Lectin Purification through Affinity Chromatography Exploiting Macroporous Monolithic Adsorbents
Abstract
:1. Introduction
2. Lectin Activity
3. Lectin-Carbohydrate Interaction
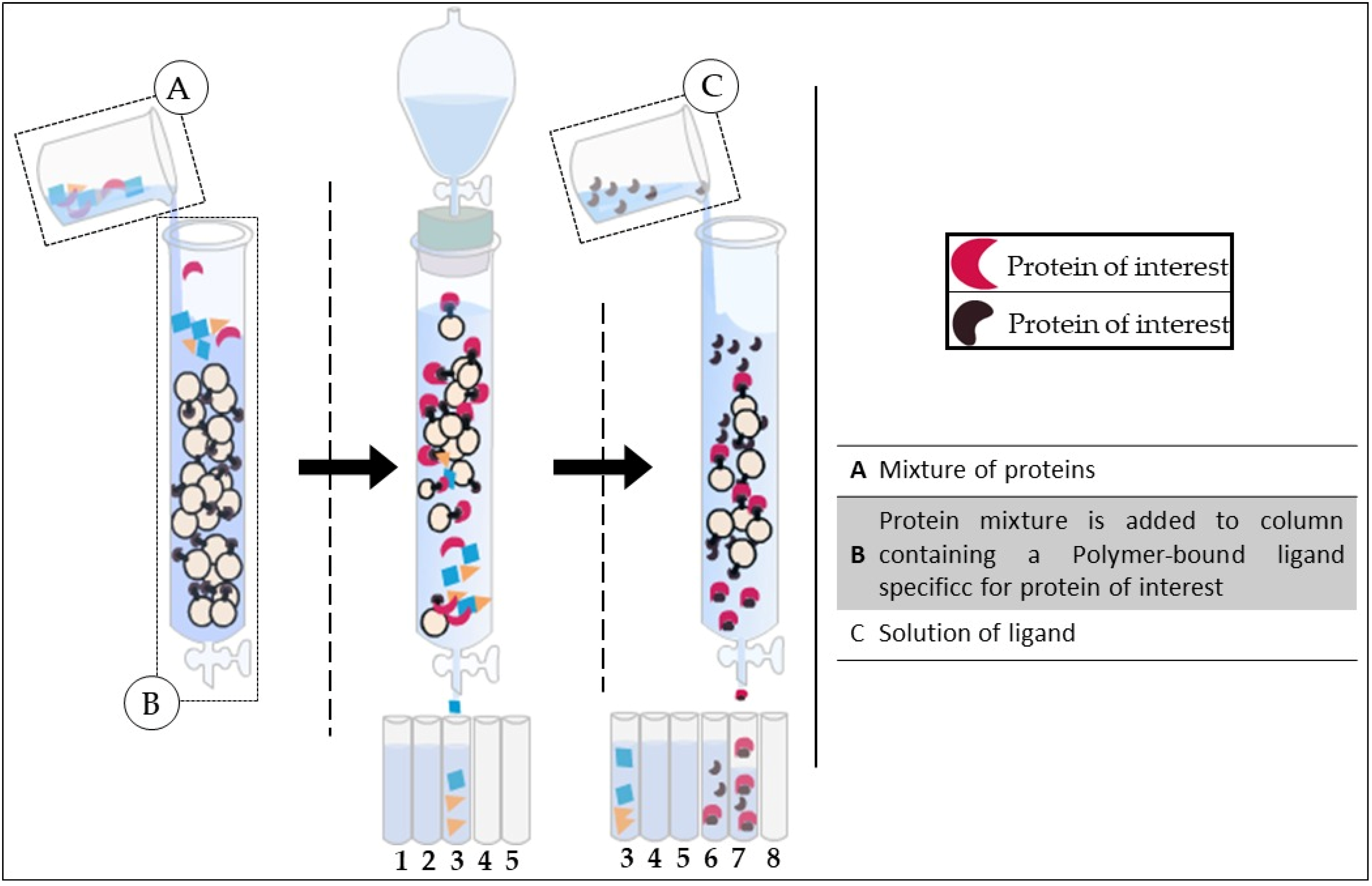
4. Purification of Lectins
5. Monolithic Polymeric Cryogels
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Janson, J.C. Principles, High Resolution Methods, and Applications. In Protein Purification; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 3–50. ISBN 978-0-471-74661-4. [Google Scholar]
- Dainiak, M.B.; Allan, I.U.; Savina, I.N.; Cornelio, L.; James, E.S.; James, S.L.; Mikhalovsky, S.v.; Jungvid, H.; Galaev, I.Y. Gelatin-Fibrinogen Cryogel Dermal Matrices for Wound Repair: Preparation, Optimisation and in Vitro Study. Biomaterials 2010, 31, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Perçin, I.; Khalaf, R.; Brand, B.; Morbidelli, M.; Gezici, O. Strong Cation-Exchange Chromatography of Proteins on a Sulfoalkylated Monolithic Cryogel. J. Chromatogr. A 2015, 1386, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.P.; Wang, C.; Sun, Y. Coating of Nanoparticles on Cryogel Surface and Subsequent Double-Modification for Enhanced Ion-Exchange Capacity of Protein. J. Chromatogr. A 2014, 1359, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Ünlüer, Ö.B.; Ersöz, A.; Denizli, A.; Demirel, R.; Say, R. Separation and Purification of Hyaluronic Acid by Embedded Glucuronic Acid Imprinted Polymers into Cryogel. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2013, 934, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Chaki, S.; Bhowal, J.; Chatterjee, B.P. Mucin Binding Mitogenic Lectin from Freshwater Indian Gastropod Belamyia Bengalensis: Purification and Molecular Characterization. Arch. Biochem. Biophys. 2004, 421, 125–134. [Google Scholar] [CrossRef]
- Oliveira, J.T.A.; Melo, V.M.M.; Câmara, M.F.L.; Vasconcelos, I.M.; Beltramini, L.M.; Machado, O.L.T.; Gomes, V.M.; Pereira, S.P.; Fernandes, C.F.; Nunes, E.P.; et al. Purification and Physicochemical Characterization of a Cotyledonary Lectin from Luetzelburgia Auriculata. Phytochemistry 2002, 61, 301–310. [Google Scholar] [CrossRef]
- Roy, I.; Sardar, M.; Gupta, M.N. Cross-Linked Alginate-Guar Gum Beads as Fluidized Bed Affinity Media for Purification of Jacalin. Biochem. Eng. J. 2005, 23, 193–198. [Google Scholar] [CrossRef]
- Gonçalves, G.R.F.; Gandolfi, O.R.R.; Santos, C.M.S.; Bonomo, R.C.F.; Veloso, C.M.; Fontan, R.d.C.I. Development of Supermacroporous Monolithic Adsorbents for Purifying Lectins by Affinity with Sugars. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1033–1034, 406–412. [Google Scholar] [CrossRef]
- Ourth, D.D.; Rose, W.M. Purification, Characterization and Seasonal Variation of Mannose-Binding C-Type Lectin in Ictalurid Catfish. Aquaculture 2011, 321, 191–196. [Google Scholar] [CrossRef]
- Jung, W.K.; Park, P.J.; Kim, S.K. Purification and Characterization of a New Lectin from the Hard Roe of Skipjack Tuna, Katsuwonus Pelamis. Int. J. Biochem. Cell Biol. 2003, 35, 255–265. [Google Scholar] [CrossRef]
- Arora, S.; Saxena, V.; Ayyar, B.V. Affinity Chromatography: A Versatile Technique for Antibody Purification. Methods 2017, 116, 84–94. [Google Scholar] [CrossRef]
- Mallik, R.; Hage, D.S. Affinity Monolith Chromatography. J. Sep. Sci. 2006, 29, 1686–1704. [Google Scholar] [CrossRef]
- Pfaunmiller, E.L.; Paulemond, M.L.; Dupper, C.M.; Hage, D.S. Affinity Monolith Chromatography: A Review of Principles and Recent Analytical Applications. Anal. Bioanal. Chem. 2013, 405, 2133–2145. [Google Scholar] [CrossRef] [Green Version]
- Ferreira da Silva, J.; Lemos da Silva, D.; Gomes Nascimento, R.; Ayra Alcântara Veríssimo, L.; Martins Veloso, C.; Ferreira Bonomo, R.C.; da Costa Ilhéu Fontan, R. Enhancements in Sugar Immobilization in Polymeric Macroporous Matrices for Affinity Capture. J. Appl. Polym. Sci. 2019, 136, 47956. [Google Scholar] [CrossRef]
- Gondim, A.C.S.; Romero-Canelón, I.; Sousa, E.H.S.; Blindauer, C.A.; Butler, J.S.; Romero, M.J.; Sanchez-Cano, C.; Sousa, B.L.; Chaves, R.P.; Nagano, C.S.; et al. The Potent Anti-Cancer Activity of Dioclea Lasiocarpa Lectin. J. Inorg. Biochem. 2017, 175, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Abe, T.; Umehara, K.; Choi, J.H.; Hirai, H.; Dohra, H.; Kawagishi, H. Purification and Characterization of a Lectin from the Mushroom Hypsizigus Marmoreus. Mycoscience 2015, 56, 359–363. [Google Scholar] [CrossRef] [Green Version]
- Selvaprakash, K.; Chen, Y.C. Functionalized Gold Nanoparticles as Affinity Nanoprobes for Multiple Lectins. Colloids Surf. B Biointerfaces 2018, 162, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Matoba, Y.; Sato, Y.; Oda, K.; Hatori, Y.; Morimoto, K. Lectins Engineered to Favor a Glycan-Binding Conformation Have Enhanced Antiviral Activity. J. Biol. Chem. 2021, 296. [Google Scholar] [CrossRef]
- Gajbhiye, V.; Gong, S. Lectin Functionalized Nanocarriers for Gene Delivery. Biotechnol. Adv. 2013, 31, 552–562. [Google Scholar] [CrossRef]
- He, S.; Shi, J.; Walid, E.; Zhang, H.; Ma, Y.; Xue, S.J. Reverse Micellar Extraction of Lectin from Black Turtle Bean (Phaseolus Vulgaris): Optimisation of Extraction Conditions by Response Surface Methodology. Food Chem. 2015, 166, 93–100. [Google Scholar] [CrossRef]
- Lavín de Juan, L.; García Recio, V.; Jiménez López, P.; Girbés Juan, T.; Cordoba-Diaz, M.; Cordoba-Diaz, D. Pharmaceutical Applications of Lectins. J. Drug Deliv. Sci. Technol. 2017, 42, 126–133. [Google Scholar] [CrossRef]
- Santos, A.L.E.; Júnior, C.P.S.; Neto, R.N.M.; Santos, M.H.C.; Santos, V.F.; Rocha, B.A.M.; Sousa, E.M.; Carvalho, R.C.; Menezes, I.R.A.; Oliveira, M.R.C.; et al. Machaerium Acutifolium Lectin Inhibits Inflammatory Responses through Cytokine Modulation. Proc. Biochem. 2020, 97, 149–157. [Google Scholar] [CrossRef]
- Carrillo, C.; Cordoba-Diaz, D.; Cordoba-Diaz, M.; Girbés, T.; Jiménez, P. Effects of Temperature, PH and Sugar Binding on the Structures of Lectins Ebulin f and SELfd. Food Chem. 2017, 220, 324–330. [Google Scholar] [CrossRef]
- Singh, A.; Trans, K.S.-C.S. Effect of Temperature, PH and Denaturing Agents on Biological Activity of MCJ Lectin. Chem. Sci. Trans. 2013, 2. [Google Scholar] [CrossRef] [Green Version]
- Sharon, N.; Lis, H. Legume Lectins—A Large Family of Homologous Proteins. FASEB J. 1990, 4, 3198–3208. [Google Scholar] [CrossRef]
- Kanellopoulos, P.N.; Tucker, P.A.; Pavlou, K.; Agianian, B.; Hamodrakas, S.J. A Triclinic Crystal Form of the Lectin Concanavalin A. J. Struct. Biol. 1996, 117, 16–23. [Google Scholar] [CrossRef] [Green Version]
- de Lima, L.R.A.; da Silva, L.P.B.G.; de Almeida, S.M.V.; Cahú, T.B.; Beltrão, E.I.C.; de Carvalho Júnior, L.B. Lectin-Carbohydrate Complex Evaluation by Chemiluminescence. Anal. Biochem. 2018, 548, 91–95. [Google Scholar] [CrossRef]
- Morokutti, A.; Redlberger-Fritz, M.; Nakowitsch, S.; Krenn, B.M.; Wressnigg, N.; Jungbauer, A.; Romanova, J.; Muster, T.; Popow-Kraupp, T.; Ferko, B. Validation of the Modified Hemagglutination Inhibition Assay (MHAI), a Robust and Sensitive Serological Test for Analysis of Influenza Virus-Specific Immune Response. J. Clin. Virol. 2013, 56, 323–330. [Google Scholar] [CrossRef]
- Adamová, L.; Malinovská, L.; Wimmerová, M. New Sensitive Detection Method for Lectin Hemagglutination Using Microscopy. Microsc Res. Tech. 2014, 77, 841–849. [Google Scholar] [CrossRef]
- Brustein, V.P.; Souza-Araájo, F.v.; Vaz, A.F.M.; Araájo, R.V.S.; Paiva, P.M.G.; Coelho, L.C.B.B.; Carneiro-Leão, A.M.A.; Teixeira, J.A.; Carneiro-Da-Cunha, M.G.; Correia, M.T.S. A Novel Antimicrobial Lectin from Eugenia Malaccensis That Stimulates Cutaneous Healing in Mice Model. Inflammopharmacology 2012, 20, 315–322. [Google Scholar] [CrossRef]
- Carvalho, B.M.A.; Carvalho, L.M.; Silva, W.F.; Minim, L.A.; Soares, A.M.; Carvalho, G.G.P.; da Silva, S.L. Direct Capture of Lactoferrin from Cheese Whey on Supermacroporous Column of Polyacrylamide Cryogel with Copper Ions. Food Chem. 2014, 154, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Baieli, M.F.; Urtasun, N.; Miranda, M.v.; Cascone, O.; Wolman, F.J. Efficient Wheat Germ Agglutinin Purification with a Chitosan-Based Affinity Chromatographic Matrix. J. Sep. Sci. 2012, 35, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Rüdiger, H.; Gabius, H.J. Plant Lectins: Occurrence, Biochemistry, Functions and Applications. Glycoconj. J. 2002, 18, 589–613. [Google Scholar] [CrossRef] [PubMed]
- Chacko, B.K.; Appukuttan, P.S. Peanut (Arachis Hypogaea) Lectin Recognizes α-Linked Galactose, but Not N-Acetyl Lactosamine in N-Linked Oligosaccharide Terminals. Int. J. Biol. Macromol. 2001, 28, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gao, F.; Huang, H.; Zeng, L.; Huang, X.; Zhu, G.; Li, Z. Determination of Trace Alkaline Phosphatase by Solid-Substrate Room-Temperature Phosphorimetry Based on Triticum Vulgare Lectin Labeled with Fullerenol. Chem. Biodivers. 2008, 5, 606–616. [Google Scholar] [CrossRef]
- Kabir, S. The Isolation and Characterisation of Jacalin [Artocarpus Heterophyllus (Jackfruit) Lectin] Based on Its Charge Properties. Int. J. Biochem. Cell Biol. 1995, 27, 147–156. [Google Scholar] [CrossRef]
- Simmons, B.M.; Russell, J.H. A Single Affinity Column Step Method for the Purification of Ricin Toxin from Castor Beans (Ricinus Communis). Anal. Biochem. 1985, 146, 206–210. [Google Scholar] [CrossRef]
- Habibi, J.; Backus, E.A.; Huesing, J.E. Effects of Phytohemagglutinin (PHA) on the Structure of Midgut Epithelial Cells and Localization of Its Binding Sites in Western Tarnished Plant Bug, Lygus Hesperus Knight. J. Insect Physiol. 2000, 46, 611–619. [Google Scholar] [CrossRef]
- Koshte, V.L.; van Dijk, W.; van der Stelt, M.E.; Allbers, R.C. Isolation and Characterization of BanLec-I, a Mannoside-Binding Lectin from Musa Paradisiac (Banana). Biochem. J. 1990, 272, 721–726. [Google Scholar] [CrossRef] [Green Version]
- Francis, F.; Jaber, K.; Colinet, F.; Portetelle, D.; Haubruge, E. Purification of a New Fungal Mannose-Specific Lectin from Penicillium Chrysogenum and Its Aphicidal Properties. Fungal Biol. 2011, 115, 1093–1099. [Google Scholar] [CrossRef]
- Singh, R.S.; Thakur, S.R.; Kennedy, J.F. Purification and Characterisation of a Xylose-Specific Mitogenic Lectin from Fusarium Sambucinum. Int. J. Biol. Macromol. 2020, 152, 393–402. [Google Scholar] [CrossRef]
- Gaofu, Q.; Shiqing, M.; Fayin, Z.; Zhiniu, Y.; Xiuyun, Z. In Vitro Assessment of Plant Lectins with Anti-Pinwood Nematode Activity. J. Invertebr. Pathol. 2008, 98, 40–45. [Google Scholar] [CrossRef]
- Rahaie, M.; Kazemi, S.S. Lectin-Based Biosensors: As Powerful Tools in Bioanalytical Applications. Biotechnology 2011, 10, 438–453. [Google Scholar] [CrossRef]
- Tullis, R.H.; Duffin, R.P.; Handley, H.H.; Sodhi, P.; Menon, J.; Joyce, J.A.; Kher, V. Reduction of Hepatitis C Virus Using Lectin Affinity Plasmapheresis in Dialysis Patients. Blood Purif. 2009, 27, 64–69. [Google Scholar] [CrossRef]
- Boyle, J. Lehninger Principles of Biochemistry: Nelson, D., and Cox, M. Biochem. Mol. Biol. Educ. 2005, 33, 74–75. [Google Scholar] [CrossRef]
- Duarte, P.L. Purificação e Caracterização Bioquímica de Uma Lectina Extraída Do Fluido Celomático Do Ouriço-Do-Mar, Echinometra Lucunter, Exposto a Indução Bacteriana; Universidade Federal do Ceará: Fortaleza, Brazil, 2018. [Google Scholar]
- Alborés, S.; Mora, P.; Bustamante, M.J.; Cerdeiras, M.P.; Franco Fraguas, L. Purification and Applications of a Lectin from the Mushroom Gymnopilus Spectabilis. Appl. Biochem. Biotechnol. 2014, 172, 2081–2090. [Google Scholar] [CrossRef]
- Maranhão, P.A.C.; Teixeira, C.S.; Sousa, B.L.; Barroso-Neto, I.L.; Monteiro-Júnior, J.E.; Fernandes, A.V.; Ramos, M.V.; Vasconcelos, I.M.; Gonçalves, J.F.C.; Rocha, B.A.M.; et al. CDNA Cloning, Molecular Modeling and Docking Calculations of L-Type Lectins from Swartzia Simplex Var. Grandiflora (Leguminosae, Papilionoideae), a Member of the Tribe Swartzieae. Phytochemistry 2017, 139, 60–71. [Google Scholar] [CrossRef]
- Machado, A.P.d.F.; Minim, L.A.; Fontan, R.d.C.I.; Minim, V.P.R.; Gonçalves, G.R.F.; Mól, P.C.G. Adsorptive Behavior of α-Lactalbumin on Cation-Exchange Supermacroporous Monolithic Column. Fluid Phase Equilib. 2015, 401, 64–69. [Google Scholar] [CrossRef]
- Gonçalves, G.R.F.; Gandolfi, O.R.R.; Santos, L.S.; Bonomo, R.C.F.; Veloso, C.M.; Veríssimo, L.A.A.; Fontan, R.; da Costa Ilhéu Fontan, R. Immobilization of Sugars in Supermacroporous Cryogels for the Purification of Lectins by Affinity Chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1068–1069, 71–77. [Google Scholar] [CrossRef]
- Zhou, W.; Gao, Y.; Xu, S.; Yang, Z.; Xu, T. Purification of a Mannose-Binding Lectin Pinellia Ternata Agglutinin and Its Induction of Apoptosis in Bel-7404 Cells. Protein Expr. Purif. 2014, 93, 11–17. [Google Scholar] [CrossRef]
- Che, A.F.; Huang, X.J.; Xu, Z.K. Polyacrylonitrile-Based Nanofibrous Membrane with Glycosylated Surface for Lectin Affinity Adsorption. J. Memb. Sci. 2011, 366, 272–277. [Google Scholar] [CrossRef]
- Absar, N.; Yeasmin, T.; Raza, M.S.; Sarkar, S.K.; Arisaka, F. Single Step Purification, Characterization and N-Terminal Sequences of a Mannose Specific Lectin from Mulberry Seeds. Protein J. 2005, 24, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Mól, P.C.G.; Veríssimo, L.A.A.; Eller, M.R.; Minim, V.P.R.; Minim, L.A. Development of an Affinity Cryogel for One Step Purification of Lysozyme from Chicken Egg White. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1044–1045, 17–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, M.; Singh, K.; Rup, P.J.; Kamboj, S.S.; Saxena, A.K.; Sharma, M.; Bhagat, M.; Sood, S.K.; Singh, J. A Tuber Lectin from Arisaema Jacquemontii Blume with Anti-Insect and Anti-Proliferative Properties. J. Biochem. Mol. Biol. 2006, 39, 432–440. [Google Scholar] [CrossRef] [Green Version]
- Kvennefors, E.C.E.; Leggat, W.; Hoegh-Guldberg, O.; Degnan, B.M.; Barnes, A.C. An Ancient and Variable Mannose-Binding Lectin from the Coral Acropora Millepora Binds Both Pathogens and Symbionts. Dev. Comp. Immunol. 2008, 32, 1582–1592. [Google Scholar] [CrossRef]
- Savina, I.N.; Gun’Ko, V.M.; Turov, V.V.; Dainiak, M.; Phillips, G.J.; Galaev, I.Y.; Mikhalovsky, S.V. Porous Structure and Water State in Cross-Linked Polymer and Protein Cryo-Hydrogels. Soft Matter 2011, 7, 4276–4283. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, L.; Liang, Y.; Wang, J.; Zhao, M.; Li, Y. Polyethylene Glycol Diacrylate-Based Supermacroporous Monolithic Cryogel as High-Performance Liquid Chromatography Stationary Phase for Protein and Polymeric Nanoparticle Separation. J. Chromatogr. A 2008, 1182, 128–131. [Google Scholar] [CrossRef]
- Tekin, K.; Uzun, L.; Şahin, Ç.A.; Bektaş, S.; Denizli, A. Preparation and Characterization of Composite Cryogels Containing Imidazole Group and Use in Heavy Metal Removal. React. Funct. Polym. 2011, 71, 985–993. [Google Scholar] [CrossRef]
- Vakifli, A.; Demirel, G.B.; Caykara, T. Swelling Characteristics of Poly(Nisopropylmethacrylamide-Co-Itaconic Acid) Gels Prepared in Various Conditions. J. Appl. Polym. Sci. 2010, 117, 817–823. [Google Scholar] [CrossRef]
- Akkaya, B.; Akkaya, R. A Crosslinked Carboxylic Acid Containing Cation Exchange Monolithic Cryogel for Human Serum Albumin Separation. J. Macromol. Sci. Part A Pure Appl. Chem. 2012, 49, 736–743. [Google Scholar] [CrossRef]
- Çimen, D.; Türkmen, D.; Denizli, A. Poly-(L)-Histidine Immobilized Cryogels for Lysozyme Purification. Ad Sci. Tech. 2016, 34, 469–487. [Google Scholar] [CrossRef]
- Haider, Z.; Haleem, A.; Ahmad, R.U.S.; Farooq, U.; Shi, L.; Claver, U.P.; Memon, K.; Fareed, A.; Khan, I.; Mbogba, M.K.; et al. Highly Porous Polymer Cryogel Based Tribopositive Material for High Performance Triboelectric Nanogenerators. Nano Energy 2020, 68, 104294. [Google Scholar] [CrossRef]
- Bereli, N.; Şener, G.; Yavuz, H.; Denizli, A. Oriented Immobilized Anti-LDL Antibody Carrying Poly(Hydroxyethyl Methacrylate) Cryogel for Cholesterol Removal from Human Plasma. Mater. Sci. Eng. C 2011, 31, 1078–1083. [Google Scholar] [CrossRef]
- Okay, O. Polymeric Cryogels Macroporous Gels with Remarkable Properties. Advs Pol. Sci. 2014, 263, iii–iv. [Google Scholar] [CrossRef]
- Plieva, F.M.; Savina, I.N.; Deraz, S.; Andersson, J.; Galaev, I.Y.; Mattiasson, B. Characterization of Supermacroporous Monolithic Polyacrylamide Based Matrices Designed for Chromatography of Bioparticles. J. Chromatogr. B Analyt. Tech. Biomed. Life Sci. 2004, 807, 129–137. [Google Scholar] [CrossRef]
- Plieva, F.M.; Galaev, I.Y.; Noppe, W.; Mattiasson, B. Cryogel Applications in Microbiology. Trends Microbiol. 2008, 16, 543–551. [Google Scholar] [CrossRef]
- Privar, Y.; Malakhova, I.; Pestov, A.; Fedorets, A.; Azarova, Y.; Schwarz, S.; Bratskaya, S. Polyethyleneimine Cryogels for Metal Ions Sorption. Chem. Eng. J. 2018, 334, 1392–1398. [Google Scholar] [CrossRef]
- Dragan, E.S.; Lazar, M.M.; Dinu, M.V.; Doroftei, F. Macroporous Composite IPN Hydrogels Based on Poly(Acrylamide) and Chitosan with Tuned Swelling and Sorption of Cationic Dyes. Chem. Eng. J. 2012, 204–205, 198–209. [Google Scholar] [CrossRef]
- Amin, R.; Alam, F.; Dey, B.K.; Mandhadi, J.R.; Emran, T.B.; Khandaker, M.U.; Safi, S.Z. Multidimensional Chromatography and Its Applications in Food Products, Biological Samples and Toxin Products: A Comprehensive Review. Separations 2022, 9, 326. [Google Scholar] [CrossRef]
- Kumar, A. Supermacroporous Cryogels: Biomedical and Biotechnological Applications; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781482228823. [Google Scholar]
- Bibi, N.S.; Singh, N.K.; Dsouza, R.N.; Aasim, M.; Fernández-Lahore, M. Synthesis and Performance of Megaporous Immobilized Metal-Ion Affinity Cryogels for Recombinant Protein Capture and Purification. J. Chromatogr. A 2013, 1272, 145–149. [Google Scholar] [CrossRef]
- Yun, J.; Kirsebom, H.; Galaev, I.Y.; Mattiasson, B. Modeling of Protein Breakthrough Performance in Cryogel Columns by Taking into Account the Overall Axial Dispersion. J. Sep. Sci. 2009, 32, 2601–2607. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, L.; Zhang, D.; Li, F.; Wang, M.; Huang, M.; Zhang, H.; Song, L. A Novel C-Type Lectin from Crab Eriocheir Sinensis Functions as Pattern Recognition Receptor Enhancing Cellular Encapsulation. Fish. Shellfish Immunol. 2013, 34, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Andaç, M.; Galaev, I.Y.; Denizli, A. Affinity Based and Molecularly Imprinted Cryogels: Applications in Biomacromolecule Purification. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1021, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Talbert, J.N.; Goddard, J.M. Enzymes on Material Surfaces. Colloids Surf. B Biointerfaces 2012, 93, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Ingavle, G.C.; Baillie, L.W.J.; Zheng, Y.; Lis, E.K.; Savina, I.N.; Howell, C.A.; Mikhalovsky, S.V.; Sandeman, S.R. Affinity Binding of Antibodies to Supermacroporous Cryogel Adsorbents with Immobilized Protein A for Removal of Anthrax Toxin Protective Antigen. Biomaterials 2015, 50, 140–153. [Google Scholar] [CrossRef] [Green Version]
- Klein, M.P.; Fallavena, L.P.; Schöffer, J.D.N.; Ayub, M.A.Z.; Rodrigues, R.C.; Ninow, J.L.; Hertz, P.F. High Stability of Immobilized β-d-Galactosidase for Lactose Hydrolysis and Galactooligosaccharides Synthesis. Carbohydr. Polym. 2013, 95, 465–470. [Google Scholar] [CrossRef] [Green Version]
- Arvidsson, P.; Plieva, F.M.; Savina, I.N.; Lozinsky, V.I.; Fexby, S.; Bülow, L.; Yu Galaev, I.; Mattiasson, B. Chromatography of Microbial Cells Using Continuous Supermacroporous Affinity and Ion-Exchange Columns. J. Chromatogr. A 2002, 977, 27–38. [Google Scholar] [CrossRef]
- Mallik, R.; Jiang, T.; Hage, D.S. High-Performance Affinity Monolith Chromatography: Development and Evaluation of Human Serum Albumin Columns. Anal. Chem. 2005, 76, 7013–7022. [Google Scholar] [CrossRef]
- Belokon, Y.N.; Kochetkov, K.A.; Plieva, F.M.; Ikonnikov, N.S.; Maleev, V.I.; Parmar, V.S.; Kumar, R.; Lozinsky, V.I. Enantioselective Hydrolysis of a Schiff’s Base of D, L-Phenylalanine Ethyl Ester in Water-Poor Media via the Reaction Catalyzed with α-Chymotrypsin Immobilized on Hydrophilic Macroporous Gel Support. Appl. Biochem. Biotech.—Part. A Enzym. Eng. Biotechnol. 2000, 88, 97–106. [Google Scholar] [CrossRef]
- Hanora, A.; Plieva, F.M.; Hedström, M.; Galaev, I.Y.; Mattiasson, B. Capture of Bacterial Endotoxins Using a Supermacroporous Monolithic Matrix with Immobilized Polyethyleneimine, Lysozyme or Polymyxin B. J. Biotechnol. 2005, 118, 421–433. [Google Scholar] [CrossRef]
- Arrua, R.D.; Strumia, M.C.; Igarzabal, C.I.A. Macroporous Monolithic Polymers: Preparation and Applications. Materials 2009, 2, 2429–2466. [Google Scholar] [CrossRef] [Green Version]
- Uygun, M.; Akduman, B.; Ergönül, B.; Aktaş Uygun, D.; Akgöl, S.; Denizli, A. Immobilization of Amyloglucosidase onto Macroporous Cryogels for Continuous Glucose Production from Starch. J. Biomater. Sci. Polym. Ed. 2015, 26, 1112–1125. [Google Scholar] [CrossRef]
- Baydemir, G.; Türkoğlu, E.A.; Andaç, M.; Perçin, I.; Denizli, A. Composite cryogels for lysozyme purification. Biotechnol. Appl. Biochem. 2015, 62, 200–207. [Google Scholar] [CrossRef]
- 20Bulut, E.; Sargin, I.; Arslan, O.; Odabasi, M.; Akyuz, B.; Kaya, M. In situ chitin isolation from body parts of a centipede and lysozyme adsorption studies. Mater. Sci. Eng. C 2017, 70, 552–563. [Google Scholar] [CrossRef]
- Kaetsu, I. Radiation Synthesis of Polymeric Materials for Biomedical and Biochemical Applications. Recent Trends in Radiation Polymer Chemistry; Springer: Berlin/Heidelberg, Germany, 1993; pp. 81–97. [Google Scholar] [CrossRef]
- Kumakura, M. Preparation method of porous polymer materials by radiation technique and its application. Polym. Adv. Technol. 2001, 12, 415–421. [Google Scholar] [CrossRef]
- Lusta, K.; Chung, I.; Sul, I.; Park, H.-S.; Shin, D.-I. Immobilization of fungus Aspergillus sp. by a novel cryogel technique for production of extracellular hydrolytic enzymes. Process Biochem. 2000, 35, 1177–1182. [Google Scholar] [CrossRef]
- Cocquempot, M.F.; Thomas, D.; Champigny, M.L.; Moyse, A. Immobilization of thylakoids in porous particles and stabilization of the photochemical processes by glutaraldehyde action at subzero temperature. Eur. J. Appl. Microbiol. Biotechnol. 1979, 8, 37–41. [Google Scholar] [CrossRef]
- Scardi, V. Immobilization of enzymes and microbial cells in gelatine. Methods Enzymol. 1987, 135B, 293–299. [Google Scholar] [CrossRef]
- Luo, Q.; Zou, H.; Zhang, Q.; Xiao, X.; Ni, J. High-Performance Affinity Chromatography with Immobilization of Protein A and L-Histidine on Molded Monolith. Biotechnol. Bioeng. 2002, 80, 481–489. [Google Scholar] [CrossRef]


| Lectins | Source | Extract | Specific Binders | References |
|---|---|---|---|---|
| Con A | Canavalia ensiformis | Pork bean | α-D-mannosil and α-D-glycosil | [34] |
| PNA | Arachis hypogaea | Peanut | N-acetylgalactosamine | [35] |
| WGA | Triticum vulgare | Wheat germ | N-acetylglycosamine | [36] |
| Jacalina | Artocarpus integrifólia | Jackfruit | α-galactopyranosids and methyl-α-D-galactopyranoside | [37] |
| Ricin | Ricinus communis | Castor bean | β-D-galactosyl | [38] |
| Emal | Eugenia malaccensis | Red Jambo | Glucose | [31] |
| PHA | Phaseolus vulgaris | Bean | N-acetyl-D-galactosamine | [39] |
| BanLec | Musa paradisiaca | Banana | Mannose-binding | [40] |
| PeCL | Penicillium chrysogenum | Fungus | Mannose-binding | [41] |
| FSL | Fusarium sambucinum | Fungus | D-xylose, L-fucose D-mannose, N-acetyl-D-glucosamine | [42] |
| PTA e LRA | Pinellia ternata Lycoris radiata | Plant | Mannose ligand | [43] |
| Technique | Lectin | Food | References |
|---|---|---|---|
| Hydrophobic interaction chromatography | Tethya sp | Marine sponge | [47] |
| Ion exchange chromatography | Jacalina (Artocarpus integrifólia) | Jackfruit | [15] |
| Affinity chromatography | Con A | Pork beans | [26] |
| Gel filtration chromatography | PeCL | Fungus (Penicillium chrysogenum) | [41] |
| Affinity chromatography followed by ion exchange | Gymnopilus spectabilis (GSL) | Mushroom | [48] |
| Ion exchange chromatography | BanLec | Banana | [40] |
| Exclusion chromatography in gel | FSL (Fusarium sambucinum) | Fungus | [42] |
| Ion exchange chromatography | PTA e LRA | Pinellia ternata and Lycoris radiata | [43] |
| Molecular exclusion (PD-10 Desalting) | Swartzia laevicarpa | Legume | [49] |
| Technique | Biomolecule | Ligand | References |
|---|---|---|---|
| Chromatography by Affinity | Concanavalin A (Con A); Peanut (PNA) | Immobilized glucose | [53] |
| Blackberry lectins | Concanavalin A | [54] | |
| Lizosima | Tris (hydroxyethyl)aminomethane | [51] | |
| N-acetyl-D-glycosamine (D-GlcNAc) | [55] | ||
| Gymnopilus spectabilis (GSL) | Manosil-Sepharose | [48] | |
| BanLec | [40] | ||
| Arisaema jacquemontii Blume (AJL) | Syapituin | [56] | |
| lectin (‘Millectin’) | Mannose | [57] |
| Monomers and Reticulates | Solvent | Catalyst | T (°C) | References |
|---|---|---|---|---|
| Methacrylic acid + oligo ethylene glycol Diacrylate | Water | APS + TEMED | −20 | [59] |
| 2-hydroxymethylmethacrylate + N-vinyl imidazole + ethylene glycolDimethacrylae | Water | APS + TEMED | −16 | [60] |
| N-isopropilacrilamide + itaconic acid + N, N’-methylene-bis-acrylamide | Water | APS + TEMED | −22 | [61] |
| Acrylamide + N, N’-methylene-bis-acrylamide + Alil- glycidyl ether | Water | APS + TEMED | −12 | [9,51] |
| N-isopropilacrilamide + acrylic acid + N, N’-methylene-bis-acrylamide | Water | APS + TEMED | −22 | [62] |
| Poly- (L) -histidine + glycidil methacrylate (PGMA) + N’-methylene-bis-acrylamide + HEMA | Water | APS + TEMED | 16 | [63] |
| Acrylamide + lauryl acrylate + ethylene glycol dimethacrylate (EGDMA) | Water | PDMS + PBPO | −18 | [64] |
| Poly (2-hydroxyethyl methacrylate) (PHEMA) | Water | APS + TEMED | −12 | [65] |
| Technique | Binders | Application | References |
|---|---|---|---|
| Glutaraldehyde method | β-D-Galactosidase immobilized in Chitosan | Continuous lactose hydrolysis and synthesis of galactooligosaccharides (GOS) | [79] |
| Cellular immobilization | poly (vinyl alcohol) PVA | Continuous synthesis of alkyl galactosides by transgli cosiled | [68] |
| Covalent ligation | Theiminodiatic cido (IDA) | Direct capture of unclarified crude oil enzymes | [80] |
| Covalent ligation | Ligands of anionic exchange [group 2-(dimethylamino)ethyl] | Purification of Escherichia coli cells | |
| Immobilization techniqueenzymatic, epoxy method | Human serum albumin (HSA) | Purification of biomolecules | [81] |
| Chemical coping | α-chymotrypsin | enantioselective hydrolysis from a D, L-Phe-OEt Schiff base (D, L-SBPH) | [82] |
| Immobilization technique by epoxy method | Polietyleneimine immobilized (PEI), polymyxin B (PMB) and lysozyme | Capture of bacterial endotoxins (BEs) | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, J.F.d.; Lima, C.M.G.; da Silva, D.L.; do Nascimento, I.S.; Rodrigues, S.d.O.; Gonçalves, L.A.; Santana, R.F.; Khalid, W.; Verruck, S.; Emran, T.B.; et al. Lectin Purification through Affinity Chromatography Exploiting Macroporous Monolithic Adsorbents. Separations 2023, 10, 36. https://doi.org/10.3390/separations10010036
Silva JFd, Lima CMG, da Silva DL, do Nascimento IS, Rodrigues SdO, Gonçalves LA, Santana RF, Khalid W, Verruck S, Emran TB, et al. Lectin Purification through Affinity Chromatography Exploiting Macroporous Monolithic Adsorbents. Separations. 2023; 10(1):36. https://doi.org/10.3390/separations10010036
Chicago/Turabian StyleSilva, Josiane F. da, Clara M. G. Lima, Débora L. da Silva, Ivonea S. do Nascimento, Sarah de O. Rodrigues, Letícia A. Gonçalves, Renata F. Santana, Waseem Khalid, Silvani Verruck, Talha Bin Emran, and et al. 2023. "Lectin Purification through Affinity Chromatography Exploiting Macroporous Monolithic Adsorbents" Separations 10, no. 1: 36. https://doi.org/10.3390/separations10010036
APA StyleSilva, J. F. d., Lima, C. M. G., da Silva, D. L., do Nascimento, I. S., Rodrigues, S. d. O., Gonçalves, L. A., Santana, R. F., Khalid, W., Verruck, S., Emran, T. B., de Menezes, I. R. A., Coutinho, H. D. M., Khandaker, M. U., Faruque, M. R. I., & Fontan, R. d. C. I. (2023). Lectin Purification through Affinity Chromatography Exploiting Macroporous Monolithic Adsorbents. Separations, 10(1), 36. https://doi.org/10.3390/separations10010036














