Separation of Cesium and Rubidium from Solution with High Concentrations of Potassium and Sodium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
2.3. Chemical Analysis
3. Results and Discussion
3.1. Solvent Extraction of Cs and Rb
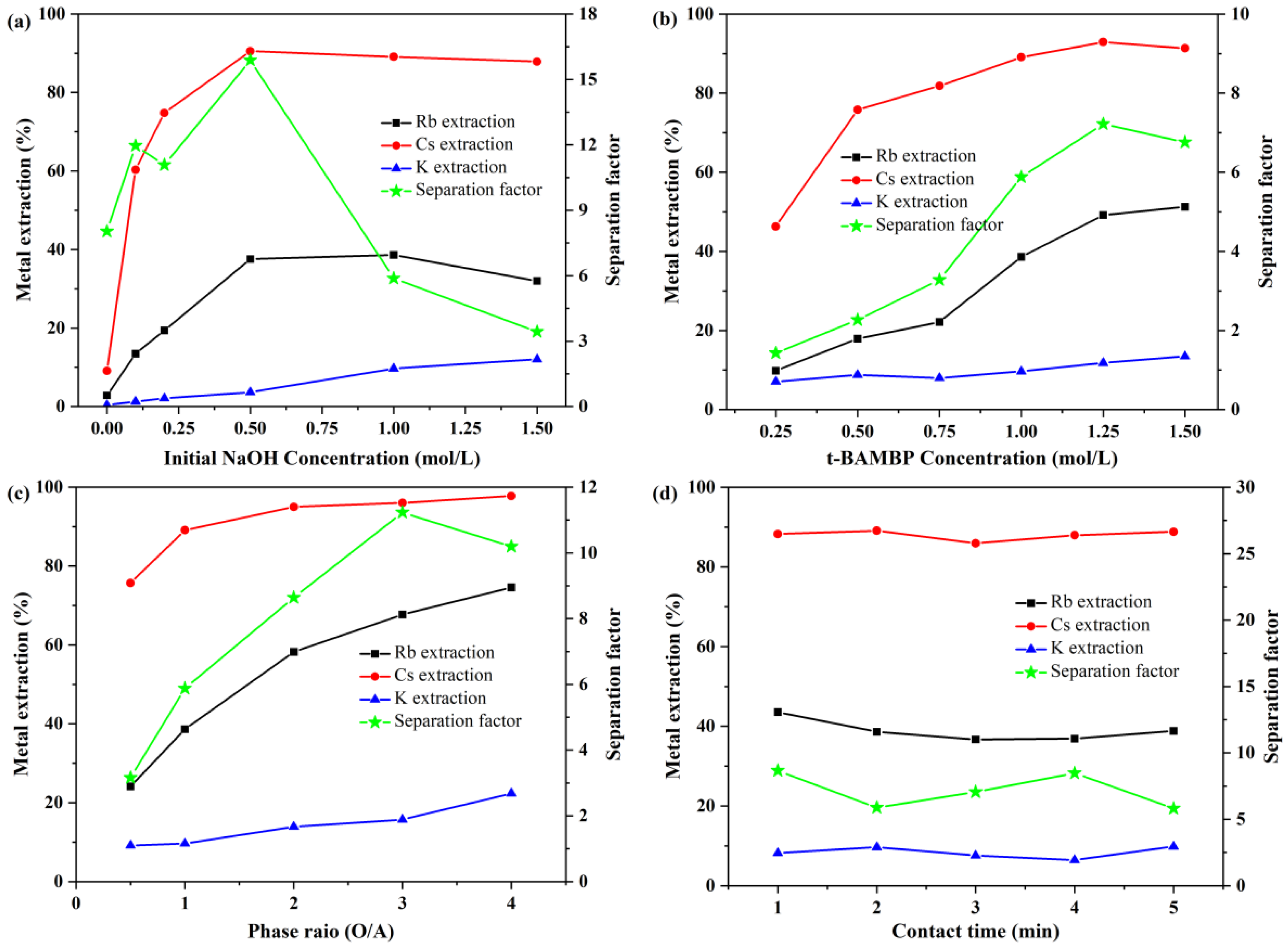
3.1.1. Effect of NaOH Concentration on EK, ERb, and ECs
3.1.2. Effect of the t-BAMBP Concentration on EK, ERb, and ECs
3.1.3. Effect of the O/A Ratio on EK, ERb, and ECs
3.1.4. Effect of the Contact Time on EK, ERb, and ECs
3.1.5. Extraction Isotherm of Rb
3.2. Scrubbing of K
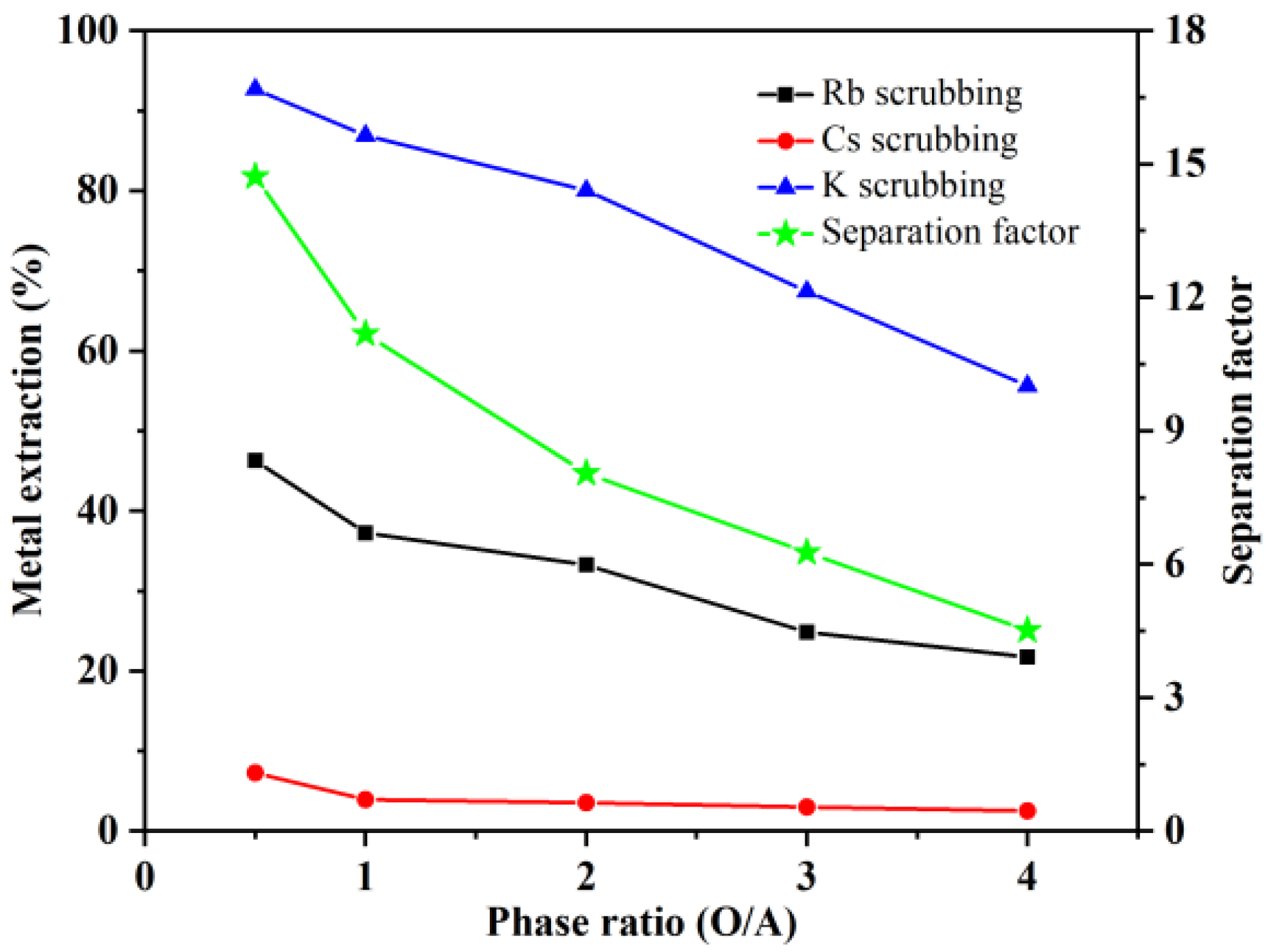
3.2.1. Effect of the O/A Ratio on K+, Rb+, and Cs+ Scrubbing
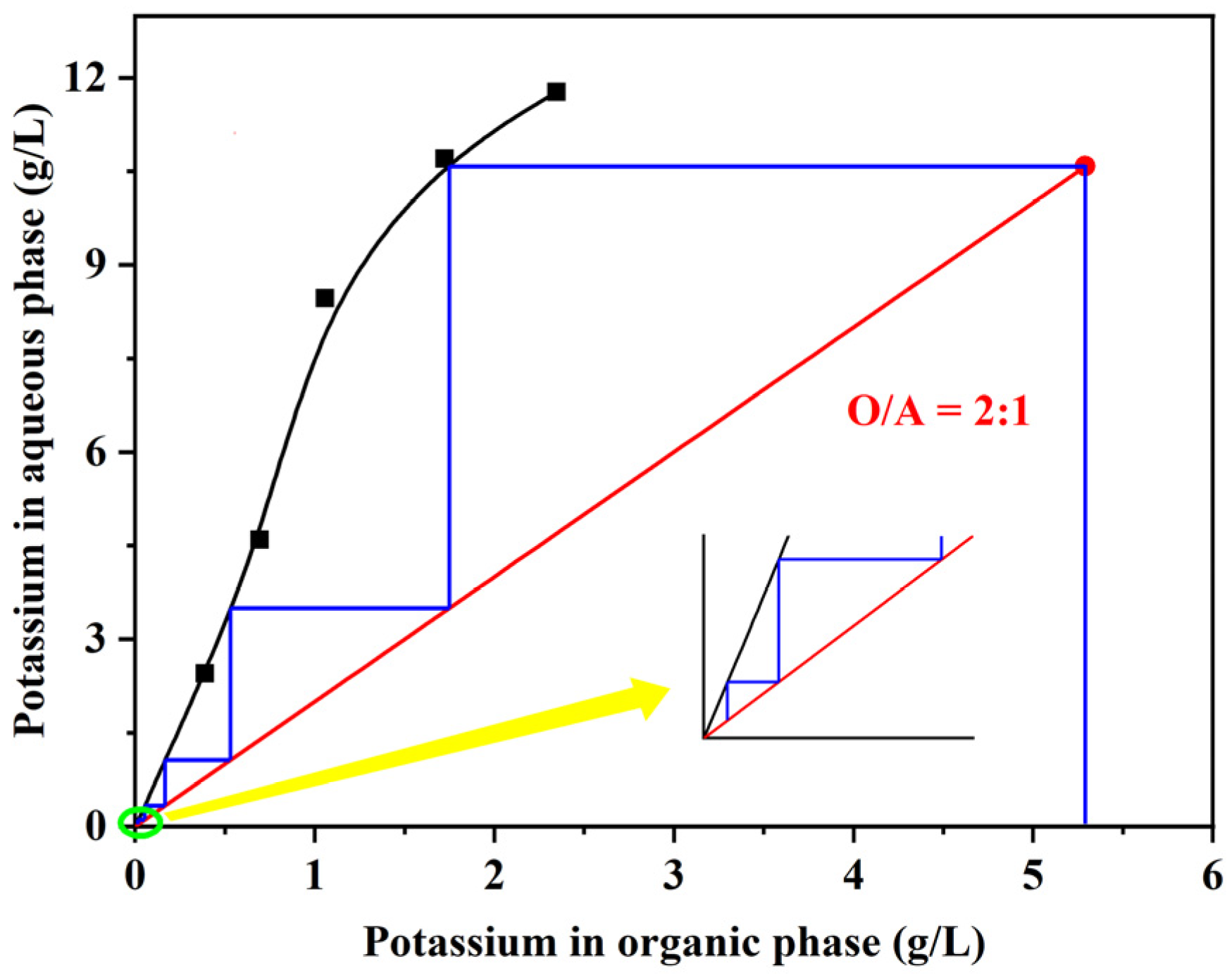
3.2.2. Scrubbing Isotherm of K
3.3. Stripping of Cs and Rb
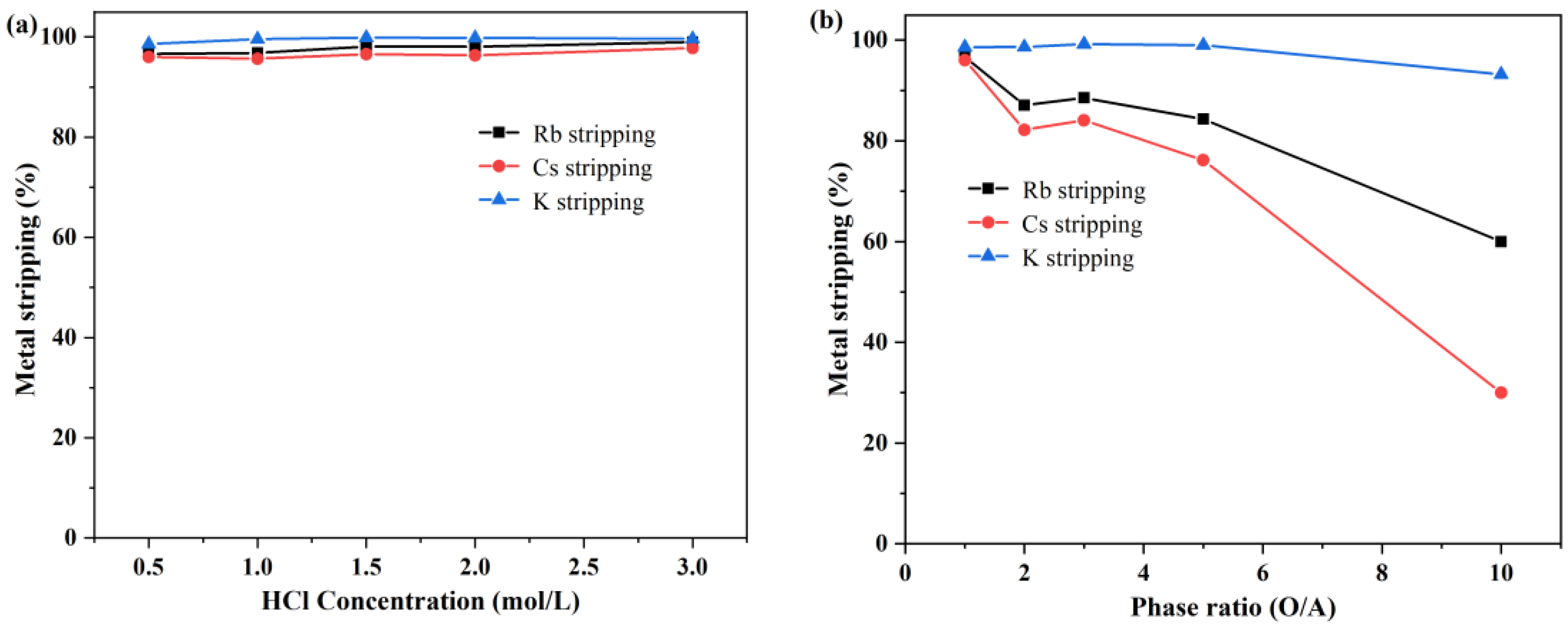
3.3.1. Effect of HCl Concentration on K+, Rb+, and Cs+ Stripping
3.3.2. Effect of the O/A Ratio on K+, Rb+ and Cs+ Stripping
3.4. Problems in the Extraction Process
3.4.1. XRD Patterns and ICP Analysis of the Solid Compound
3.4.2. Infrared Spectral Analysis
4. Conclusions
- (1)
- The optimal extraction conditions for separating Cs and Rb from high concentration of K and Na were as follows: 1 mol/L t-BAMBP extractant (in sulfonated kerosene), 0.5 mol/L NaOH, O/A ratio of 3:1, and 1 min contact time. More than 99% of Cs and 98% of Rb were recovered by a five-stage countercurrent extraction.
- (2)
- The organic phase was scrubbed with deionized water at an O/A ratio of 2:1, and 99.32% of K was removed after five-stage countercurrent scrubbing. In the stripping stage, 0.5 mol/L HCl solution was sufficient to strip rubidium and cesium, and after two-stage countercurrent stripping at an O/A ratio of 3:1, more than 99% of Cs and Rb were stripped from the organic phase.
- (3)
- A solid compound may appear when the t-BAMBP/metal molar ratio in the organic phase is less than 4. The amount of solid compound increased with a decrease in the t-BAMBP/metal molar ratio.
- (4)
- The formation of the solid compound is related to intermolecular hydrogen bonds, resulting in an increased melting point of the organic phase.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, D.Z.; Wang, Y.P.; Liu, H.N.; Zhang, H.F.; Ye, X.S.; Li, Q.; Li, J.; Wu, Z.J. Efficient extraction of Rb+ and Cs+ by a precipitation flotation process with ammonium phosphowolframate as precipitant. Colloids Surf. A 2021, 608, 125581. [Google Scholar] [CrossRef]
- Ivanova, Z.G.; Zavadil, J.; Kostka, P.; Djouama, T.; Reinfelde, M. Photoluminescence properties of Er-doped Ge-In(Ga)-S glasses modified by caesium halides. Phys. Status Solidi B 2017, 254, 1600662. [Google Scholar] [CrossRef]
- Xu, X.L.; Singh, H.P.; Wang, L.; Qi, D.L.; Poulos, B.K.; Abramson, D.H.; Jhanwar, S.C.; Cobrinik, D. Rb suppresses human cone-precursor-derived retinoblastoma tumours. Nature 2014, 514, 385–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saliba, M.; Matsui, T.; Domanski, K.; Seo, J.; Ummadisingu, A.; Zakeeruddin, S.M.; Correa-Baena, J.; Tress, W.R.; Abate, A.; Hagfeldt, A.; et al. Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance. Science 2016, 354, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Harikesh, P.C.; Mulmudi, H.K.; Ghosh, B.; Goh, T.W.; Teng, Y.T.; Thirumal, K.; Lockrey, M.; Weber, K.; Koh, T.M.; Li, S.Z.; et al. Rb as an Alternative Cation for Templating Inorganic Lead-Free Perovskites for Solution Processed Photovoltaics. Chem. Mater. 2016, 28, 7496–7504. [Google Scholar] [CrossRef]
- Chen, Q.; Peng, C.G.; Du, L.; Hou, T.; Yu, W.J.; Chen, D.; Shu, H.; Huang, D.J.; Zhou, X.Q.; Zhang, J.Y.; et al. Synergy of mesoporous SnO2 and RbF modification for high-efficiency and stable perovskite solar cells. J. Energy Chem. 2022, 66, 250–259. [Google Scholar] [CrossRef]
- Zabeti, M.; Gupta, K.; Raman, G.; Lefferts, L.; Schallmoser, S.; Lercher, J.A.; Seshan, K. Aliphatic Hydrocarbons from Lignocellulose by Pyrolysis over Cesium-Modified Amorphous Silica Alumina Catalysts. ChemCatChem 2015, 7, 3386–3396. [Google Scholar] [CrossRef]
- Formichella, V.; Camparo, J.; Tavella, P. Mitigation of Lamplight-Induced Frequency Jumps in Space Rubidium Clocks. In IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control; IEEE: Piscataway, NJ, USA, 2018; Volume 65, pp. 911–918. [Google Scholar] [CrossRef]
- Zhang, X.F.; Aldahri, T.; Tan, X.M.; Liu, W.Z.; Zhang, L.Z.; Tang, S.W. Efficient co-extraction of lithium, rubidium, cesium and potassium from lepidolite by process intensification of chlorination roasting. Chem. Eng. Process. Process Intensif. 2020, 1477, 107777. [Google Scholar] [CrossRef]
- Zhang, X.F.; Qin, Z.F.; Aldahri, T.; Rohani, S.; Ren, S.; Liu, W.Z. Separation and recovery of cesium sulfate from the leach solution obtained in the sulfuric acid baking process of lepidolite concentrate. Hydrometallurgy 2021, 199, 105537. [Google Scholar] [CrossRef]
- Jeppesen, T.; Shu, L.; Keir, G.; Jegatheesan, V. Metal recovery from reverse osmosis concentrate. J. Clean. Prod. 2009, 17, 703–707. [Google Scholar] [CrossRef]
- Yan, Q.X.; Li, X.H.; Wang, Z.X.; Wu, X.F.; Guo, H.J.; Hu, Q.Y.; Peng, W.; Wang, J.X. Extraction of valuable metals from lepidolite. Hydrometallurgy 2012, 117–118, 116–118. [Google Scholar] [CrossRef]
- Wang, J.L.; Zhuang, S.T. Removal of cesium ions from aqueous solutions using various separation technologies. Rev. Environ. Sci. Biotechnol. 2019, 18, 231–269. [Google Scholar] [CrossRef]
- Jandová, J.; Dvořák, P.; Formánek, J.; Vu, H.N. Recovery of rubidium and potassium alums from lithium-bearing minerals. Hydrometallurgy 2012, 119–120, 73–76. [Google Scholar] [CrossRef]
- Liu, J.L.; Yin, Z.L.; Li, X.H.; Hu, Q.Y.; Liu, W. A novel process for the selective precipitation of valuable metals from lepidolite. Miner. Eng. 2019, 135, 29–36. [Google Scholar] [CrossRef]
- Naidu, G.; Nur, T.; Loganathan, P.; Kandasamy, J.; Vigneswaran, S. Selective sorption of rubidium by potassium cobalt hexacyanoferrate. Sep. Purif. Technol. 2016, 163, 238–246. [Google Scholar] [CrossRef]
- Lian, L.S.; Zhang, S.Y.; Ma, N.; Dai, W. Well-designed a novel phosphomolybdic-acid@PCN-224 composite with efficient simultaneously capture towards rubidium and cesium ions. Polyhedron 2021, 207, 115402. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, A.Y.; Su, J.Y.; Chen, R.R. Selective adsorption of potassium and rubidium by metal-organic frameworks supported supramolecular materials. J. Radioanal. Nucl. Chem. 2021, 329, 359–370. [Google Scholar] [CrossRef]
- Wang, J.L.; Zhuang, S.T.; Liu, Y. Metal hexacyanoferrates-based adsorbents for cesium removal. Coord. Chem. Rev. 2018, 374, 430–438. [Google Scholar] [CrossRef]
- Xu, C.; Wang, J.C.; Chen, J. Solvent Extraction of Strontium and Cesium: A Review of Recent Progress. Solvent Extr. Ion Exch. 2012, 30, 623–650. [Google Scholar] [CrossRef]
- Jagasia, P.; Mohapatra, P.K.; Dhami, P.S.; Patil, A.B.; Adya, V.C.; Sengupta, A.; Gandhi, P.M.; Wattal, P.K. Studies on the radiolytic stability of newly developed solvent systems containing four calix-crown-6 ligands for radio-cesium recovery. J. Radioanal. Nucl. Chem. 2014, 302, 1087–1093. [Google Scholar] [CrossRef]
- Huang, D.F.; Zheng, H.; Liu, Z.Y.; Bao, A.M.; Li, B. Extraction of rubidium and cesium from brine solutions using a room temperature ionic liquid system containing 18-crown-6. Pol. J. Chem. Technol. 2018, 20, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.M.; Liu, H.H.; Huang, Y.J.; Yang, W.J. Solvent extraction of rubidium and cesium from salt lake brine with t-BAMBP-kerosene solution. Trans. Nonferrous. Met. Soc. China 2015, 25, 329–334. [Google Scholar] [CrossRef]
- Li, Z.; Pranolo, Y.; Zhu, Z.W.; Cheng, C.Y. Solvent extraction of cesium and rubidium from brine solutions using 4-tert-butyl-2-(α-methylbenzyl)-phenol. Hydrometallurgy 2017, 171, 1–7. [Google Scholar] [CrossRef]
- Xing, P.; Wang, G.D.; Wang, C.Y.; Ma, B.Z.; Chen, Y.Q. Separation of rubidium from potassium in rubidium ore liquor by solvent extraction with t-BAMBP. Miner. Eng. 2018, 121, 158–163. [Google Scholar] [CrossRef]
- Zhang, A.Y.; Hu, Q.H. Removal of cesium by countercurrent solvent extraction with a calix[4]crown derivative. Sep. Sci. Technol. 2017, 52, 1670–1679. [Google Scholar] [CrossRef]
- Mohite, B.S.; Burungale, S.H. Separation of Rubidium from Associated Elements by Solvent Extraction with Dibenzo-24-crown-8. Anal. Lett. 1999, 32, 173–183. [Google Scholar] [CrossRef]
- Zhang, X.F.; Qin, Z.F.; Aldahri, T.; Ren, S.; Liu, W.Z. Solvent extraction of rubidium from a sulfate solution with high concentrations of rubidium and potassium using 4-tert-butyl-2-(α-methylbenzyl)-phenol. J. Taiwan Inst. Chem. E 2020, 116, 43–50. [Google Scholar] [CrossRef]
- Xing, P.; Wang, C.Y.; Chen, Y.Q.; Ma, B.Z. Separation of potassium from sodium in alkaline solution by solvent extraction with 4-tert-butyl-2-(α-methylbenzyl) phenol. J. Cent. South Univ. 2021, 28, 2003–2009. [Google Scholar] [CrossRef]
- Wang, J.W.; Che, D.H.; Qin, W. Extraction of rubidium by t-BAMBP in cyclohexane. Chin. J. Chem. Eng. 2015, 23, 1110–1113. [Google Scholar] [CrossRef]
- Chen, W.S.; Lee, C.H.; Chung, Y.F.; Tien, K.W.; Chen, Y.J.; Chen, Y.A. Recovery of Rubidium and Cesium Resources from Brine of Desalination through t-BAMBP Extraction. Metals 2020, 10, 607. [Google Scholar] [CrossRef]
- Gao, L.; Ma, G.H.; Zheng, Y.X.; Tang, Y.; Xie, G.S.; Yu, J.W.; Liu, B.X.; Duan, J.Y. Research Trends on Separation and Extraction of Rare Alkali Metal from Salt Lake Brine: Rubidium and Cesium. Solvent Extr. Ion Exch. 2020, 38, 753–776. [Google Scholar] [CrossRef]
- Ross, W.J.; White, J.C. Determination of Cesium and Rubidium after Extraction with 4-sec-Butyl-2(α-methylbenzyl) phenol. Anal. Chem. 1964, 36, 1998–2000. [Google Scholar] [CrossRef]
- Lv, Y.W.; Ma, B.Z.; Liu, Y.B.; Wang, C.Y.; Zhang, W.J.; Chen, Y.Q. Selective extraction of cesium from high concentration rubidium chloride leach liquor of lepidolite. Desalination 2022, 530, 115673. [Google Scholar] [CrossRef]
- Wang, J.H.; Shen, C.; Liu, Y.C.; Luo, J.; Duan, Y.J. Effects of hydrogen bond on the melting point of azole explosives. J. Mol. Struct. 2018, 1163, 54–60. [Google Scholar] [CrossRef]


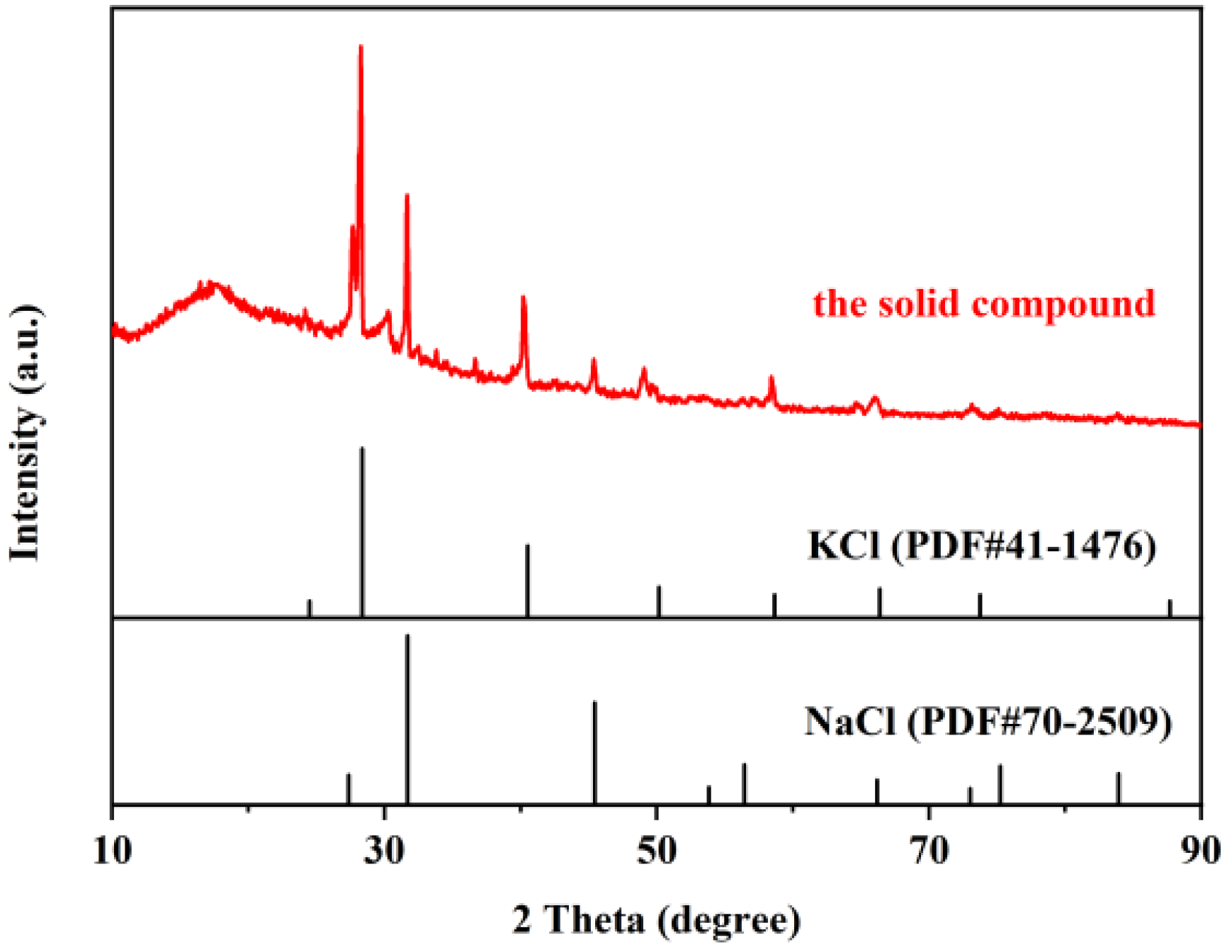
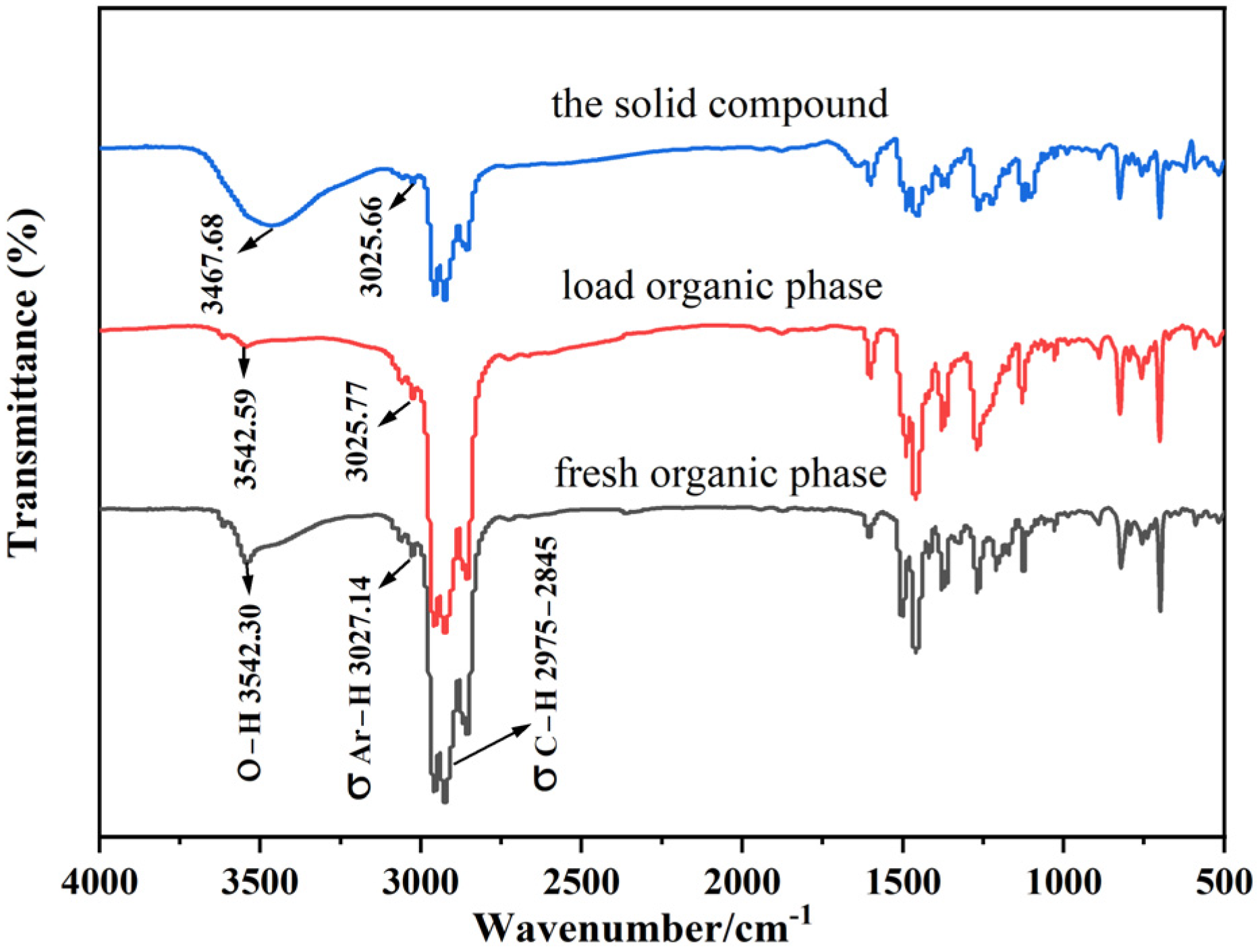
| Element | Na | K | Rb | Cs | Cl | Br | I |
|---|---|---|---|---|---|---|---|
| Concentration (g/L) | 86.13 | 82.18 | 6.54 | 7.71 | 191.39 | 20.98 | 40.99 |
| Material | Preprocessing | Element Mass Percentage (%) | |||
|---|---|---|---|---|---|
| Solid compound | Without scrubbing | Na | K | Rb | Cs |
| 2.35 | 2.66 | 1.39 | 8.23 | ||
| After scrubbing | Na | K | Rb | Cs | |
| / | 0.16 | 0.32 | 8.05 | ||
| O/A | Raffinate Concentration (g/L) | Organic Concentration (g/L) | Molar Ratio of t-BAMBP/Metal | Appearance of the Solid Compound | ||||
|---|---|---|---|---|---|---|---|---|
| K | Rb | Cs | K | Rb | Cs | |||
| 1:3 | 77.13 | 5.53 | 2.34 | 15.16 | 3.04 | 16.12 | 1.84 | Large quantity |
| 1:2 | 74.66 | 4.96 | 1.87 | 15.04 | 3.16 | 11.67 | 1.96 | Large quantity |
| 1:1 | 74.23 | 4.01 | 0.84 | 7.95 | 2.53 | 6.87 | 3.52 | Small quantity |
| 2:1 | 70.76 | 2.73 | 0.39 | 5.71 | 1.90 | 3.66 | 5.10 | No |
| 3:1 | 69.25 | 2.11 | 0.31 | 4.31 | 1.48 | 2.47 | 6.85 | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, J.; Li, K.; Shi, Z.; Min, C.; Li, S.; Yin, Z.; Ma, R. Separation of Cesium and Rubidium from Solution with High Concentrations of Potassium and Sodium. Separations 2023, 10, 42. https://doi.org/10.3390/separations10010042
Xie J, Li K, Shi Z, Min C, Li S, Yin Z, Ma R. Separation of Cesium and Rubidium from Solution with High Concentrations of Potassium and Sodium. Separations. 2023; 10(1):42. https://doi.org/10.3390/separations10010042
Chicago/Turabian StyleXie, Junjie, Kang Li, Zhuonan Shi, Changli Min, Shina Li, Zichen Yin, and Ruixin Ma. 2023. "Separation of Cesium and Rubidium from Solution with High Concentrations of Potassium and Sodium" Separations 10, no. 1: 42. https://doi.org/10.3390/separations10010042






