Preparation of Hydrolyzed Sugarcane Molasses as a Low-Cost Medium for the Mass Production of Probiotic Lactobacillus paracasei ssp. paracasei F19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Microorganism
2.3. Pretreatment and Hydrolysis Conditions of Molasses
2.4. Media and Culture Conditions for Bacteria
2.5. Analytical Methods
2.6. Experimental Design
3. Results and Discussion
3.1. Composition of Raw Material
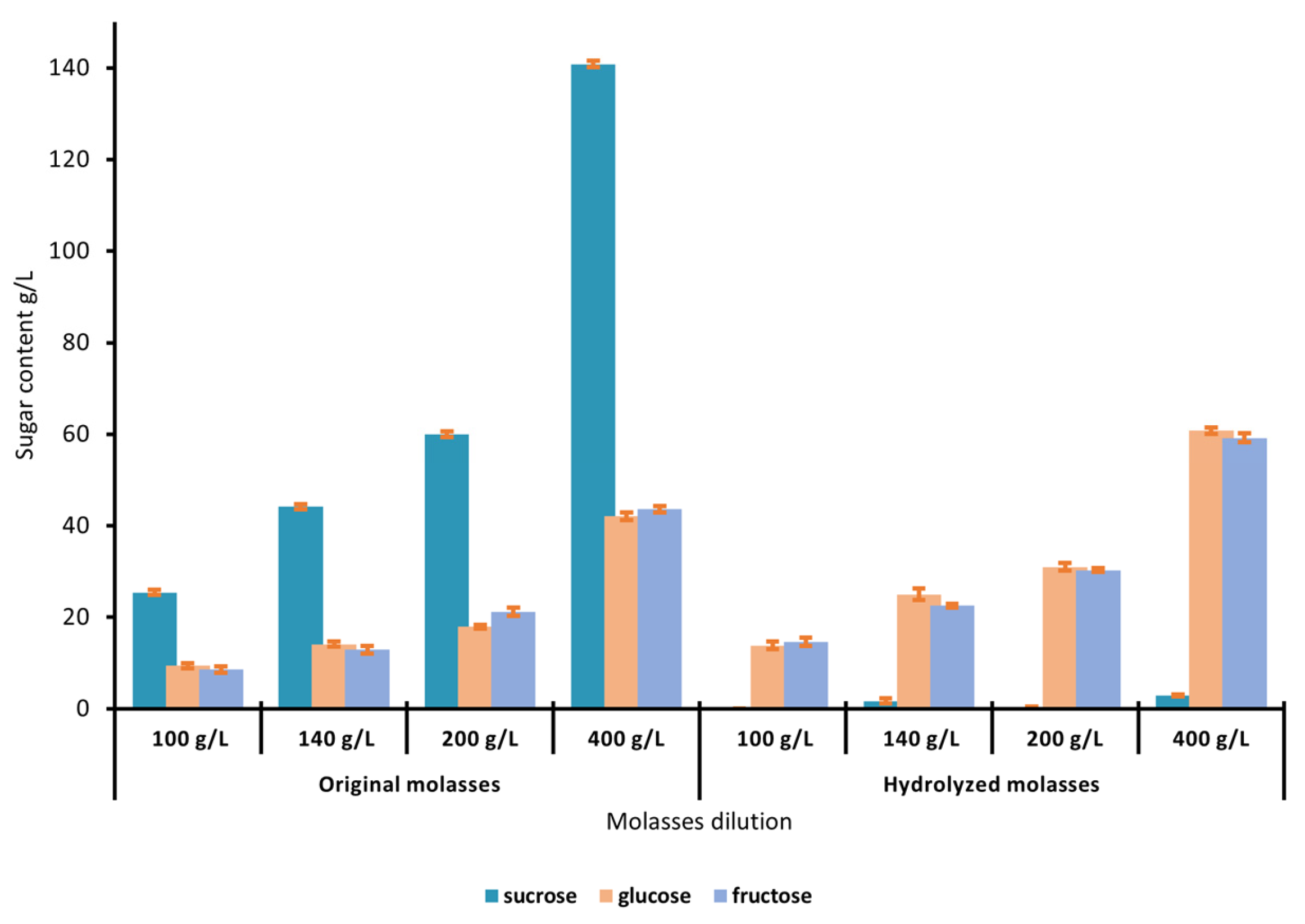
3.2. Molasses Pretreatment and Hydrolysis Conditions
3.3. Microtox Test
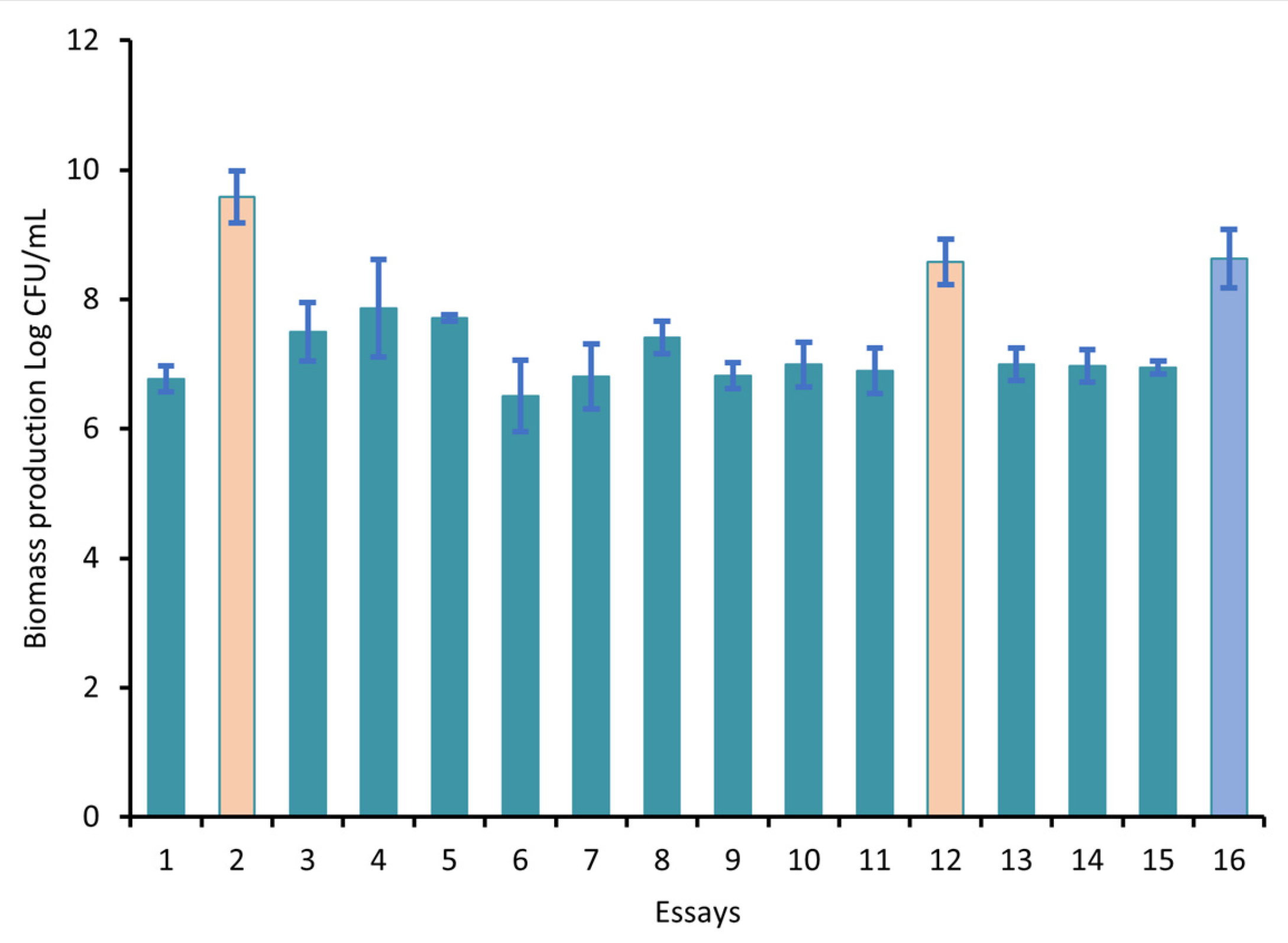
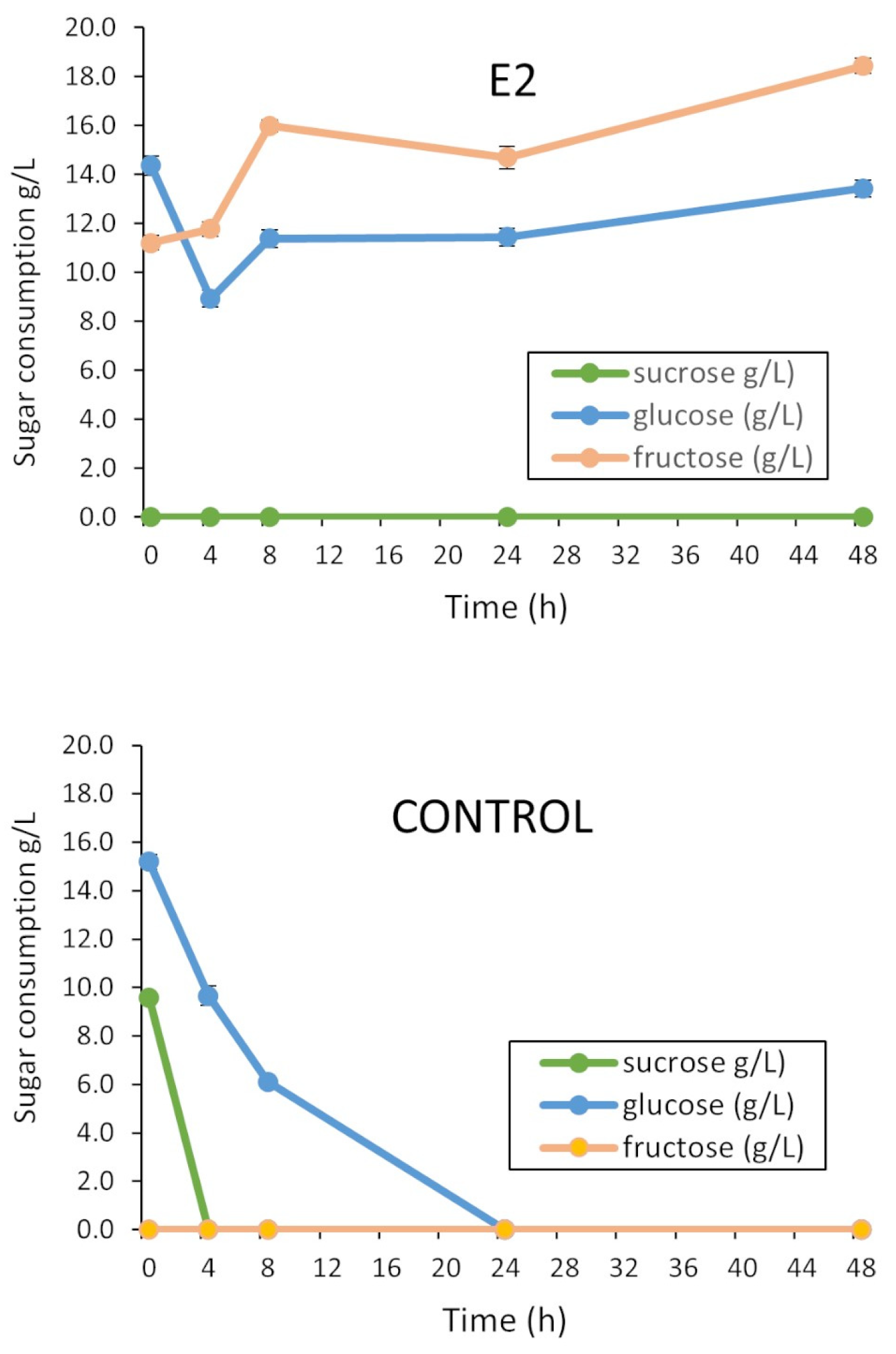
3.4. Optimization of the Culture Medium
3.5. Cost of Molasses Culture Medium (MCM)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chávarri, M.; Diez-Gutiérrez, L.; Marañón, I.; del Carmen Villarán, M.; Barrón, L.J.R. Chapter 27—The role of probiotics in nutritional health: Probiotics as nutribiotics. In Probiotics in the Prevention and Management of Human Diseases; Dwivedi, M.K., Amaresan, N., Sankaranarayanan, A., Kemp, E.H., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 397–415. [Google Scholar]
- Zendeboodi, F.; Khorshidian, N.; Mortazavian, A.M.; da Cruz, A.G. Probiotic: Conceptualization from a new approach. Curr. Opin. Food Sci. 2020, 32, 103–123. [Google Scholar] [CrossRef]
- Acosta-Piantini, E.; Villaran, M.; Lombraña, J.I. Stabilization of encapsulated probiotics from the bacterium Lactobacillus casei by different drying techniques, IDS 2018. In 21st International Drying Symposium Proceedings; Editorial Universitat Politècnica de València: Valencia, Spain, 2018; pp. 691–698. [Google Scholar] [CrossRef]
- Burgain, J.; Gaiani, C.; Linder, M.; Scher, J. Encapsulation of probiotic living cells: From laboratory scale to industrial Applications. J. Food Eng. 2011, 104, 467–483. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Rocha, V.; Teixeira, J.A.; Rodrigues, L.R. Antimicrobial and antiadhesive properties of a biosurfactant isolated from Lactobacillus paracasei ssp. paracasei A20. Lett. Appl. Microbiol. 2010, 50, 419–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hippolyte Mouafo, T. Application of response surface methodology to improve the production of antimicrobial biosurfactants by Lactobacillus paracasei subsp. tolerans N2 using sugar cane molasses as substrate. Bioresour. Bioprocess. 2018, 5, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Aouidi, F.; Gannoun, H.; Ben Othman, N.; Ayed, L.; Hamdi, M. Improvement of fermentative decolorization of olive mill wastewater by Lactobacillus paracasei by cheese whey’s addition. Process Biochem. 2009, 44, 597–601. [Google Scholar] [CrossRef]
- Hwang, C.; Chang, J.; Houng, J.; Tsai, C.; Lin, C.; Tsen, H. Optimization of medium composition for improving biomass production of Lactobacillus plantarum Pi06 using the Taguchi array design and the Box-Behnken method. Biotechnol. Bioprocess Eng. 2012, 17, 827–834. [Google Scholar] [CrossRef]
- Dumbrepatil, A.; Adsul, M.; Chaudhari, S.; Khire, J.; Gokhale, D. Utilization of molasses sugar for lactic acid production by Lactobacillus delbrueckii subsp. delbrueckii mutant Uc-3 in batch fermentation. Appl. Environ. Microbiol. 2008, 74, 333–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ai, H.; Liu, M.; Yu, P.; Zhang, S.; Suo, Y.; Luo, P.; Li, S.; Wang, J. Improved welan gum production by Alcaligenes sp. ATCC31555 from pretreated cane molasses. Carbohydr. Polym. 2015, 129, 35–43. [Google Scholar] [CrossRef]
- Rodrigues, L.R.; Teixeira, J.A.; Oliveira, R. Low-cost fermentative medium for biosurfactant production by probiotic bacteria. Biochem. Eng. J. 2006, 32, 135–142. [Google Scholar] [CrossRef]
- Lopez-Arenas, T.; Anaya-Reza, O.; Perez-Cisneros, E.S.; Sales-Cruz, M. 19—Conceptual design of sugarcane biorefinery upgrading molasses to value-added chemicals. In A–Z of Biorefinery; Thongchul, N., Kokossis, A., Assabumrungrat, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 683–712. [Google Scholar]
- Gutiérrez, M.; Etxebarria, J.; de las Fuentes, L. Evaluation of wastewater toxicity: Comparative study between Microtox® and activated sludge oxygen uptake inhibition. Water Res. 2002, 36, 919–924. [Google Scholar] [CrossRef]
- Lino, F.S.d.O.; Basso, T.O.; Sommer, M.O.A. A synthetic medium to simulate sugarcane molasses. Biotechnol. Biofuels 2018, 11, 1–12. [Google Scholar] [CrossRef]
- Ruiz, S.P.; Martinez, C.O.; Noce, A.S.; Sampaio, A.R.; Baesso, M.L.; Matioli, G. Biosynthesis of succinoglycan by Agrobacterium radiobacter NBRC 12665 immobilized on loofa sponge and cultivated in sugar cane molasses. Structural and rheological characterization of biopolymer. J. Molec. Catal. B 2015, 122, 15–28. [Google Scholar] [CrossRef]
- Xu, S.; Hao, N.; Xu, L.; Liu, Z.; Yan, M.; Li, Y.; Ouyang, P. Series fermentation production of ornithine and succinic acid from cane molasses by Corynebacterium glutamicum. Biochem. Eng. J. 2015, 99, 177–182. [Google Scholar] [CrossRef]
- Hujanen, M.; Linko, S.; Linko, Y.; Leisola, M. Optimisation of media and cultivation conditions for L (+)(S)-lactic acid production by Lactobacillus casei NRRL B-441. Appl. Microbiol. Biotechnol. 2001, 56, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Batt, C. Characteristics of the Lactobacillus Species; Cornell University, Academic Press: New York, NY, USA, 2012. [Google Scholar]
- Ishaque, A.B.; Johnson, L.; Gerald, T.; Boucaud, D.; Okoh, J.; Tchounwou, P.B. Assessment of individual and combined toxicities of four non-essential metals (As, Cd, Hg and Pb) in the microtox assay. Int. J. Environ. Res. Public Health 2006, 3, 118–120. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zheng, P.; Sun, Z.; Ni, Y.; Dong, J.; Zhu, L. Economical succinic acid production from cane molasses by Actinobacillus succinogenes. Bioresour. Technol. 2008, 99, 1736–1742. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.A. Syrus In Encyclopedia of Food Sciences and Nutrition; Academic Press: Cambridge, MA, USA, 2003; pp. 154–196. [Google Scholar]
- Xu, K.; Xu, P. Efficient production of l-lactic acid using co-feeding strategy based on cane molasses/glucose carbon sources. Bioresour. Technol. 2014, 153, 23–29. [Google Scholar] [CrossRef]
- Santos, F.; Eichler, P.; Machado, G.; De Mattia, J.; De Souza, G. By-products of the sugarcane industry. In Sugarcane Biorefinery, Technology and Perspectives; Academic Press: Washington, DC, USA, 2020; pp. 21–48. [Google Scholar]
- Xia, J.; Xu, Z.; Xu, H.; Liang, J.; Li, S.; Feng, X. Economical production of poly (ε-l-lysine) and poly (l-diaminopropionic acid) using cane molasses and hydrolysate of streptomyces cells by Streptomyces albulus PD-1. Bioresour. Technol. 2014, 164, 241–247. [Google Scholar] [CrossRef]
- Teclu, D.; Tivchev, G.; Laing, M.; Wallis, M. Determination of the elemental composition of molasses and its suitability as carbon source for growth of sulphate-reducing bacteria. J. Hazard. Mater 2009, 161, 1157–1165. [Google Scholar] [CrossRef]
- Ayangbenro, A.S.; Babalola, O.O. A New Strategy for Heavy Metal Polluted Environments: A Review of Microbial Biosorbents. Int. J. Environ. Res. Public Health 2017, 14, 94. [Google Scholar] [CrossRef]
- Hébert, E.M.; Raya, R.R.; Giori, G.S.d. Nutritional requirements of Lactobacillus delbrueckii subsp. lactis in a chemically defined medium. Curr. Microbiol. 2004, 49, 341–345. [Google Scholar] [CrossRef]
- Schär-Zammaretti, P.; Dillmann, M.; D’Amico, N.; Affolter, M.; Ubbink, J. Influence of fermentation medium composition on physicochemical surface properties of Lactobacillus acidophilus. Appl. Environ. Microbiol. 2005, 71, 8165–8173. [Google Scholar] [CrossRef] [Green Version]
- Schepers, A.W.; Thibault, J.; Lacroix, C. Lactobacillus helveticus growth and lactic acid production during pH-controlled batch cultures in whey permeate/yeast extract medium. Part I. Multiple factor kinetic analysis. Enzyme. Microb. Technol. 2002, 30, 176–186. [Google Scholar] [CrossRef]
- Brignone, D.; Radmann, P.; Behr, J.; Vogel, R.F. Boosting the growth of the probiotic strain Lactobacillus paracasei ssp. paracasei F19. Arch. Microbiol. 2017, 199, 853–862. [Google Scholar] [CrossRef]
- Wegkamp, A.; Teusink, B.; De Vos, W.M.; Smid, E.J. Development of a minimal growth medium for Lactobacillus plantarum. Lett. Appl. Microbiol. 2010, 50, 57–64. [Google Scholar] [CrossRef]
- Grobben, G.J.; Boels, I.C.; Sikkema, J.; Smith, M.R.; de Bont, J.A. Influence of ions on growth and production of exopolysaccharides by Lactobacillus delbrueckii subsp. bulgaricus NCFB 2772. J. Dairy Res. 2000, 67, 131–135. [Google Scholar] [CrossRef] [Green Version]
- Fitzpatrick, J.J.; Ahrens, M.; Smith, S. Effect of manganese on Lactobacillus casei fermentation to produce lactic acid from whey permeate. Process Biochem. 2001, 36, 671–675. [Google Scholar] [CrossRef]
- Saeed, A.H.; Salam, A.I. Current limitations and challenges with lactic acid bacteria: A review. Food Nutr. Sci. 2013, 2013, 73–87. [Google Scholar]
- Gerdtzen, Z.P. Medium Design, Culture Management, and the PAT Initiative. In Applied Bioengineering: Innovations and Future Directions; Wiley-VCH: Weinheim, Germany, 2017; pp. 383–416. [Google Scholar]
- Mladenović, D.D.; Djukić-Vuković, A.P.; Kocić-Tanackov, S.D.; Pejin, J.D.; Mojović, L.V. Lactic acid production on a combined distillery stillage and sugar beet molasses substrate. J. Chem. Technol. Biotechnol. 2016, 91, 2474–2479. [Google Scholar] [CrossRef]
- Krzywonos, M.; Eberhard, T. High density process to cultivate Lactobacillus plantarum biomass using wheat stillage and sugar beet molasses. EJB 2011, 14, 6. [Google Scholar] [CrossRef]
- Kadam, S.R.; Patil, S.S.; Bastawde, K.B.; Khire, J.M.; Gokhale, D.V. Strain improvement of Lactobacillus delbrueckii NCIM 2365 for lactic acid production. Process Biochem. 2006, 41, 120–126. [Google Scholar] [CrossRef]
- Vidra, A.; Tóth, A.J.; Németh, Á. Lactic acid production from cane molasses. Waste Treat. Recovery 2017, 2, 13–16. [Google Scholar] [CrossRef]
- INAZUCAR. Institutional Memory. 2020, Dominican Republic. Available online: https://www.inazucar.gov.do/transparencia/index.php/plan-estrategico/memorias-institucionales?download=252:memoria-anual-2020 (accessed on 14 January 2022).
- Peters, M.S.; Timmerhaus, K.D.; West, R.E. Analysis of Cost Estimation. In Plant Design and Economics for Chemical Engineers, 5th ed.; McGraw Hill Education: Chennai, India, 2011; pp. 224–278. [Google Scholar]



| RF Power (W) | 1550 |
|---|---|
| Plasma gas flow (L min−1) | 15 |
| Carrier gas flow (L min−1) | 0.85–0.90 |
| Sample flow rate (mL min−1) | 0.1 |
| He flow rate (mL min−1) | 4.3 |
| Extraction lens 1 (V) | 2 |
| Extraction lens 2 (V) | −140 |
| Omega bias (V) | −30 |
| Omega lens (V) | 1 |
| Cell input (V) | −34 |
| QP focus | 2 |
| Cell output (V) | −30 |
| Octopole RF (V) | 150 |
| Octopole bias | −6 |
| QP bias | −3 |
| Data acquisition | (Dwell time, 300 ms) |
| Sweeps per replicate | 8 |
| Replicates | 3 |
| Detection mode | Peak hopping |
| Isotopes | 75As, 111Cd, 59Co, 52Cr, 63Cu, 56Fe, 55Mn, 96Mo, 56Ni 208, 207, 206Pb, 51V and 66Zn |
| Essay | Yeast Extract, g |
Minerals (% Max Level *) | Surfactant Agent, mL |
Biomass & (log CFU/mL) |
|---|---|---|---|---|
| E1 | 0 | 50 | 1 | 6.77 a |
| E2 | 4 | 50 | 0 | 9.58 b |
| E3 | 4 | 50 | 1 | 7.50 a |
| E4 | 0 | 50 | 0 | 7.86 a |
| E5 | 2 | 0 | 0 | 7.71 a |
| E6 | 0 | 0 | 0.5 | 6.51 a |
| E7 | 2 | 0 | 1 | 6.81 a |
| E8 | 4 | 100 | 0.5 | 7.41 a |
| E9 | 0 | 100 | 0.5 | 6.82 a |
| E10 | 2 | 100 | 1 | 6.99 a |
| E11 | 4 | 0 | 0.5 | 6.90 a |
| E12 | 2 | 100 | 0 | 8.58 c |
| E13 | 2 | 50 | 0.5 | 7.00 a |
| E14 | 2 | 50 | 0.5 | 6.97 a |
| E15 | 2 | 50 | 0.5 | 6.94 a |
| E16 # | CF | CF | CF | 8.63 c |
| Sample | TU | EC50, % |
|---|---|---|
| Molasses without hydrolysis | 21 | 4.8 |
| Molasses with hydrolysis, pH = 6.5 | 18 | 5.6 |
| Molasses with hydrolysis and centrifugation, pH = 6.5 | 19 | 5.3 |
| Molasses with hydrolysis, pH = 8.5 | 15 | 6.7 |
| Molasses with hydrolysis and centrifugation, pH = 8.5 | 7.1 | 14 |
| Metal * | Concentration (µg /L) | Reduction (%) after Pre-Treatment |
|---|---|---|
| V | 72.5 | 61.2 |
| Cr | 73.2 | 31.7 |
| Mn | 6835.0 | 57.5 |
| Fe | 24,963.0 | 72.9 |
| Co | 135.0 | 55.0 |
| Ni | 446.0 | 33.9 |
| Cu | 2027.0 | 63.0 |
| Zn | 1160.0 | 51.6 |
| As | 15.3 | 50.3 |
| Mo | 30.1 | 40.2 |
| Cd | 1.4 | 64.3 |
| Pb | 23.9 | 60.0 |
| Raw Material |
Quantity/L of MCM | Laboratory | Industry | ||
|---|---|---|---|---|---|
| Market Price * | Cost of MCM (EUR/L) | Market Price * | Cost of MCM (EUR/L) | ||
| Molasses | 400 g | 0.669 ** | 0.0706 | 0.669 ** | 0.0706 |
| Yeast extract | 4.00 g | 42.14/500 g | 0.3371 | 125/25 kg | 0.0200 |
| Dipotassium hydrogen phosphate | 2.00 g | 40.32/kg | 0.0806 | 592.50/25 kg | 0.0474 |
| Sodium acetate trihydrate | 5.00 g | 19.21/kg | 0.0961 | 197.50/25 kg | 0.0395 |
| Triammonium citrate | 2.00 g | 38.70/250 g | 0.3096 | 38.70/250 g | 0.3096 |
| Magnesium sulfate heptahydrate | 0.20 g | 15.30/100 g | 0.0306 | 142.50/25 kg | 0.0011 |
| Manganese sulfate tetrahydrate | 0.05 g | 26.60/100 g | 0.0133 | 24.70/kg | 0.0012 |
| Surfactant agent | 1.00 mL | 23.50/25 mL | 0.9400 | 138.6/L | 0.1386 |
| Molasses Pre-treatment | |||||
| Sulfuric acid 98% | 8.80 mL | 20.26/L | 0.1783 | 20.26/L | 0.1783 |
| Sodium hydroxide | 0.67 g | 155/5 kg | 0.0208 | 155/5 kg | 0.0208 |
| Total | 1.2310 | 0.8271 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acosta-Piantini, E.; Rodríguez-Díez, E.; Chavarri, M.; López-de-Armentia, I.; Villaran, M.C.; Lombraña, J.I. Preparation of Hydrolyzed Sugarcane Molasses as a Low-Cost Medium for the Mass Production of Probiotic Lactobacillus paracasei ssp. paracasei F19. Separations 2023, 10, 33. https://doi.org/10.3390/separations10010033
Acosta-Piantini E, Rodríguez-Díez E, Chavarri M, López-de-Armentia I, Villaran MC, Lombraña JI. Preparation of Hydrolyzed Sugarcane Molasses as a Low-Cost Medium for the Mass Production of Probiotic Lactobacillus paracasei ssp. paracasei F19. Separations. 2023; 10(1):33. https://doi.org/10.3390/separations10010033
Chicago/Turabian StyleAcosta-Piantini, Elsa, Elena Rodríguez-Díez, María Chavarri, Iratxe López-de-Armentia, M. Carmen Villaran, and José Ignacio Lombraña. 2023. "Preparation of Hydrolyzed Sugarcane Molasses as a Low-Cost Medium for the Mass Production of Probiotic Lactobacillus paracasei ssp. paracasei F19" Separations 10, no. 1: 33. https://doi.org/10.3390/separations10010033
APA StyleAcosta-Piantini, E., Rodríguez-Díez, E., Chavarri, M., López-de-Armentia, I., Villaran, M. C., & Lombraña, J. I. (2023). Preparation of Hydrolyzed Sugarcane Molasses as a Low-Cost Medium for the Mass Production of Probiotic Lactobacillus paracasei ssp. paracasei F19. Separations, 10(1), 33. https://doi.org/10.3390/separations10010033








