Ins and Outs of Rocker Switch Mechanism in Major Facilitator Superfamily of Transporters
Abstract
:1. Introduction
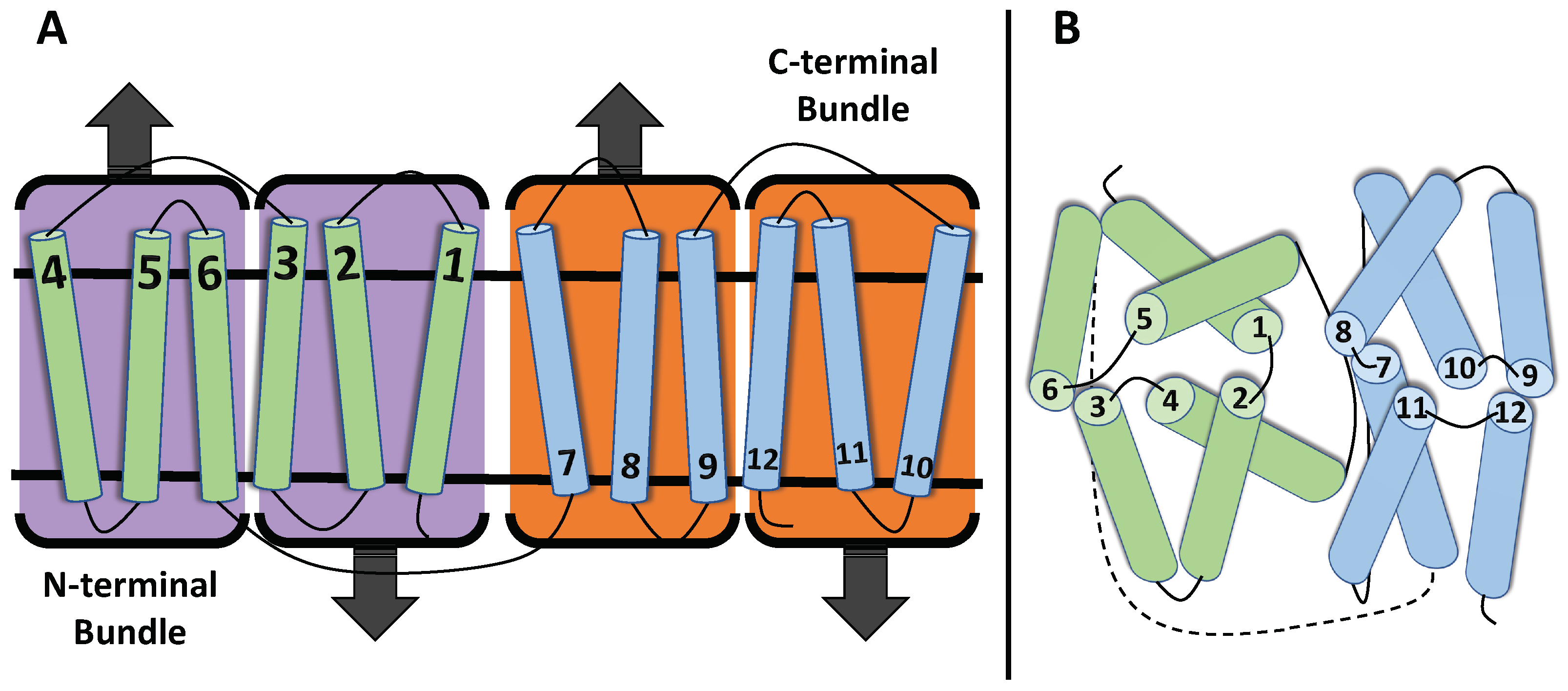
1.1. Structure of MFS Transporters
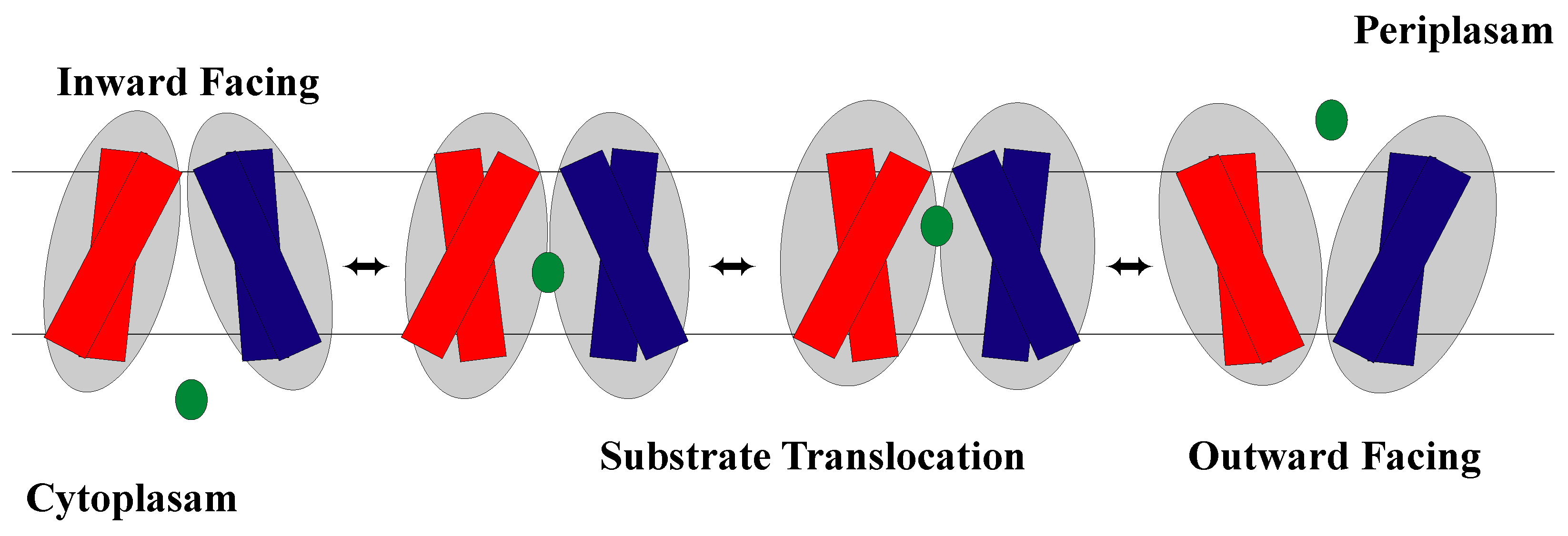
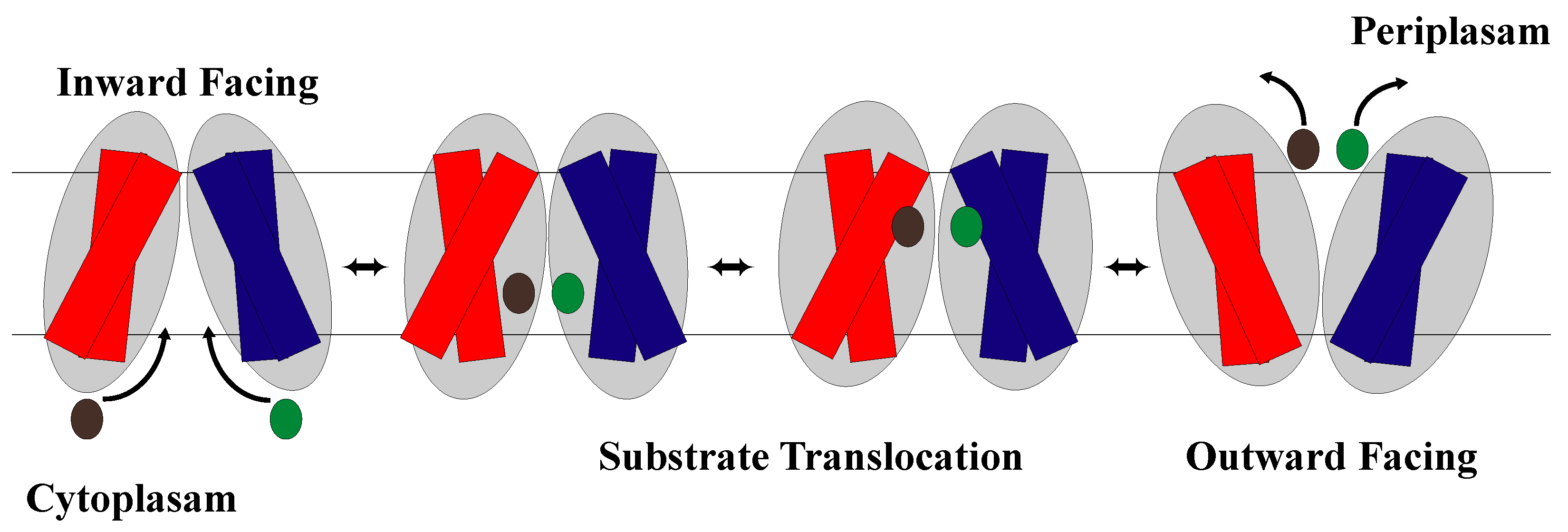
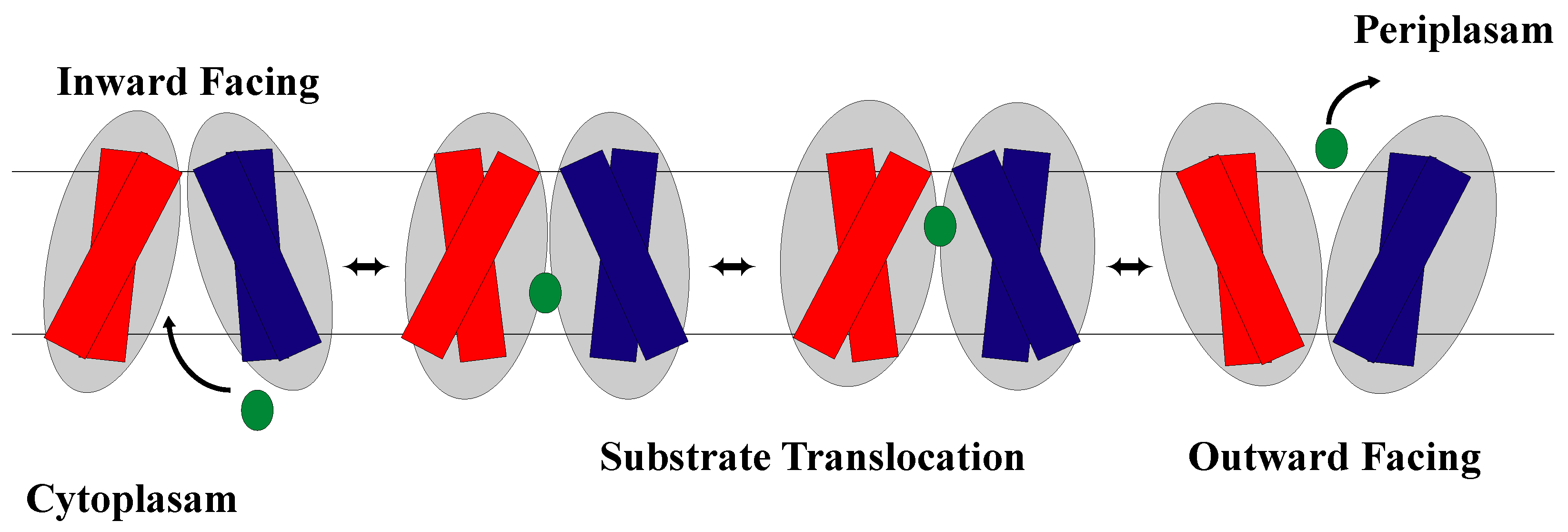
1.2. The Rocker-Switch Mechanism
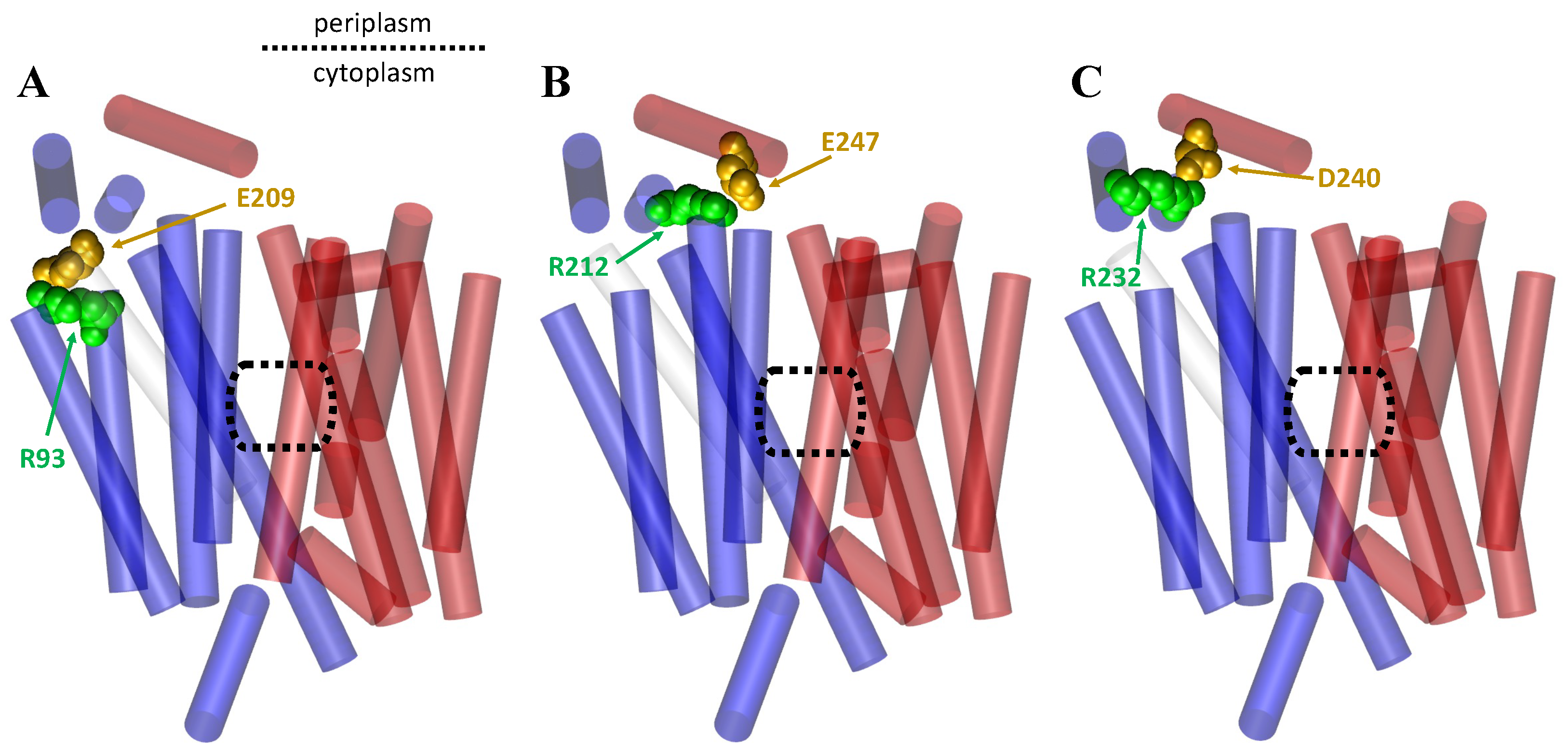
1.3. Importance of Salt Bridges for the MFS
2. Significant Structural and Conformational Differences in the Rocker-Switch Mechanism across the Major Facilitator Superfamily of Transporters
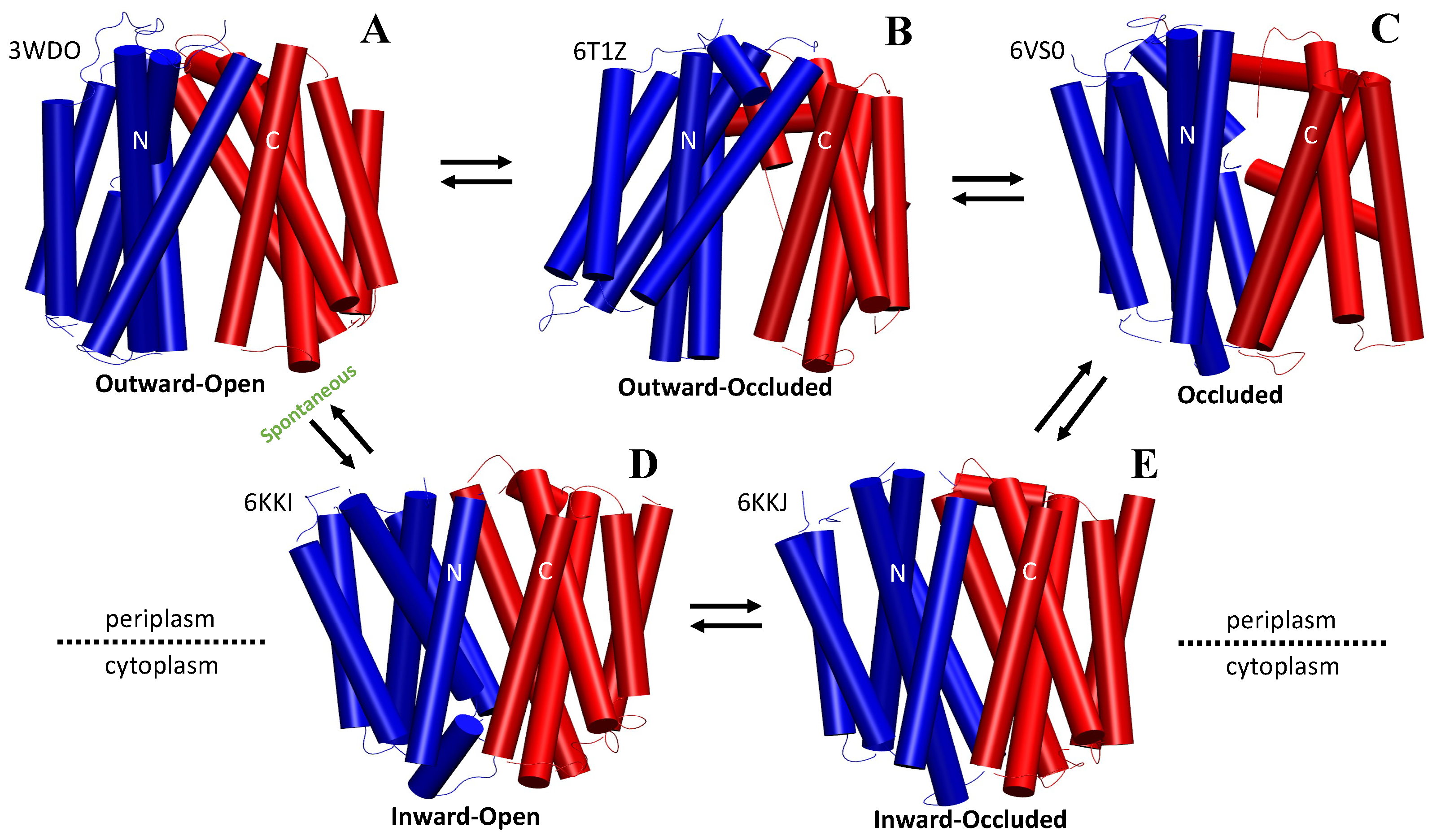
2.1. The Rocker-Switch Mechanism for Antiporters
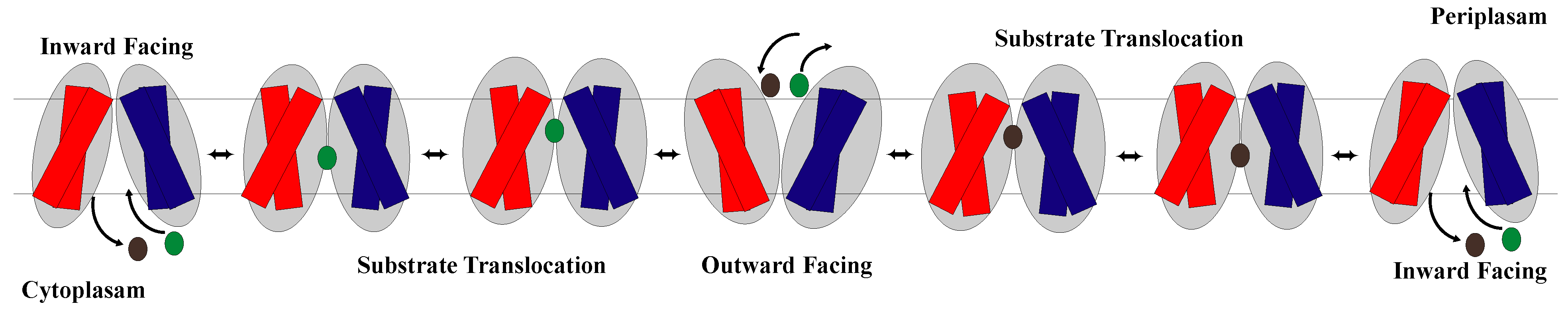
2.2. The Rocker-Switch Mechanism for Symporters
2.3. The Rocker-Switch Mechanism for Uniporters
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
- The following abbreviations are used in this manuscript:
| Major facilitator superfamily | MFS |
| Adenosine triphosphate | ATP |
| Inward facing | IF |
| Outward facing | OF |
| The Transporter Classification Database | TCDB |
| Major Intrinsic Protein | MIP |
| Transmembrane segments | TM |
| N-terminal domain | NTD |
| C-terminal domain | CTD |
| Lactose symporter | LacY |
| Glycerol-3-phosphate-antiporter | GlpT |
| Oxalate transporter | OxlT |
| Melibiose permease | MelB |
| Transmembrane domains | TMDs |
| Proton-dependent oligopeptide transporter | POT |
| Peptide transport | PTR |
| Peptide transporters 1 | PepT1 |
| Peptide transporters 2 | PepT2 |
| Serotonin transporter | SERT |
| Dopamine transporter | DAT |
| Excitatory Amino Acid Transporter | EAAT3 |
| Lactose permease | LacY |
| Maltose transporter | MalFGK2 |
| Uracil transporter | UraA |
| Xanthine transporter | XanQ |
| Purine transporter | PUP1 |
| Proton-motive force | PMF |
| Sugar porter | SP |
| Glucose transporter 1 | GLUT1 |
References
- Stillwell, W. Membrane Transport. In An Introduction to Biological Membranes; Elsevier: Amsterdam, The Netherlands, 2016; pp. 423–451. [Google Scholar] [CrossRef]
- Frans, M. Solute Transport: Basic Concepts. Plant Physiol. Funct. 2017, 6, 1–31. [Google Scholar] [CrossRef]
- Xi, C.; Peter, P. The Balance of Fluid and Osmotic Pressures across Active Biological Membranes with Application to the Corneal Endothelium. PLoS ONE 2015, 10, e0145422. [Google Scholar] [CrossRef]
- Timney, B.; Raveh, B.; Mironska, R.; Trivedi, J.; Kim, S.; Russel, D.; Wente, S.; Sali, A.; Rout, M. Simple rules for passive diffusion through the nuclear pore complex. J. Cell Biol. 2016, 215, 57–76. [Google Scholar] [CrossRef]
- Camenisch, G.; Alsenz, J.; ven de Waterbeemd, H.; Folkers, G. Estimation of permeability by passive diffusion through Caco-2 cell monolayers using the drugs’ lipophilicity and molecular weight. Eur. J. Pharm. Sci. 1998, 6, 313–319. [Google Scholar] [CrossRef]
- Heidi, E.; Janet, E.; Locksley, M. Osmotic Transport across Cell Membranes in Nondilute Solutions: A New Nondilute Solute Transport Equation. Biophys. J. 2009, 96, 2559–2571. [Google Scholar] [CrossRef]
- Forrest, L.; Krämer, R.; Ziegler, C. The structural basis of secondary active transport mechanisms. Biochim. Et Biophys. Acta Bioenerg. 2011, 1807, 167–188. [Google Scholar] [CrossRef] [PubMed]
- Sweadner, K.; Goldin, S. Active Transport of Sodium and Potassium Ions—Mechanism, Function, and Regulation. N. Engl. J. Med. 1980, 302, 777–783. [Google Scholar] [CrossRef]
- Kyte, J. Molecular considerations relevant to the mechanism of active transport. Nature 1981, 292, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Boyer, P. A Model For Conformational Coupling Of Membrane Potential and Proton Translocation To ATP Synthesis and To Active Transport. Fed. Eur. Biochem. Soc. Lett. 1975, 58, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.; Grider, M. Physiology, Adenosine Triphosphate; StatPearls: Tampa, FL, USA, 2023. [Google Scholar]
- Kulkarni, T.; Slaughter, G. Application of Semipermeable Membranes in Glucose Biosensing. Membranes 2016, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kamcev, J.; Robeson, L.; Menachem, E.; Freeman, B. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, eaab0530. [Google Scholar] [CrossRef] [PubMed]
- Starzak, M. Membranes, Synthetic (Chemistry). In Encyclopedia of Physical Science and Technology, 3rd ed.; Elsevier Science Ltd.: Cambridge, MA, USA, 2003; pp. 345–354. [Google Scholar] [CrossRef]
- Lee, V. Membrane transporters. Eur. J. Pharm. Sci. 2000, 11, S41–S50. [Google Scholar] [CrossRef]
- Drew, D.; Boudker, O. Shared Molecular Mechanisms of Membrane Transporters. Annu. Rev. Biochem. 2016, 85, 543–572. [Google Scholar] [CrossRef] [PubMed]
- Stieger, B.; Steiger, J.; Locher, K. Membrane lipids and transporter function. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2021, 1867, 166079. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; He, G.; He, T. Specifically Targeted Transport of Plasma Membrane Transporters: From Potential Mechanisms for Regulating Cell Health or Disease to Applications. Membranes 2021, 11, 736. [Google Scholar] [CrossRef]
- Denning, E.; Beckstein, O. Influence of lipids on protein-mediated transmembrane transport. Chem. Phys. Lipids 2013, 169, 57–71. [Google Scholar] [CrossRef]
- Huang, Y.; Lemieux, M.; Song, J.; Auer, M.; Wang, D. Structure and Mechanism of the Glycerol-3-Phosphate Transporter from Escherichia coli. Science 2003, 301, 616–620. [Google Scholar] [CrossRef]
- Chan, M.; Shukla, D. Markov state modeling of membrane transport proteins. J. Struct. Biol. 2021, 213, 107800. [Google Scholar] [CrossRef]
- Beckstein, O.; Naughton, F. General principles of secondary active transporter function. Biophys. Rev. 2021, 3, 011307. [Google Scholar] [CrossRef]
- Pao, S.; Paulsen, I.; Saier, M. Major Facilitator Superfamily. Microbiol. Mol. Biol. Rev. 1998, 62, 1–34. [Google Scholar] [CrossRef]
- Drew, D.; North, R.; Nagarathinum, K.; Tanabe, M. Structures and General Transport Mechanisms by the Major Facilitator Superfamily (MFS). Chem. Rev. 2021, 121, 5289–5335. [Google Scholar] [CrossRef]
- Law, C.; Maloney, P.; Wang, D.N. Ins and Outs of Major Facilitator Superfamily Antiporters. Annu. Rev. Microbiol. 2008, 62, 289–305. [Google Scholar] [CrossRef]
- Moradi, M.; Babin, V.; Roland, C.; Sagui, C. The Adaptively Biased Molecular Dynamics method revisited: New capabilities and an application. J. Phys. Conf. Ser. 2015, 640, 12020. [Google Scholar] [CrossRef]
- Madej, M.G. Function, Structure, and Evolution of the Major Facilitator Superfamily: The LacY Manifesto. Adv. Biol. 2014, 2014, 523591. [Google Scholar] [CrossRef]
- Feng, J.; Selvam, B.; Shukla, D. How do antiporters exchange substrates across the cell membrane? An atomic-level description of the complete exchange cycle in NarK. Structure 2021, 29, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Penderson, B.; Kumar, H.; Waight, A.; Risenmay, A.; Roe-Zurz, Z.; Chau, B.; Schlessinger, A.; Bonomi, M.; Harries, W.; Sali, A.; et al. Crystal structure of a eukaryotic phosphate transporter. Nature 2013, 496, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Weyand, S.; Shimamura, T.; Beckstein, O.; Sansom, M.; Iwata, S.; Henderson, P.; Cameron, A. The alternating access mechanism of transport as observed in the sodium-hydantoin transporter Mhp1. J. Synchrotron Radiat. 2011, 18, 20–23. [Google Scholar] [CrossRef]
- Shimamura, T.; Weyand, S.; Beckstein, O.; Rutherford, N.; Hadden, J.; Sharples, D.; Sansom, M.; Iwata, S.; Henderson, P.; Cameron, A. Molecular Basis of Alternating Access Membrane Transport by the Sodium-Hydantoin Transporter Mhp1. Science 2010, 328, 470–473. [Google Scholar] [CrossRef]
- Nomura, N.; Verdon, G.; Kang, H.J.; Shimamura, T.; Nomura, Y.; Sonoda, Y.; Hussien, S.A.; Qureshi, A.A.; Coincon, M.; Sato, Y.; et al. Structure and mechanism of the mammalian fructose transporter GLUT5. Nature 2015, 526, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Kanai, Y.; Clémençon, B.; Simonin, A.; Leuenberger, M.; Lochner, M.; Weisstanner, M.; Hediger, M.A. The SLC1 high-affinity glutamate and neutral amino acid transporter family. Mol. Asp. Med. 2013, 34, 108–120. [Google Scholar] [CrossRef]
- Singh, S.K.; Yamashita, A.; Gouaux, E. Antidepressant binding site in a bacterial homologue of neurotransmitter transporters. Nature 2007, 448, 952–956. [Google Scholar] [CrossRef]
- Shechter, E. Secondary Active Transport. Biochimie 1986, 86, 357–365. [Google Scholar] [CrossRef]
- Quistgaard, E.; Löw, C.; Guettou, F.; Norlund, P. Understanding transport by the major facilitator superfamily (MFS): Structures pave the way. Nat. Rev. Mol. Cell Biol. 2016, 17, 123–132. [Google Scholar] [CrossRef]
- Yan, N. Structural Advances for the Major Facilitator Superfamily (MFS) Transporters. Trends Biochem. Sci. 2013, 38, 151–159. [Google Scholar] [CrossRef]
- Lee, J.; Sands, Z.; Biggin, P. A Numbering System for MFS Transporter Proteins. Front. Mol. Biosci. 2016, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Teruhisa, H.; Jürgen, H.; Peter, M.; Sriram, S. Structural Model for 12-Helix Transporters Belonging to the Major Facilitator Superfamily. J. Bacteriol. 2003, 185, 1712–1718. [Google Scholar] [CrossRef]
- Madej, M.; Kaback, H. Evolutionary Mix-and-Match with MFS Transporters II. Proc. Natl. Acad. Sci. USA 2013, 110, E4831–E4838. [Google Scholar] [CrossRef]
- Mittal, S.; Dutta, S.; Shukla, D. Reconciling membrane protein simulations with experimental DEER spectroscopy data. Phys. Chem. Chem. Phys. 2023, 25, 6253–6262. [Google Scholar] [CrossRef] [PubMed]
- Fowler, P.W.; Orwick-Rydmark, M.; Radestock, S.; Solcan, N.; Dijkman, P.M.; Lyons, J.A.; Kwok, J.; Caffrey, M.; Watts, A.; Forrest, L.R.; et al. Gating Topology of the Proton-Coupled Oligopeptide Symporters. Structure 2015, 23, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.; Maksim, A.; Castillo, R.; Sun, E.; Saier, M. The Major Facilitator Superfamily (MFS) Revisited. Fed. Eur. Biochem. Soc. J. 2012, 279, 2022–2035. [Google Scholar] [CrossRef]
- Saier, M.; Beatty, J.; Goffeau, A.; Harley, K.; Heijne, W.; Jack, D.; Jähn, P.; Lew, K.; Liu, J.; Pao, S.; et al. The Major Facilitator Superfamily. J. Mol. Microbiol. Biotechnol. 1999, 1, 257–279. [Google Scholar] [PubMed]
- Abramson, J.; Smirnova, I.; Vladimir, K.; Verner, G.; Kaback, R.; Iwata, S. Structure and Mechanism of the Lactose Permease of Escherichia coli. Science 2003, 301, 610–615. [Google Scholar] [CrossRef]
- Heymann, J.; Sarker, R.; Teruhisa, H.; Shi, D.; Milne, J.; Maloney, P.; Subramaniam, S. Projection Structure and Molecular Architecture of OxIT, a Bacterial Membrane Transporter. Eur. Mol. Biol. Organ. J. 2001, 20, 4409–4413. [Google Scholar] [CrossRef]
- Hacksell, I.; Rigaud, J.; Purhonen, P.; Pourcher, T.; Hebert, H.; Leblanc, G. Projection Structure at 8 Å Resolution of the Melibiose Permease, an Na-sugar Co-transporter from Escherichia coli. Eur. Mol. Biol. Organ. J. 2002, 21, 3569–3574. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P. A General Theory of Membrane Transport from Studies of Bacteria. Nature 1957, 180, 134–136. [Google Scholar] [CrossRef] [PubMed]
- Durana, A.; Meilera, J. Inverted Topologies in Membrane Proteins: A Mini-Review. Comput. Struct. Biotechnol. J. 2013, 8, e201308004. [Google Scholar] [CrossRef] [PubMed]
- Radestock, S.; Forrest, L. The Alternating-Access Mechanism of MFS Transporters Arises from Inverted-Topology Repeats. J. Mol. Biol. 2011, 407, 698–715. [Google Scholar] [CrossRef]
- Forrest, L. Structural Symmetry in Membrane Proteins. Annu. Rev. Biophys. 2015, 44, 311–337. [Google Scholar] [CrossRef]
- Ubarretxena-Belandia, I.; Baldwin, J.; Schuldiner, S.; Tate, C. Three-Dimensional Structure of the Bacterial Multidrug Transporter EmrE Shows it is an Asymmetric Homodimer. Eur. Mol. Biol. Organ. J. 2003, 22, 6175–6181. [Google Scholar] [CrossRef]
- Selvam, B.; Mittal, S.; Shukla, D. Free Energy Landscape of the Complete Transport Cycle in a Key Bacterial Transporter. Am. Chem. Soc. Cent. Sci. 2018, 4, 1146–1154. [Google Scholar] [CrossRef]
- Mittal, S.; Shukla, D. Predicting Optimal DEER Label Positions to Study Protein Conformational Heterogeneity. J. Phys. Chem. 2017, 121, 9761–9770. [Google Scholar] [CrossRef]
- Solcan, N.; Kwok, J.; Fowler, P.W.; Cameron, A.D.; Drew, D.; Iwata, S.; Newstead, S. Alternating access mechanism in the POT family of oligopeptide transporters. Eur. Mol. Biol. Organ. J. 2012, 31, 3411–3421. [Google Scholar] [CrossRef]
- Mitchell, P. Osmochemistry of solute translocation. Res. Microbiol. 1990, 141, 286–289. [Google Scholar] [CrossRef]
- Patlak, C. Contributions to the theory of active transport: II. The gate type non-carrier mechanism and generalizations concerning tracer flow, efficiency, and measurement of energy expenditure. Bull. Math. Biophys. 1957, 19, 209–235. [Google Scholar] [CrossRef]
- Vidaver, G. Inhibition of parallel flux and augmentation of counter flux shown by transport models not involving a mobile carrier. J. Theor. Biol. 1966, 10, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Jardetzky, O. Simple Allosteric Model for Membrane Pumps. Nature 1966, 211, 969–970. [Google Scholar] [CrossRef]
- Renae, R.; Robert, V. Elevating the alternating-access model. Nat. Struct. Mol. Biol. 2016, 23, 187–189. [Google Scholar] [CrossRef]
- Forrest, L.; Rudnick, G. The Rocking Bundle: A Mechanism for Ion-Coupled Solute Flux by Symmetrical Transporters. Physiology 2009, 24, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Alisa, G.; Dirk, S. Elevator-type mechanisms of membrane transport. Viochem. Soc. Trans. 2020, 48, 1227–1241. [Google Scholar] [CrossRef]
- Lee, C.; Kang, H.J.; von Ballmoos, C.; Newstead, S.; Uzdavinys, P.; Dotson, D.; Iwata, S.; Beckstein, O.; Cameron, A.; Drew, D. A two-domain elevator mechanism for sodium/proton antiport. Nature 2013, 501, 573–577. [Google Scholar] [CrossRef]
- Ogden, D.; Immadisetty, K.; Moradi, M. Conformational Transition Pathways in Major Facilitator Superfamily Transporters. bioRxiv 2019. [Google Scholar] [CrossRef]
- Lukas, S.; Philip, F.; Mark, S.; Oliver, B. Flexible Gates Generate Occluded Intermediates in the Transport Cycle of LacY. J. Mol. Biol. 2014, 426, 735–751. [Google Scholar] [CrossRef]
- Kumar, S.; Nussinov, R. Salt bridge stability in monomeric proteins. J. Mol. Biol. 1999, 293, 1241–1255. [Google Scholar] [CrossRef]
- Kumar, S.; Nussinov, R. Close-Range Electrostatic Interactions in Proteins. ChemBioChem 2002, 3, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Bosshard, H.R.; Marti, D.; Jelesarov, I. Protein stabilization by salt bridges: Concepts, experimental approaches and clarification of some misunderstandings. J. Mol. Recognit. 2004, 17, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Donald, J.; Kulp, D.; DeGrado, W. Salt bridges: Geometrically specific, designable interactions. Proteins 2010, 79, 898–915. [Google Scholar] [CrossRef]
- Pylaeva, S.; Brehm, M.; Sebastiani, D. Salt Bridge in Aqueous Solution: Strong Structural Motifs but Weak Enthalpic Effect. Sci. Rep. 2018, 8, 13626. [Google Scholar] [CrossRef]
- Fox, J.; Kang, K.; Sherman, W.; Héroux, A.; Sastry, G.M.; Baghbanzadeh, M.; Lockett, M.; Whitesides, G. Interactions between Hofmeister Anions and the Binding Pocket of a Protein. J. Am. Chem. Soc. 2015, 137, 3859–3866. [Google Scholar] [CrossRef]
- Smith, M.D.; Cruz, L. Effect of Ionic Aqueous Environments on the Structure and Dynamics of the Aβ21–30 Fragment: A Molecular-Dynamics Study. J. Phys. Chem. 2013, 117, 6614–6624. [Google Scholar] [CrossRef]
- Petrauskas, V.; Maximowitsch, E.; Matulis, D. Thermodynamics of Ion Pair Formations Between Charged Poly(Amino Acid)s. J. Phys. Chem. 2015, 199, 12164–12171. [Google Scholar] [CrossRef]
- Law, C.; Yang, Q.; Soudant, C.; Maloney, P.; Wang, D.N. Kinetic Evidence Is Consistent with the Rocker-Switch Mechanism of Membrane Transport by GlpT. Biochemistry 2007, 46, 12190–12197. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Heng, J.; Jiang, D. Energy coupling mechanisms of MFS transporters. Protein Sci. 2015, 24, 1560–1579. [Google Scholar] [CrossRef] [PubMed]
- Newstead, S.; Drew, D.; Cameron, A.D.; Postis, V.L.G.; Xia, X.; Fowler, P.W.; Ingram, J.C.; Carpenter, E.P.; Sansom, M.S.P.; McPherson, M.J.; et al. Crystal structure of a prokaryotic homologue of the mammalian oligopeptide–proton symporters, PepT1 and PepT2. Eur. Mol. Biol. Organ. J. 2011, 30, 417–426. [Google Scholar] [CrossRef]
- Immadisetty, K.; Polasa, A.; Shelton, R.; Moradi, M. Elucidating the molecular basis of spontaneous activation in an engineered mechanosensitive channel. Comput. Struct. Biotechnol. J. 2022, 20, 2539–2550. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.; Iwata, S.; Kaback, H.R. Lactose permease as a paradigm for membrane transport proteins (Review). Mol. Membr. Biol. 2004, 21, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Guellec, J.; Elbahnsi, A.; Le Tertre, M.; Uguen, K.; Gourlaouen, I.; Férec, C.; Ka, C.; Callebaut, I.; Le Gac, G. Molecular model of the ferroportin intracellular gate and implications for the human iron transport cycle and hemochromatosis type 4A. Fed. Am. Soc. Exp. Biol. 2019, 33, 14625–14635. [Google Scholar] [CrossRef]
- Tortosa, V.; Bonaccorsi di Patti, C.; Iacovelli, F.; Pasquadibisceglie, A.; Falconi, M.; Musci, G.; Polticelli, F. Dynamical Behavior of the Human Ferroportin Homologue from Bdellovibrio bacteriovorus: Insight into the Ligand Recognition Mechanism. Int. J. Mol. Sci. 2020, 21, 6785. [Google Scholar] [CrossRef]
- Mohan, S.; Sheena, A.; Poulose, N.; Anilkuma, G. Molecular Dynamics Simulation Studies of GLUT4: Substrate-Free and Substrate-Induced Dynamics and ATP-Mediated Glucose Transport Inhibition. PLoS ONE 2010, 5, e14217. [Google Scholar] [CrossRef] [PubMed]
- Dastvan, R.; Rasouli, A.; Dehghani-Ghahnaviyeh, S.; Gies, S.; Tajkhorshid, E. Proton-driven alternating access in a spinster lipid transporter. Nat. Commun. 2022, 13, 5161. [Google Scholar] [CrossRef]
- Ethayathulla, A.; Yousef, M.; Amin, A.; Leblanc, G.; Kaback, R.; Guan, L. Structure-based mechanism for Na+/melibiose symport by MelB. Nat. Commun. 2014, 5, 3009. [Google Scholar] [CrossRef]
- Fukuda, M.; Takeda, H.; Kato, H.; Doki, S.; Ito, K.; Maturana, A.; Ishitani, R.; Nureki, O. Structural basis for dynamic mechanism of nitrate/nitrite antiport by NarK. Nat. Commun. 2015, 6, 7097. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.; Lindahl, L.; Zengel, J. Effects on translation pausing of alterations in protein and RNA components of the ribosome exit tunnel. J. Bacteriol. 2008, 190, 5862–5869. [Google Scholar] [CrossRef]
- Elvin, C.M.; Hardy, C.M.; Rosenberg, H. Pi exchange mediated by the GlpT-dependent sn-glycerol-3-phosphate transport system in Escherichia coli. J. Bacteriol. 1985, 161, 1054–1058. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, J.; Huang, Y.; Weng, D. Glycerol-3-phosphate transporter of Escherichia coli: Structure, function and regulation. Res. Microbiol. 2004, 188, 623–629. [Google Scholar] [CrossRef]
- Law, C.; Almqvist, J.; Berstein, A.; Goetz, R.; Huang, Y.; Soudant, C.; Laaksonen, A.; Hovmöller, S.; Wang, D. Salt-bridge Dynamics Control Substrate-induced Conformational Change in the Membrane Transporter GlpT. J. Mol. Biol. 2008, 378, 828–839. [Google Scholar] [CrossRef]
- Lemieux, J. Eukaryotic major facilitator superfamily transporter modeling based on the prokaryotic GlpT crystal structure (Review). Mol. Membr. Biol. 2007, 24, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Iyalomhe, O.; Khantwal, C.; Kang, D.C. The Structure and Function of OxlT, the Oxalate Transporter of Oxalobacter formigenes. J. Membr. Biol. 2015, 248, 641–650. [Google Scholar] [CrossRef]
- Forrest, L.R.; Zhang, Y.W.; Jacobs, M.T.; Gesmonde, J.; Xie, L.; Honig, B.H.; Rudnick, G. Mechanism for alternating access in neurotransmitter transporters. Proc. Natl. Acad. Sci. USA 2008, 105, 10338–10343. [Google Scholar] [CrossRef]
- Adhikary, S.; Deredge, D.J.; Nagarajan, A.; Forrest, L.R.; Wintrode, P.L.; Singh, S.K. Conformational dynamics of a neurotransmitter:sodium symporter in a lipid bilayer. Proc. Natl. Acad. Sci. USA 2017, 114, E1786–E1795. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Xu, C.; Sun, P.; Wu, J.; Yan, C.; Hu, M.; Yan, N. Crystal structure of the human glucose transporter GLUT1. Nature 2014, 510, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Weyand, S.; Shimamura, T.; Yajima, S.; Suzuki, S.; Mirza, O.; Krusong, K.; Carpenter, E.P.; Rutherford, N.G.; Hadden, J.M.; O’Reilly, J.; et al. Structure and Molecular Mechanism of a Nucleobase–Cation–Symport-1 Family Transporter. Science 2008, 322, 709–713. [Google Scholar] [CrossRef]
- Lu, F.; Li, S.; Jiang, Y.; Jiang, J.; Fan, H.; Lu, G.; Deng, D.; Dang, S.; Zhang, X.; Wang, J.; et al. Structure and mechanism of the uracil transporter UraA. Nature 2011, 472, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, H.; Gouaux, E. X-ray Structures of LeuT in Substrate-Free Outward-Open and apo Inward-Open States. Nature 2012, 481, 469–474. [Google Scholar] [CrossRef]
- Galochkina, T.; Ng Fuk Chong, M.; Challali, L.; Abbar, S.; Etchebest, C. New insights into GluT1 mechanics during glucose transfer. Sci. Rep. 2019, 9, 998. [Google Scholar] [CrossRef]
- Chen, X.Z.; Zhu, T.; Smith, D.E.; Hediger, M.A. Stoichiometry and Kinetics of the High-affinity H+-coupled Peptide Transporter PepT2. J. Biol. Chem. 1999, 274, 2773–2779. [Google Scholar] [CrossRef]
- Agu, R.; Cowley, E.; Shao, D.; MacDonald, C.; Kirkpatrick, D.; Renton, K.; Massoud, E. Proton-Coupled Oligopeptide Transporter (POT) Family Expression in Human Nasal Epithelium and Their Drug Transport Potential. Mol. Pharm. 2011, 8, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Doki, S.; Kato, H.E.; Solcan, N.; Iwaki, M.; Koyama, M.; Hattori, M.; Iwase, N.; Tsukazaki, T.; Sugita, Y.; Kandori, H.; et al. Structural basis for dynamic mechanism of proton-coupled symport by the peptide transporter POT. Proc. Natl. Acad. Sci. USA 2013, 110, 11343–11348. [Google Scholar] [CrossRef]
- Ganapathy, V.; Leibach, F.H. Role of pH gradient and membrane potential in dipeptide transport in intestinal and renal brush-border membrane vesicles from the rabbit. Studies with L-carnosine and glycyl-L-proline. J. Biol. Chem. 1983, 258, 14189–14192. [Google Scholar] [CrossRef] [PubMed]
- Daniel, H.; Spanier, B.; Kottra, G.; Weitz, D. From Bacteria to Man: Archaic Proton-Dependent Peptide Transporters at Work. Physiology 2006, 21, 93–102. [Google Scholar] [CrossRef]
- Fei, Y.J.; Kanai, Y.; Nussberger, S.; Ganapathy, V.; Leibach, F.H.; Romero, M.F.; Singh, S.K.; Boron, W.F.; Hediger, M.A. Expression cloning of a mammalian proton-coupled oligopeptide transporter. Nature 1994, 368, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Covitz, K.M.Y.; Amidon, G.L.; Sadée, W. Membrane Topology of the Human Dipeptide Transporter, hPEPT1, Determined by Epitope Insertions. Biochemistry 1998, 37, 15214–15221. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; He, X.; Szewczyk, P.; Nguyen, T.; Chang, G. Structure of the Multidrug Transporter EmrD from Escherichia coli. Science 2006, 312, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Daniel, H.; Kottra, G. The proton oligopeptide cotransporter family SLC15 in physiology and pharmacology. Pflüg. Arch. Eur. J. Physiol. 2004, 447, 610–618. [Google Scholar] [CrossRef]
- Weitz, D.; Harder, D.; Casagrande, F.; Fotiadis, D.; Obrdlik, P.; Kelety, B.; Daniel, H. Functional and Structural Characterization of a Prokaryotic Peptide Transporter with Features Similar to Mammalian PEPT1. J. Biol. Chem. 2007, 282, 2832–2839. [Google Scholar] [CrossRef] [PubMed]
- Killer, M.; Wald, J.; Pieprzyk, J.; Marlovits, T.C.; Löw, C. Structural snapshots of human PepT1 and PepT2 reveal mechanistic insights into substrate and drug transport across epithelial membranes. Sci. Adv. 2021, 7, eabk3259. [Google Scholar] [CrossRef]
- Coleman, J.A.; Green, E.M.; Gouaux, E. X-ray structures and mechanism of the human serotonin transporter. Nature 2016, 532, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Elferich, J.; Gouaux, E. Structures of LeuT in bicelles define conformation and substrate binding in a membrane-like context. Nat. Struct. Mol. Biol. 2012, 19, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Grewer, C.; Watzke, N.; Wiessner, M.; Rauen, T. Glutamate translocation of the neuronal glutamate transporter EAAC1 occurs within milliseconds. Proc. Natl. Acad. Sci. USA 2000, 97, 9706–9711. [Google Scholar] [CrossRef] [PubMed]
- Kaback, H.R.; Sahin-Tóth, M.; Weinglass, A.B. The kamikaze approach to membrane transport. Nat. Rev. Mol. Cell Biol. 2001, 2, 610–620. [Google Scholar] [CrossRef]
- Oldham, M.L.; Khare, D.; Quiocho, F.A.; Davidson, A.L.; Chen, J. Crystal structure of a catalytic intermediate of the maltose transporter. Nature 2007, 450, 515–521. [Google Scholar] [CrossRef]
- Wadiche, J.I.; Amara, S.G.; Kavanaugh, M.P. Ion fluxes associated with excitatory amino acid transport. Neuron 1995, 15, 721–728. [Google Scholar] [CrossRef]
- Singh, S.K.; Piscitelli, C.L.; Yamashita, A.; Gouaux, E. A Competitive Inhibitor Traps LeuT in an Open-to-Out Conformation. Science 2008, 322, 1655–1661. [Google Scholar] [CrossRef]
- Yan, R.; Zhao, X.; Lei, J.; Zhou, Q. Structure of the human LAT1–4F2hc heteromeric amino acid transporter complex. Nature 2019, 568, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Johnson, Z.L.; Cheong, C.G.; Lee, S.Y. Crystal structure of a concentrative nucleoside transporter from Vibrio cholerae at 2.4 Å. Nature 2012, 483, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Xuejun, Z.; Lei, H. Uniporter substrate binding and transport: Reformulating mechanistic questions. Biophys. Rep. 2016, 2, 45–54. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell; Garland Science: New York, NY, USA, 2002; Volume 4. [Google Scholar]
- Simpson, I.; Vannucci, S.; Maher, F. Glucose Transporters in Mammalian Brain. Biochem. Soc. Trans. 1994, 22, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Vollers, S.; Carruthers, A. Sequence Determinants of GLUT1-Mediated Accelerated-Exchange Transport. J. Biol. Chem. 2012, 287, 42533–42544. [Google Scholar] [CrossRef] [PubMed]
- Longo, N.; Griffin, L.; Elsas, L. Influx and Efflux of 3-O-methyl-D-glucose by Cultured Human Fibroblasts. Am. J. Physiol. 1988, 254, 628–633. [Google Scholar] [CrossRef]
- Sun, L.; Zeng, X.; Yan, C.; Sun, X.; Gong, X.; Rao, Y.; Yan, N. Crystal structure of a bacterial homologue of glucose transporters GLUT1–4. Nature 2012, 490, 361–366. [Google Scholar] [CrossRef]
- Qureshi, A.; Suades, A.; Matsuoka, R.; Brock, J.; McComas, S.; Nji, E.; Orellana, L.; Claesson, M.; Delemotte, L.; Drew, D. The Molecular Basis for Sugar Import in Malaria Parasites. Nature 2020, 578, 321–325. [Google Scholar] [CrossRef]
- Deng, D.; Sun, P.; Yan, C.; Ke, M.; Jiang, X.; Xiong, L.; Ren, W.; Hirata, K.; Yamamoto, M.; Fan, S.; et al. Molecular Basis of Ligand Recognition and Transport by Glucose Transporters. Nature 2015, 526, 391–396. [Google Scholar] [CrossRef]
- Kapoor, K.; Finer-Moore, J.S.; Pedersen, B.P.; Caboni, L.; Waight, A.; Hillig, R.C.; Bringmann, P.; Heisler, I.; Müller, T.; Siebeneicher, H.; et al. Mechanism of inhibition of human glucose transporter GLUT1 is conserved between cytochalasin B and phenylalanine amides. Proc. Natl. Acad. Sci. USA 2016, 113, 4711–4716. [Google Scholar] [CrossRef] [PubMed]
- Salas-Burgos, A.; Iserovich, P.; Zuniga, F.; Vera, J.C.; Fischbarg, J. Predicting the Three-Dimensional Structure of the Human Facilitative Glucose Transporter Glut1 by a Novel Evolutionary Homology Strategy: Insights on the Molecular Mechanism of Substrate Migration, and Binding Sites for Glucose and Inhibitory Molecules. Biophys. J. 2004, 87, 2990–2999. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Yan, N. GLUT, SGLT, and SWEET: Structural and mechanistic investigations of the glucose transporters. Protein Sci. 2016, 25, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, D.; Forrest, L.; Schuldiner, S. The Ins and Outs of Vesicular Monoamine Transporters. J. Gen. Physiol. 2018, 150, 671–682. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sauve, S.; Williamson, J.; Polasa, A.; Moradi, M. Ins and Outs of Rocker Switch Mechanism in Major Facilitator Superfamily of Transporters. Membranes 2023, 13, 462. https://doi.org/10.3390/membranes13050462
Sauve S, Williamson J, Polasa A, Moradi M. Ins and Outs of Rocker Switch Mechanism in Major Facilitator Superfamily of Transporters. Membranes. 2023; 13(5):462. https://doi.org/10.3390/membranes13050462
Chicago/Turabian StyleSauve, Stephanie, Joseph Williamson, Adithya Polasa, and Mahmoud Moradi. 2023. "Ins and Outs of Rocker Switch Mechanism in Major Facilitator Superfamily of Transporters" Membranes 13, no. 5: 462. https://doi.org/10.3390/membranes13050462
APA StyleSauve, S., Williamson, J., Polasa, A., & Moradi, M. (2023). Ins and Outs of Rocker Switch Mechanism in Major Facilitator Superfamily of Transporters. Membranes, 13(5), 462. https://doi.org/10.3390/membranes13050462






