolent video games on heart rate variability, sleep, and emotions in adolescents with different violent gaming habits. Psychosom. Med. 2013, 75, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Kostoglou-Athanassiou, I.; Treacher, D.F.; Wheeler, M.J.; Forsling, M.L. Bright light exposure and pituitary hormone secretion. Clin. Endocrinol. 1998, 48, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Leproult, R.; Colecchia, E.F.; L’Hermite-Balériaux, M.; Van Cauter, E. Transition from dim to bright light in the morning induces an immediate elevation of cortisol levels. J. Clin. Endocrinol. Metab. 2001, 86, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, S.; Paquet, J.; Selmaoui, B.; Rufiange, M.; Dumont, M. Vigilance levels during and after bright light exposure in the first half of the night. Chronobiol. Int. 2003, 20, 1019–1038. [Google Scholar] [CrossRef] [PubMed]
- Reigal, R.E.; Barrero, S.; Morales-Sánchez, V.; Juárez-Ruiz de Mier, R.; Hernández-Mendo, A. Relationships between reaction time, selective attention, physical activity, and physical fitness in children. Front. Psychol. 2019, 10, 481543. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Miguel, P.A.; Sevil-Serrano, J.; Sánchez-Oliva, D.; Tapia-Serrano, M.A. School and non-school day screen time profiles and their differences in health and educational indicators in adolescents. Scand. J. Med. Sci. Sports 2022, 32, 1668–1681. [Google Scholar] [CrossRef] [PubMed]
- Touitou, Y.; Touitou, D.; Reinberg, A. Disruption of adolescents’ circadian clock: The vicious circle of media use, exposure to light at night, sleep loss and risk behaviors. J. Physiol. Paris 2016, 110, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Mougharbel, F.; Goldfield, G.S. Psychological correlates of sedentary screen time behaviour among children and adolescents: A narrative review. Curr. Obes. Rep. 2020, 9, 493–511. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, M.P.; Tyler, R.; Mackintosh, K.A.; Stratton, G. Relationship between sedentary time, physical activity and multiple lifestyle factors in children. J. Funct. Morphol. Kinesiol. 2018, 3, 15. [Google Scholar] [CrossRef]
- Edelson, L.R.; Mathias, K.C.; Fulgoni, V.L.; Karagounis, L.G. Screen-based sedentary behavior and associations with functional strength in 6–15 year-old children in the United States. BMC Public Health 2015, 16, 116. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.J.; Peeling, P.; Dawson, B.; Halson, S.; Miller, J.; Dunican, I.; Clarke, M.; Goodman, C.; Eastwood, P. Evening electronic device use: The effects on alertness, sleep and next-day physical performance in athletes. J. Sports Sci. 2018, 36, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Souissi, N.; Sesboüé, B.; Gauthier, A.; Larue, J.; Davenne, D. Effects of one night’s sleep deprivation on anaerobic performance the following day. Eur. J. Appl. Physiol. 2003, 89, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Reilly, T.; Piercy, M. The effect of partial sleep deprivation on weight-lifting performance. Ergonomics 1994, 37, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Engle-Friedman, M. The effects of sleep loss on capacity and effort. Sleep Sci. 2014, 7, 213–224. [Google Scholar] [CrossRef]
- Olds, T.; Maher, C.; Dumuid, D. Life on holidays: Differences in activity composition between school and holiday periods in Australian children. BMC Public Health 2019, 19, 450. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.; Maher, C.; Tomkinson, G.R.; Golley, R.; Fraysse, F.; Dumuid, D.; Lewthwaite, H.; Olds, T. Life on holidays: Study protocol for a 3-year longitudinal study tracking changes in children’s fitness and fatness during the in-school versus summer holiday period. BMC Public Health 2019, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Martinez, J.-M.; Castillo-Martinez, A.; Medina-Merodio, J.-A.; Aguado-Delgado, J.; Martinez-Herraiz, J.-J. Smartphones as a light measurement tool: Case of study. Appl. Sci. 2017, 7, 616. [Google Scholar] [CrossRef]
| BL | AC | RC | ANOVA (F(2,26), p, ηp2) | |
|---|---|---|---|---|
| SOL (min) | 12.62 ± 9.82 | 14.15 ± 6.97 | 12.77 ± 15.27 | (0.072, 0.93, 0.21) |
| SE (%) | 88.14 ± 5.66 | 85.59 ± 7.17 | 81.5 ± 2.12 | (2.87, 0.076, 0.22) |
| TIB (min) | 551.23 ± 26.18 | 541.46 ± 22.53 | 541.46 ± 24.28 | (0.71, 0.49, 0.04) |
| TST (min) | 487.08 ± 40.2 | 458.38 ± 40.9 *** | 440.08 ± 38.92 *** | (11.16, 0.01, 0.4) |
| WASO (min) | 23.69 ± 2.21 | 27.23 ± 4.23 ** | 33.62 ± 5.38 ***,## | (19.02, <0.001, 0.83) |
| BL | AC | RC | ANOVA (F(1,13), p, ηp2) | |
|---|---|---|---|---|
| Cortisol (ng/mL) | 3.48 ± 1.97 | 3.42 ± 1.69 | 2.69 ± 0.89 | (1.96, p = 0.19, 0.26) |
| BL | AC | RC | ANOVA (F(2,26), p, ηp2) | |
|---|---|---|---|---|
| CMJ | ||||
| Flight time (s) | 0.36 ± 0.04 | 0.36 ± 0.01 | 0.32 ± 0.04 *,# | (6.44, p < 0.01, 0.35) |
| Jump height (cm) | 14.08 ± 1.2 | 13.79 ± 1.55 | 12.85 ± 1.63 ** | (5.24, 0.013, 0.3) |
| 30 m sprint speed | ||||
| Time (s) | 7.31 ± 0.56 | 7.38 ± 0.49 | 7.75 ± 0.34 *,# | (5.27, 0.013, 0.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abid, R.; Ammar, A.; Maaloul, R.; Boudaya, M.; Souissi, N.; Hammouda, O. Nocturnal Smartphone Use Affects Sleep Quality and Cognitive and Physical Performance in Tunisian School-Age Children. Eur. J. Investig. Health Psychol. Educ. 2024, 14, 856-869. https://doi.org/10.3390/ejihpe14040055
Abid R, Ammar A, Maaloul R, Boudaya M, Souissi N, Hammouda O. Nocturnal Smartphone Use Affects Sleep Quality and Cognitive and Physical Performance in Tunisian School-Age Children. European Journal of Investigation in Health, Psychology and Education. 2024; 14(4):856-869. https://doi.org/10.3390/ejihpe14040055
Chicago/Turabian StyleAbid, Rihab, Achraf Ammar, Rami Maaloul, Mariem Boudaya, Nizar Souissi, and Omar Hammouda. 2024. "Nocturnal Smartphone Use Affects Sleep Quality and Cognitive and Physical Performance in Tunisian School-Age Children" European Journal of Investigation in Health, Psychology and Education 14, no. 4: 856-869. https://doi.org/10.3390/ejihpe14040055
APA StyleAbid, R., Ammar, A., Maaloul, R., Boudaya, M., Souissi, N., & Hammouda, O. (2024). Nocturnal Smartphone Use Affects Sleep Quality and Cognitive and Physical Performance in Tunisian School-Age Children. European Journal of Investigation in Health, Psychology and Education, 14(4), 856-869. https://doi.org/10.3390/ejihpe14040055









