Comparison of Digestion Methods Using Atomic Absorption Spectrometry for the Determination of Metal Levels in Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Methods of Digestion
2.2.1. Nitric Acid Digestion (1% and 10%)
2.2.2. Acid Mixtures Digestion
2.3. Trace Metal Element Analysis
2.4. Statistical Analysis
3. Results
3.1. Cadmium Assay
3.2. Manganese Assay
3.3. Magnesium Assay
3.4. Aluminum Assay
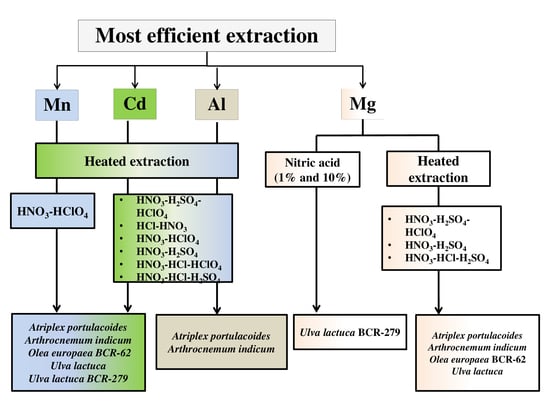
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Uddin, A.H.; Khalid, R.S.; Alaama, M.; Abdualkader, A.M.; Kasmuri, A.; Abbas, S.A. Comparative study of three digestion methods for elemental analysis in traditional medicine products using atomic absorption spectrometry. J. Anal. Sci. Technol. 2017, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Micó, C.; Recatalá, L.; Peris, M.; Sanchez, J. A comparison of two digestion methods for the analysis of heavy metals by flame atomic absorption spectroscopy. Spectrosc. Eur. 2007, 19, 23–26. [Google Scholar]
- Azcue, J.; Mudroch, A. Comparison of different washing, ashing and digestion methods for the analysis of trace elements in vegetation. Int. J. Environ. Anal. Chem. 1994, 57, 151–262. [Google Scholar] [CrossRef]
- Duyusen, E.G.; Görkem, A. Comparison of acid digestion techniques to determine heavy metals in sediment and soil samples. Gazi Univ. J. Sci. 2011, 24, 29–34. [Google Scholar]
- Andersen, K.J.; Kisser, M.I. Digestion of Solid Matrices Desk Study. Horizontal 2004, 18, 1–39. [Google Scholar]
- Santoro, A.; Held, A.; Linsinger, T.P.; Perez, A.; Ricci, M. Comparison of total and aqua regia extractability of heavy metals in sewage sludge: The case study of a certified reference material. Trends Analyt. Chem. 2017, 89, 34–40. [Google Scholar] [CrossRef]
- Hseu, Z.Y. Evaluating heavy metal contents in nine composts using four digestion methods. Bioresour. Technol. 2004, 95, 53–59. [Google Scholar] [CrossRef]
- Asher, A.S.; Samuel, K.E.; Mary, D.S. Analytical method for comparison of suitable wet digestion methods for heavy metal analysis in soil around a cement industry. Int. J. Res. Innov. Sci. 2020, 7, 41–47. [Google Scholar]
- Tangahu, B.V.; Abdullah, S.R.S.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A Review on Heavy Metals (As, Pb, and Hg) Uptake by Plants through Phytoremediation. Int. J. Chem. Eng. 2011, 2011, 939161. [Google Scholar] [CrossRef]
- Weis, P.; Windham, L.; Burke, D.J.; Weis, J.S. Release into the environment of metals by two vascular salt plants. Mar. Environ. Res. 2002, 54, 325–329. [Google Scholar] [CrossRef]
- Windham, L.; Weis, J.S.; Weis, P. Uptake and distribution of metals in two dominant salt marsh macrophytes, spartina alterniflora (cordgrass) and phragmites australis (common reed). Estuar. Coast. Shelf Sci. 2003, 56, 63–72. [Google Scholar] [CrossRef]
- Caçador, I.; Valo, C.; Catarino, F. Seasonal variation of Zn, Pb, Cu and Cd concentrations in the roots-sediment system of spartima maritima and halimione portulacoides from Tagus estuary salt marches. Mar. Environ. Res. 2000, 49, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Kouki, R.; Ayachi, R.; Ferreira, R.; Sleimi, N. Behavior of cucumis sativus L. in presence of Aluminum stress: Germination, plant growth and antioxidant enzymes. Food Sci. Nutr. 2021, 9, 3280–3288. [Google Scholar] [CrossRef] [PubMed]
- Dridi, N.; Ferreira, R.; Bouslimi, H.; Brito, P.; Martins-Dias, S.; Caçador, I.; Sleimi, N. Assessment of tolerance to lanthanum and cerium in helianthus annuus plant: Effect on growth, mineral nutrition and secondary metabolism. Plants 2022, 11, 988. [Google Scholar] [CrossRef]
- Dridi, N.; Brito, P.; Bouslimi, H.; Ferreira, R.; Martins-Dias, S.; Caçador, I.; Sleimi, N. Physiological and biochemical behaviours and antioxidant response of helianthus annuus under lanthanum and cerium stress. Sustainability 2022, 14, 4153. [Google Scholar] [CrossRef]
- Duarte, B.; Vaz, N.; Valentim, J.M.; Dias, J.M.; Silva, H.; Marques, J.C.; Sleimi, N.; Caçador, I. Revisiting the outwelling hypothesis: Modelling salt marsh detrital metal exports under extreme climatic events. Mar. Chem. 2017, 191, 24–33. [Google Scholar] [CrossRef]
- Fonseca, V.F.; França, S.; Duarte, B.; Caçador, I.; Cabral, H.N.; Mieiro, C.L.; Coelho, J.P.; Pereira, E.; Reis-Santos, P. Spatial variation in mercury bioaccumulation and magnification in a temperate estuarine food web. Front. Mar. Sci 2019, 6, 117. [Google Scholar] [CrossRef]
- Duarte, B.; Reboreda, R.; Caçador, I. Seasonal variation of Extracellular Enzymatic Activity (EEA) and its influence on metal speciation in a polluted salt marsh. Chemosphere 2008, 73, 1056–1063. [Google Scholar] [CrossRef]
- Sleimi, N.; Bankaji, I.; Kouki, R.; Dridi, N.; Duarte, B.; Caçador, I. Assessment of extraction methods of trace metallic elements in plants: Approval of a common method. Sustainability 2022, 14, 1428. [Google Scholar] [CrossRef]
- Larson, K.D.; Dejong, T.K.; Johnson, R.S. Physiological and growth responses of mature peach trees to postharvest water stress. J. Am. Soc. Hortic. Sci. 1988, 133, 296–300. [Google Scholar] [CrossRef]
- Sghaier, B.D.; Bankaji, I.; Pedro, S.; Caçador, I.; Sleimi, N. Photosynthetic behaviour and mineral nutrition of tamarix gallica cultivated under aluminum and NaCl combined stress. Phyton-Int. J. Exp. Bot. 2019, 88, 239–252. [Google Scholar] [CrossRef]
- Labidi, O.; Vives-Peris, V.; Gómez-Cadenas, A.; Pérez-Clemente, R.M.; Sleimi, N. Assessing of growth, antioxidant enzymes and phytohormone regulation in cucurbita pepo under cadmium stress. Food Sci. Nutr. 2021, 9, 2021–2031. [Google Scholar] [CrossRef] [PubMed]
- Sleimi, N.; Kouki, R.; Hadj Ammar, M.; Ferreira, R.; Perez-Clemente, R. Barium effect on germination, plant growth, and antioxidant enzymes in cucumis sativus L. plants. Food Sci. Nutr. 2021, 9, 2086–2094. [Google Scholar] [CrossRef] [PubMed]
- Abbruzzini, T.F.; Silva, C.A.; de Andrade, D.A.; de Oliveira Carneiro, W.J. Influence of digestion methods on the recovery of iron, zinc, nickel, chromium, cadmium and lead contents in 11 organic residues. Rev. Bras. Ciênc. Solo 2014, 38, 166–176. [Google Scholar] [CrossRef]
- Caçador, I.; Caetano, M.; Duarte, B.; Vale, C. Stock and losses of trace metals from salt marsh plants. Mar. Environ. Res. 2009, 67, 75–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Momen, A.A.; Zachariadis, G.A.; Anthemidis, A.N.; Stratis, J.A. Investigation of four digestion procedures for multi-element determination of toxic and nutrient elements in legumes by inductively coupled plasma-optical emission spectrometry. Anal. Chim. Acta 2006, 565, 81–88. [Google Scholar] [CrossRef]
- Adamczyk-Szabela, D.; Anielak, P.; Wolf, W.M. Influence of digestion procedure and residual carbon on manganese, copper, and zinc determination in herbal matrices by atomic absorption spectrometry. J. Anal. Meth. Chem. 2017, 2017, 6947376. [Google Scholar] [CrossRef] [Green Version]
- Zheljazkov, V.D.; Warman, P.R. Comparison of Three Digestion Methods For the Recovery of 17 Plant Essential Nutrients And Trace Elements from Six Composts. Compost. Sci. Util. 2002, 10, 197–203. [Google Scholar] [CrossRef]
- Sastre, J.; Sahuquillo, A.; Vidal, M.; Rauret, G. Determination of Cd, Cu, Pb and Zn in environmental samples microwave-assisted total digestion versus aqua regia and nitric acid extraction. Anal. Chim. Acta 2002, 462, 59–72. [Google Scholar] [CrossRef]
- Schmid, R. The effects of sample processing on amounts of nutrients and pollutants in composts. Assoc. Ger. Agric. Investig. Res. Inst. Ser. Congr. Rep. 1990, 32, 357–362. [Google Scholar]
- Shaibur, M.R.; Shamim, A.H.M.; ImamulHuq, S.M.; Kawai, S. Comparison of digesting capacity of nitric acid and nitric acid-perchloric acid mixture and the effect of lanthanum chloride on potassium measurement. Nat. Sci. 2010, 8, 157–162. [Google Scholar]
- Zarcinas, B.A.; Cartwright, B. Analysis of Soil and Plant Material by Inductively Coupled Plasma-Optical Emission Spectroscopy; Commonwealth Scientific and Industrial Research Organization: Canberra, Australia, 1983; p. 36.
- Warman, P.R.; Muizelaar, T. A comparison of the elemental analysis of compost using two standard methods. In Proceedings of the 5th Annual Meeting of the Composting Council of Canada, Aylmer, PQ, Canada, 1–3 November 1995.
- Sahrawat, K.L.; Kumar, G.R.; Rao, J.K. Evaluation of triacid and dry ashing procedures for determining potassium, calcium, magnesium, iron, zinc, manganese, and copper in plant materials. Commun. Soil Sci. Plant Anal. 2002, 33, 95–102. [Google Scholar] [CrossRef]




| Methods | Reagents | Proportions | Time/min |
|---|---|---|---|
| C | HNO3/H2SO4/HClO4 | 10:1:0.5, v/v/v | 120 |
| D | HCl/HNO3 | 3:1, v/v | 120 |
| E | HNO3/HClO4 | 3:1, v/v | 120 |
| F | HNO3/H2SO4 | 2:1, v/v | 120 |
| G | HNO3/HCl/HClO4 | 5:1:0.5, v/v/v | 120 |
| H | HNO3/HCl/H2SO4 | 5:1:1, v/v/v | 120 |
| Metal | Wavelength (nm) | Slit Width (nm) | Background Correction | Oxidant: Acetylene L·min−1 | Lampe Type |
|---|---|---|---|---|---|
| Cd | 228.8 | 0.7 | Yes | Air: Acetylene 10:2.5 | HCl |
| Mn | 279.48 | 0.2 | No | Air: Acetylene 10:2.5 | HCl |
| Mg | 285.21 | 0.7 | No | Air: Acetylene 10:2.5 | HCl |
| Al | 309.27 | 0.7 | No | N2O:Acetylene 06:7.5 | HCl |
| Olea europaea BCR-62 | Cd (µg·g−1 DW) | Mn (µg·g−1 DW) | Al (µg·g−1 DW) |
|---|---|---|---|
| Method A | 181.5 | 187.6 | 1345.9 |
| Method B | 175.1 | 100.8 | 1725.7 |
| Method C | 233.3 | 364.1 | 1144.6 |
| Method D | 236.4 | 442.3 | 7329.9 |
| Method E | 251.2 | 1106.1 | 1691.9 |
| Method F | 229.1 | 443.7 | 2792.9 |
| Method G | 231.7 | 366.4 | 1498.2 |
| Method H | 219.1 | 286.1 | 7051.0 |
| Certified reference value | 000.1 | 057.0 | 0450.0 |
| Ulva lactuca BCR-279 | Cd (µg·g−1 DW) |
|---|---|
| Method A | 167.875 |
| Method B | 170.833 |
| Method C | 221.062 |
| Method D | 210.322 |
| Method E | 242.534 |
| Method F | 234.414 |
| Method G | 232,081 |
| Method H | 235,996 |
| Certified reference value | 000.274 |
| Cd | ||||||||
| A | B | C | D | E | F | G | H | |
| A | 1 | |||||||
| B | 0.145 | 1 | ||||||
| C | 0.206 | −0.669 | 1 | |||||
| D | 0.412 | −0.275 | 0.809 * | 1 | ||||
| E | 0.005 | −0.569 | 0.944 ** | 0.857 * | 1 | |||
| F | −0.343 | −0.950 ** | 0.580 | 0.281 | 0.580 | 1 | ||
| G | 0.584 | −0.353 | 0.151 | 0.336 | 0.040 | 0.323 | 1 | |
| H | −0.444 | 0.330 | −0.522 | −0.823 * | −0.565 | −0.393 | −0.761 * | 1 |
| Mn | ||||||||
| A | B | C | D | E | F | G | H | |
| A | 1 | |||||||
| B | 0.994 ** | 1 | ||||||
| C | 0.974 ** | 0.973 ** | 1 | |||||
| D | 0.858 * | 0.838 * | 0.912 ** | 1 | ||||
| E | 0.952 ** | 0.929 ** | 0.962 ** | 0.844 * | 1 | |||
| F | 0.974 ** | 0.965 ** | 0.967 ** | 0.803 * | 0.987 ** | 1 | ||
| G | 0.998 ** | 0.993 ** | 0.976 ** | 0.882 * | 0.937 ** | 0.958 ** | 1 | |
| H | 0.992 ** | 0.997 ** | 0.961 ** | 0.803 * | 0.933 ** | 0.973 ** | 0.987 ** | 1 |
| Al | ||||||||
| A | B | C | D | E | F | G | H | |
| A | 1 | |||||||
| B | 0.820 * | 1 | ||||||
| C | −0.408 | 0.009 | 1 | |||||
| D | 0.764 * | 0.670 | −0.623 | 1 | ||||
| E | 0.741 * | 0.847 * | 0.288 | 0.274 | 1 | |||
| F | −0.220 | 0.312 | 0.918 ** | −0.370 | 0.436 | 1 | ||
| G | 0.050 | 0.569 | 0.706 | −0.130 | 0.600 | 0.920 ** | 1 | |
| H | 0.716 * | 0.494 | −0.812 * | 0.962 ** | 0.107 | −0.599 | −0.351 | 1 |
| Mg | ||||||||
| A | B | C | D | E | F | G | H | |
| A | 1 | |||||||
| B | 0.968 ** | 1 | ||||||
| C | −0.198 | −0.185 | 1 | |||||
| D | 0.711 * | 0.840 * | −0.025 | 1 | ||||
| E | 0.973 ** | 0.991 ** | −0.301 | 0.814 * | 1 | |||
| F | 0.732 * | 0.789 * | −0.718 * | 0.688 | 0.848 * | 1 | ||
| G | 0.946 ** | 0.971 ** | −0.414 | 0.791 * | 0.992 ** | 0.906 ** | 1 | |
| H | 0.662 | 0.810 * | −0.436 | 0.830 * | 0.813 * | 0.889 * | 0.849 * | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bankaji, I.; Kouki, R.; Dridi, N.; Ferreira, R.; Hidouri, S.; Duarte, B.; Sleimi, N.; Caçador, I. Comparison of Digestion Methods Using Atomic Absorption Spectrometry for the Determination of Metal Levels in Plants. Separations 2023, 10, 40. https://doi.org/10.3390/separations10010040
Bankaji I, Kouki R, Dridi N, Ferreira R, Hidouri S, Duarte B, Sleimi N, Caçador I. Comparison of Digestion Methods Using Atomic Absorption Spectrometry for the Determination of Metal Levels in Plants. Separations. 2023; 10(1):40. https://doi.org/10.3390/separations10010040
Chicago/Turabian StyleBankaji, Insaf, Rim Kouki, Nesrine Dridi, Renata Ferreira, Saida Hidouri, Bernardo Duarte, Noomene Sleimi, and Isabel Caçador. 2023. "Comparison of Digestion Methods Using Atomic Absorption Spectrometry for the Determination of Metal Levels in Plants" Separations 10, no. 1: 40. https://doi.org/10.3390/separations10010040
APA StyleBankaji, I., Kouki, R., Dridi, N., Ferreira, R., Hidouri, S., Duarte, B., Sleimi, N., & Caçador, I. (2023). Comparison of Digestion Methods Using Atomic Absorption Spectrometry for the Determination of Metal Levels in Plants. Separations, 10(1), 40. https://doi.org/10.3390/separations10010040