Analysis of Polycyclic Aromatic Hydrocarbons Using Magnetic Three-Dimensional Graphene Solid-Phase Extraction Coupled with Gas Chromatography–Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Apparatus
2.3. Gas Chromatography–Mass Spectrometry
2.4. Synthesis of the Adsorbent
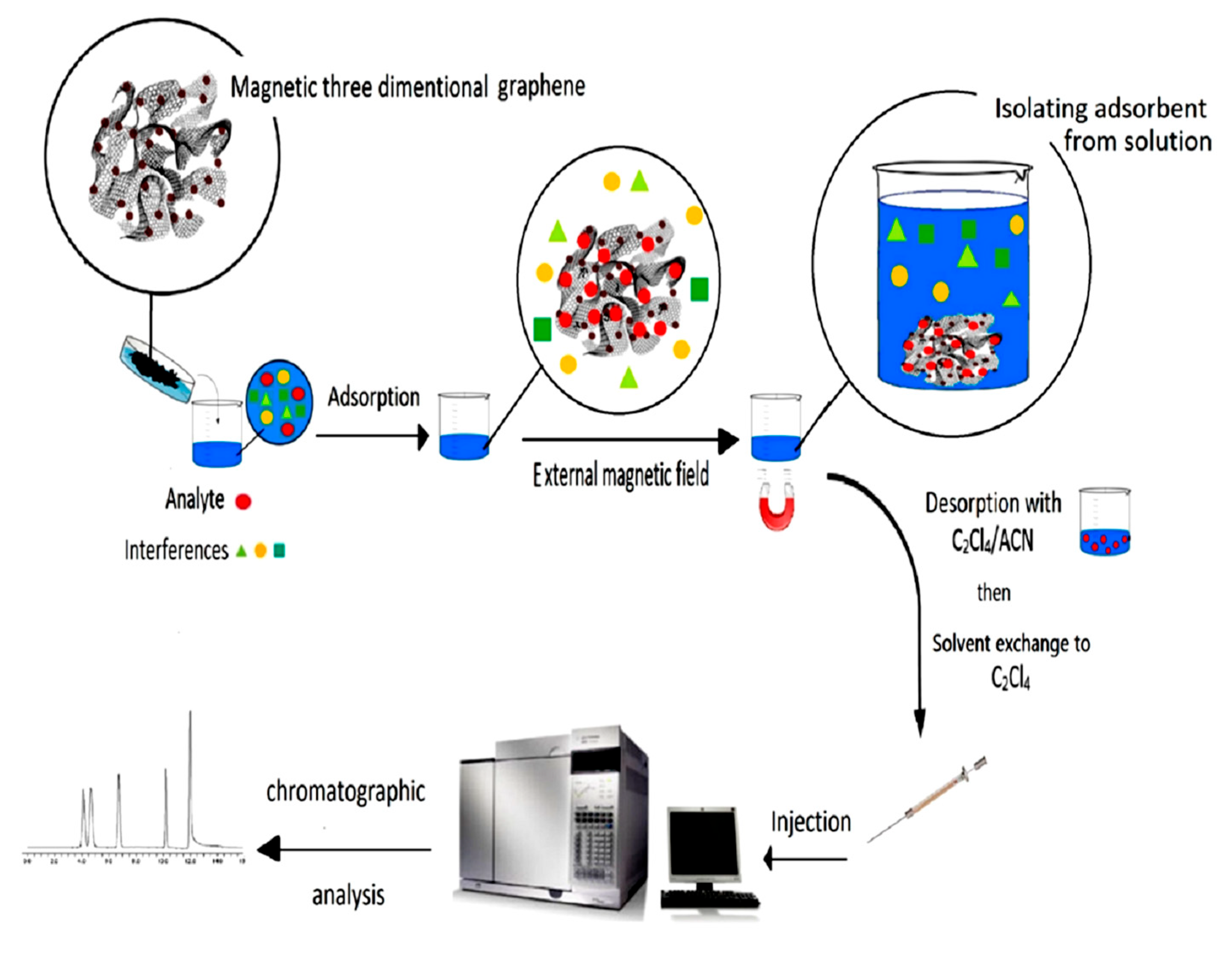
2.5. The MSPE Procedure
3. Results and Discussion
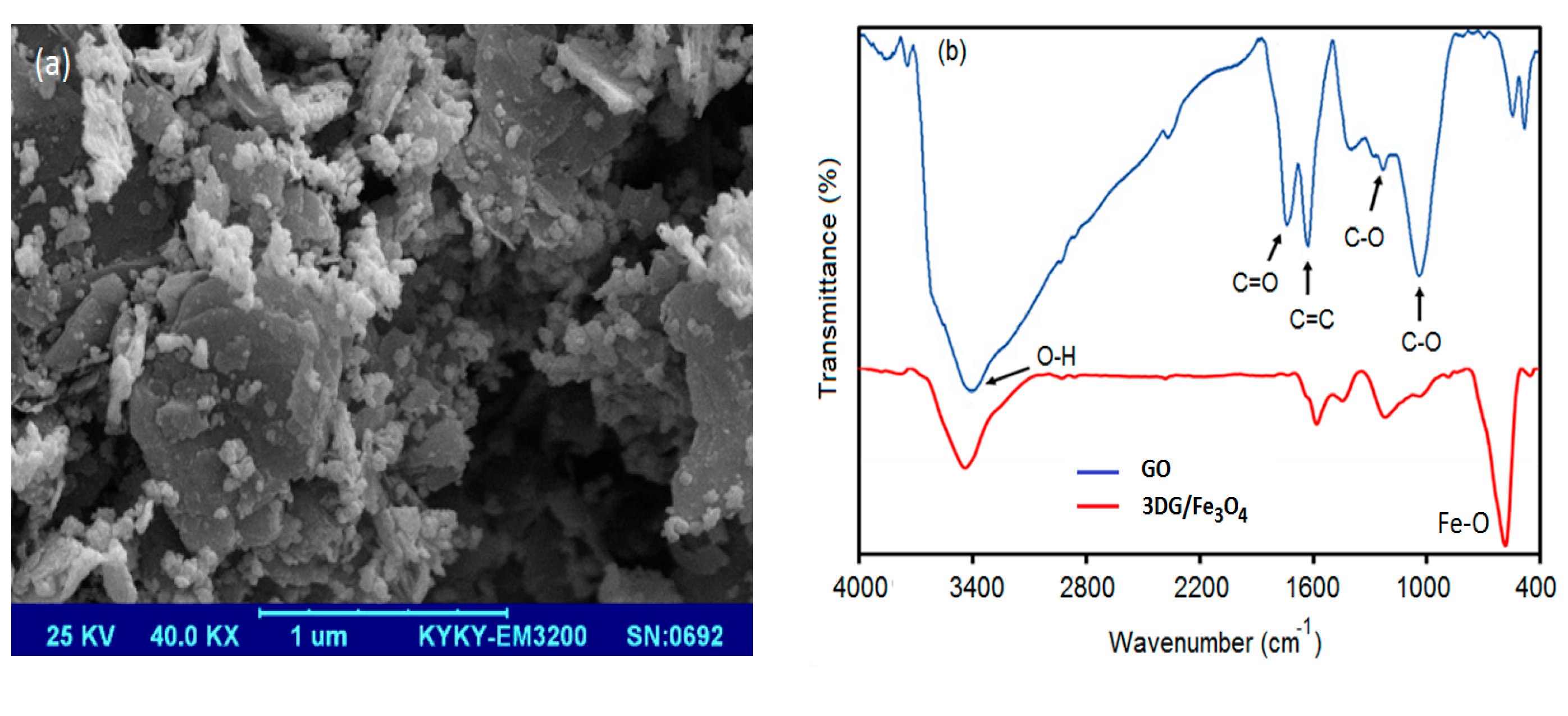
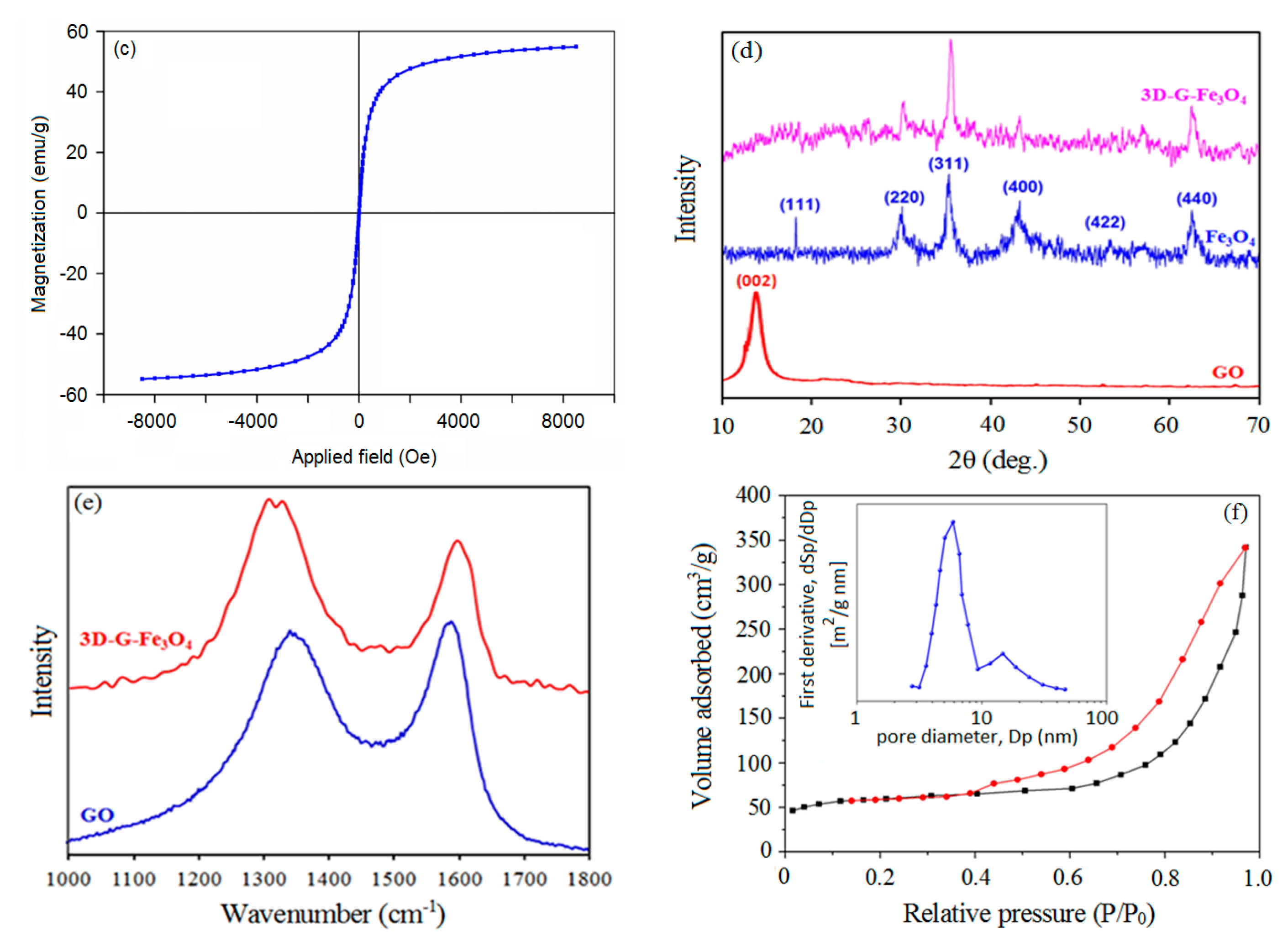
3.1. Characterization
3.2. Response Surface Modeling and Optimization
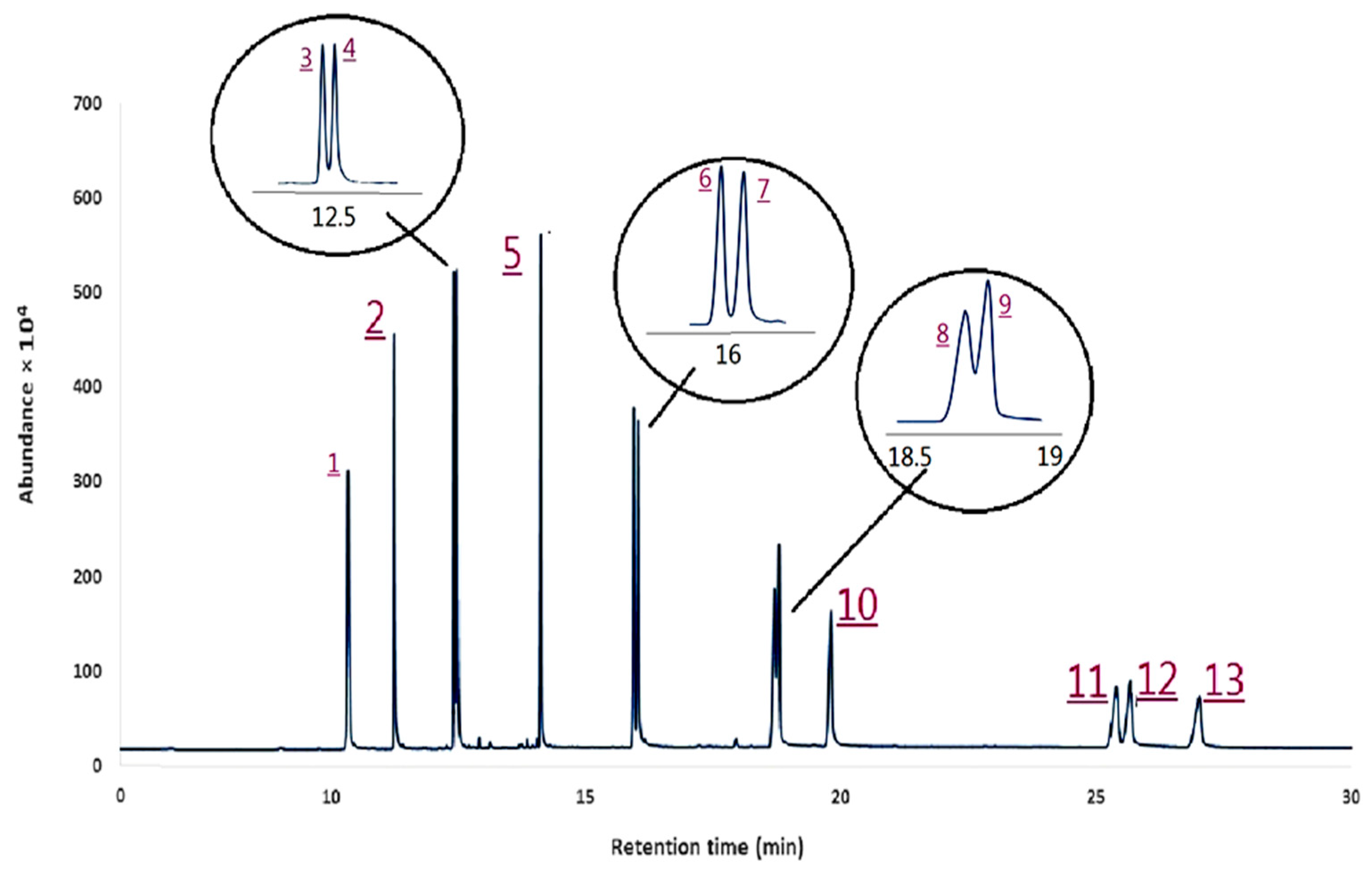
3.3. Method Validation
3.4. Reusability of the Adsorbent
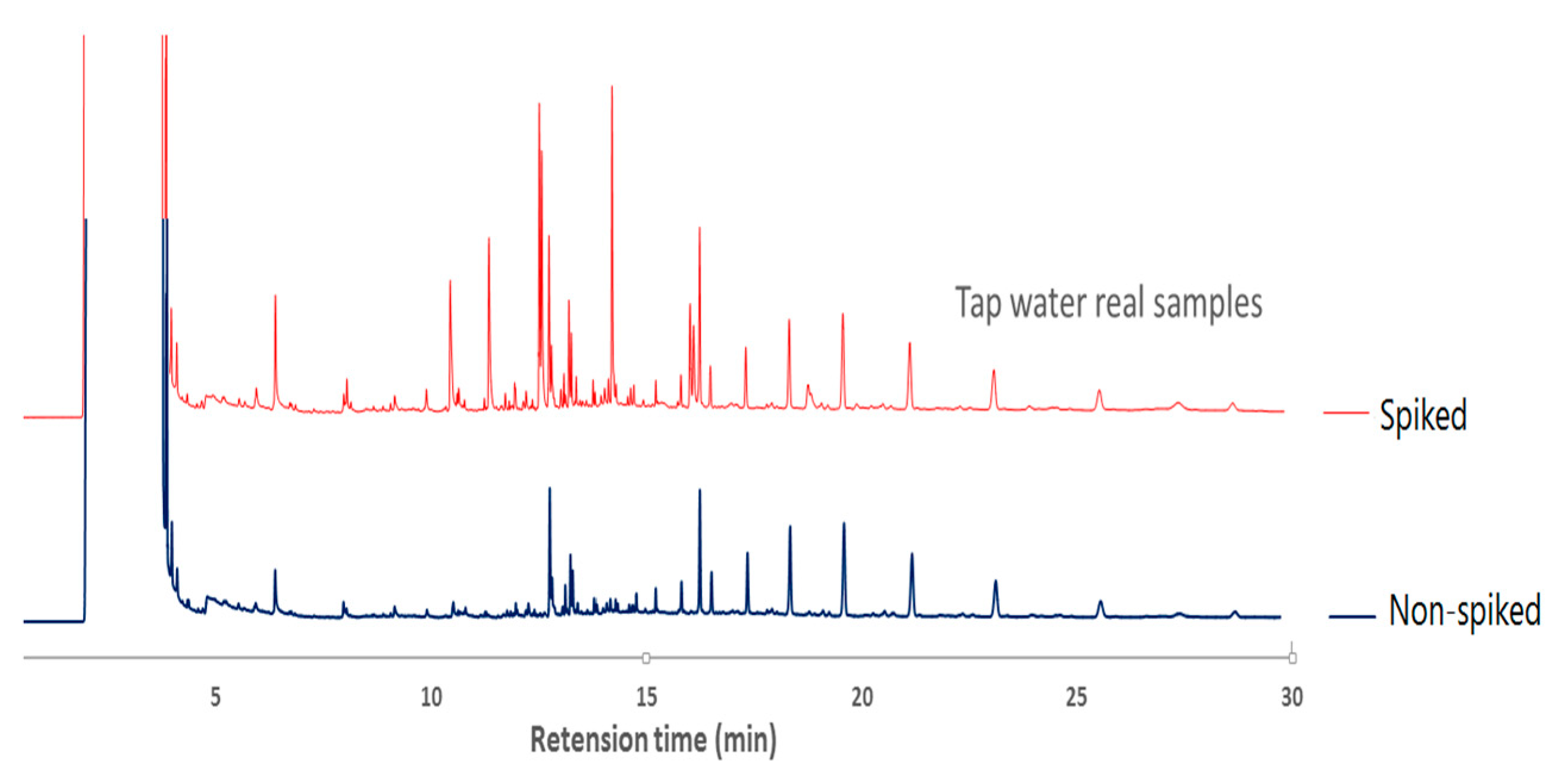
3.5. Analysis of Real Samples
3.6. Comparison with Other Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nazir, N.A.M.; Raoov, M.; Mohamad, S. Spent tea leaves as an adsorbent for micro-solid-phase extraction of polycyclic aromatic hydrocarbons (PAHs) from water and food samples prior to GC-FID analysis. Microchem. J. 2020, 159, 105581. [Google Scholar] [CrossRef]
- Hamadan, R.; Al-Taee, M.M.; Mahdi, A.B.; Hadrawi, S.K.; Dwijendra, N.K.A.; Kavitha, M.; Mustafa, Y.F.; Wadday, A.K. Entrapment of polyethylene terephthalate derived carbon in Ca-alginate beads for solid phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. Inorg. Chem. Comm. 2022, 146, 110147. [Google Scholar] [CrossRef]
- Menzie, C.A.; Potocki, B.B. Exposure to carinogenic PAHs in the Environment. Environ. Sci. Technol. 1992, 26, 1278–1284. [Google Scholar] [CrossRef]
- ATSDR. Toxicological Profile for Total Petroleum Hydrocarbons (TPH); U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 1999; 315p.
- Bagheri, A.R.; Aramesh, N.; Gong, Z.; Cerda, V.; Lee, H.K. Two-dimensional materials as a platform in extraction methods: A review. TrAC Trend. Anal. Chem. 2022, 152, 116606. [Google Scholar] [CrossRef]
- Câmara, J.S.; Perestrelo, R.; Berenguer, C.V.; Andrade, C.F.P.; Gomes, T.M.; Olayanju, B.; Kabir, A.; Rocha, C.M.R.; Teixeira, J.A.; Pereira, J.A.M. Green extraction techniques as advanced sample preparation approaches in biological, food, and environmental matrices: A review. Molecules 2022, 27, 2953. [Google Scholar] [CrossRef] [PubMed]
- Ullah, N.; Tuzen, M. A comprehensive review on recent developments and future perspectives of switchable solvents and their applications in sample preparation techniques. Green Chem. 2023, 25, 1729–1748. [Google Scholar] [CrossRef]
- Abuzar, K.; Samanidou, V. Fabric phase sorptive extraction: A paradigm shift approach in analytical and bioanalytical sample preparation. Molecules 2021, 26, 865. [Google Scholar]
- Suseela, M.N.L.; Viswanadh, M.K.; Mehata, A.K.; Priya, V.; Setia, V.A.; Malik, A.K.; Gokul, P.; Selvin, J.; Muthu, M.S. Advances in solid-phase extraction techniques: Role of nanosorbents for the enrichment of antibiotics for analytical quantification. J. Chromatogr. A 2023, 1695, 463937. [Google Scholar] [CrossRef]
- Chen, L.; Xu, S.; Li, J. Recent advances in molecular imprinting technology: Current status, challenges and highlighted applications. Chem. Soc. Rev. 2011, 40, 2922. [Google Scholar] [CrossRef]
- Lin, J.H.; Wu, Z.-H.; Tseng, W.-L. Extraction of environmental pollutants using magnetic nanomaterials. Anal. Methods 2010, 2, 1874–1879. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, X.-A.; Jin, C.-Z.; Zhang, W.-B. Ultrasonic assisted magnetic solid phase extraction of ultra-trace mercury with ionic liquid functionalized materials. Anal. Chim. Acta 2023, 1245, 340865. [Google Scholar] [CrossRef]
- Mohammad, F.; Shirani, M.; Rashidi-Nodeh, H. The recent advances in magnetic sorbents and their applications. TrAC Trend. Anal. Chem. 2021, 141, 116302. [Google Scholar]
- Somayeh, M.; Sabouri, Z.; Darroudi, M. Greener synthesis and medical applications of metal oxide nanoparticles. Ceram. Int. 2021, 47, 19632–19650. [Google Scholar]
- Zhang, T.; Wang, W.; Zhao, Y.; Bai, H.; Wen, T.; Kang, S.; Song, G.; Song, S.; Komarneni, S. Removal of heavy metals and dyes by clay-based adsorbents: From natural clays to 1D and 2D nanocomposites. Chem. Eng. J. 2021, 420, 127574. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, F.; Ji, S.; Yang, B.; Liang, X. Graphene nanoplatelets as a highly efficient solid-phase extraction sorbent for determination of phthalate esters in aqueous solution. Talanta 2014, 120, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Shahabaldin, R.; Mojiri, A.; Park, J.; Nawrot, N.; Wojciechowska, E.; Marraiki, N.; Zaghloul, N.S.S. Removal of lead ions from wastewater using lanthanum sulfide nanoparticle decorated over magnetic graphene oxide. Environ. Res. 2022, 204, 111959. [Google Scholar]
- Sadanala, K.C.; Chung, B.C. Graphene nanoplatelets as a solid phase extraction sorbent for analysis of chlorophenols in water. J. Korean Soc. Appl. Biol. Chem. 2013, 56, 673–678. [Google Scholar] [CrossRef]
- Mahpishanian, S.; Sereshti, H. Graphene oxide-based dispersive micro-solid phase extraction for separation and preconcentration of nicotine from biological and environmental water samples followed by gas chromatography-flame ionization detection. Talanta 2014, 130, 71–77. [Google Scholar] [CrossRef]
- Process, S.; Cong, H.; Ren, X.; Wang, P.; Yu, S. Macroscopic Multifunctional Graphene-Based Hydrogels and Aerogels by a Metal Ion Induced self-assembly process. ACS Nano. 2012, 6, 2693–2703. [Google Scholar]
- Mahpishanian, S.; Sereshti, H. Three-dimensional graphene aerogel-supported iron oxide nanoparticles as an efficient adsorbent for magnetic solid phase extraction of organophosphorus pesticide residues in fruit juices followed by gas chromatographic determination. J. Chromatogr. A 2016, 1443, 43–53. [Google Scholar] [CrossRef]
- Han, Q.; Wang, Z.; Xia, J.; Chen, S.; Zhang, X.; Ding, M. Facile and tunable fabrication of Fe3O4/graphene oxide nanocomposites and their application in the magnetic solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. Talanta 2012, 101, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Maddah, B.; Shamsi, J. Extraction and preconcentration of trace amounts of diazinon and fenitrothion from environmental water by magnetite octadecylsilane nanoparticles. J. Chromatogr. A 2012, 1256, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Li, X.; Peng, Q.; Wang, X.; Chen, J.; Li, Y. Monodisperse magnetic single-crystal ferrite microspheres. Angew. Chem. 2005, 117, 2842–2845. [Google Scholar] [CrossRef]
- Ma, Z.; Guan, Y.; Liu, H. Synthesis and characterization of micron-sizedmonodisperse superparamagnetic polymer particles with amino groups. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 3433–3439. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Mahpishanian, S.; Sereshti, H.; Ahmadvand, M. A nanocomposite consisting of silica-coated magnetite and phenyl-functionalized graphene oxide for extraction of polycyclic aromatic hydrocarbon from aqueous matrices. J. Environ. Sci. 2017, 55, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Razmi, H.; Jabbari, M. Development of graphene-carbon nanotube-coated magnetic nanocomposite as an efficient sorbent for HPLC determination of organophosphorus pesticides in environmental water samples. Int. J. Environ. Anal. Chem. 2015, 95, 1353–1369. [Google Scholar] [CrossRef]
- Karpierz, E.; Drabińska, A.; Bożek, R.; Kaźmierczak, P.; Wysmołek, A.; Kamińska, M.; Strupiński, W. ESR Spectroscopy of Graphene with Adsorbed NaCl Particles. Acta Phys. Pol. A 2014, 126, 1187–1189. [Google Scholar] [CrossRef]
- Mehdinia, A.; Khodaee, N.; Jabbari, A. Fabrication of graphene/Fe3O4@polythiophene nanocomposite and its application in the magnetic solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. Anal. Chim. Acta 2015, 868, 1–9. [Google Scholar] [CrossRef]
- Sarafraz-Yazdi, A.; Rokhian, T.; Amiri, A.; Ghaemi, F. Carbon nanofibers decorated with magnetic nanoparticles as a new sorbent for the magnetic solid phase extraction of selected polycyclic aromatic hydrocarbons from water samples. New J. Chem. 2015, 39, 5621–5627. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Lin, J.-M. Magnetic solid-phase extraction based on octadecyl functionalization of monodisperse magnetic ferrite microspheres for the determination of polycyclic aromatic hydrocarbons in aqueous samples coupled with gas chromatography-mass spectrometry. Talanta 2009, 77, 1037–1042. [Google Scholar] [CrossRef]
- Xu, S.-N.; Zhao, Q.; He, H.-B.; Yuan, B.-F.; Feng, Y.-Q.; Yu, Q.-W. Rapid determination of polycyclic aromatic hydrocarbons in environmental water based on magnetite nanoparticles/polypyrrole magnetic solid-phase extraction. Anal. Methods 2014, 6, 7046–7053. [Google Scholar] [CrossRef]
- Reyes-Gallardo, E.M.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Magnetic nanoparticles-nylon 6 composite for the dispersive micro solid phase extraction of selected polycyclic aromatic hydrocarbons from water samples. J. Chromatogr. A 2014, 1345, 43–49. [Google Scholar] [CrossRef]

| Compound | LDR a | R2 b | Calibration Equation | LOD c | LOQ d | RSD e (%), n = 3 |
|---|---|---|---|---|---|---|
| Acenaphthylene | 5–100 | 0.9984 | Y = 3.6x + 13.0 | 0.02 | 0.05 | 2.1 |
| Fluorene | 5–100 | 0.9990 | Y = 8.4x − 20.6 | 0.11 | 0.36 | 2.6 |
| Phenanthrene | 5–100 | 0.9997 | Y = 11.3x − 7.2 | 0.20 | 0.73 | 1.9 |
| Anthracene | 5–100 | 0.9973 | Y = 11.8x − 7.8 | 0.20 | 0.73 | 3.0 |
| Pyrene | 5–100 | 0.9989 | Y = 15.8x − 11.3 | 0.02 | 0.06 | 3.1 |
| Benz[a]anthracene | 5–100 | 0.9945 | Y = 6.9x − 5.1 | 0.14 | 0.46 | 3.6 |
| Chrysene | 5–100 | 0.9948 | Y = 7.5x − 0.04 | 0.04 | 0.13 | 3.0 |
| Benzo[b]fluoranthene | 5–100 | 0.9925 | Y = 2.5x + 7.5 | 0.20 | 0.66 | 2.9 |
| Benzo[k]fluoranthene | 5–100 | 0.9913 | Y = 2.5x + 3.6 | 0.17 | 0.56 | 3.9 |
| Benzo[a]pyrene | 5–100 | 0.9913 | Y = 1.6x − 8.4 | 0.10 | 0.33 | 3.3 |
| Indeno [123-CD]pyrene | 5–100 | 0.9925 | Y = 0.2x − 1.3 | 0.12 | 0.40 | 3.5 |
| Dibenz[ah]anthracene | 5–100 | 0.9987 | Y = 0.2x − 1.0 | 0.12 | 0.40 | 3.8 |
| benzo[ghi]perylene | 5–100 | 0.9965 | Y = 0.2x − 1.8 | 0.12 | 0.40 | 2.8 |
| Compound | Added (ng mL−1) | Found (ng mL−1) | Recovery (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Tap Water | River | Rainwater | Tap Water | River | Rainwater | Tap Water | River | Rainwater | |
| Acenaphthylene | 0 | 0 | 0 | n.d. | 2.84 | 0.6 | 106.29 | 106.62 | 81.82 |
| 25 | 25 | 25 | 43.45 | 29.49 | 21.06 | ||||
| Fluorene | 0 | 0 | 0 | n.d. | n.d. | 4.6 | 97.03 | 73.44 | 45.94 |
| 25 | 25 | 25 | 24.25 | 18.36 | 16.08 | ||||
| Phenanthrene | 0 | 0 | 0 | n.d. | n.d. | n.d. | 79.88 | 62.24 | 71.06 |
| 25 | 25 | 25 | 19.97 | 15.56 | 17.76 | ||||
| Anthracene | 0 | 0 | 0 | n.d. | n.d. | n.d. | 86.17 | 66.15 | 74.97 |
| 25 | 25 | 25 | 21.54 | 16.53 | 18.74 | ||||
| Pyrene | 0 | 0 | 0 | n.d. | n.d. | 2.3 | 64.85 | 46.42 | 61.85 |
| 25 | 25 | 25 | 16.21 | 11.6 | 17.79 | ||||
| Benz[a]anthracene | 0 | 0 | 0 | n.d. | n.d. | 2.46 | 59.1 | 17.41 | 53.33 |
| 25 | 25 | 25 | 14.77 | 4.35 | 13.33 | ||||
| Chrysene | 0 | 0 | 0 | n.d. | n.d. | 1.1 | 50.94 | 10.93 | 41.34 |
| 25 | 25 | 25 | 12.73 | 2.73 | 10.35 | ||||
| Benzo[b]fluoranthene | 0 | 0 | 0 | n.d. | 5.36 | 7.63 | 102.63 | 82.58 | 85.08 |
| 25 | 25 | 25 | 25.65 | 26.01 | 21.27 | ||||
| Benzo[k]fluoranthene | 0 | 0 | 0 | n.d. | n.d. | n.d. | 103.97 | 94.12 | 79.58 |
| 25 | 25 | 25 | 25.99 | 23.53 | 19.89 | ||||
| Benzo[a]pyrene | 0 | 0 | 0 | n.d. | n.d. | n.d. | 98.83 | 83.91 | 68.73 |
| 25 | 25 | 25 | 24.71 | 20.97 | 17.18 | ||||
| Indeno [123-CD]pyrene | 0 | 0 | 0 | n.d. | n.d. | n.d. | 109.08 | 97.53 | 82.68 |
| 25 | 25 | 25 | 27.27 | 24.38 | 20.68 | ||||
| Dibenz[ah]anthracene | 0 | 0 | 0 | n.d. | n.d. | n.d. | 109.13 | 101.54 | 84.47 |
| 25 | 25 | 25 | 27.28 | 25.38 | 21.11 | ||||
| benzo[ghi]perylene | 0 | 0 | 0 | n.d. | n.d. | n.d. | 109.97 | 101.36 | 87.59 |
| 25 | 25 | 25 | 27.49 | 25.38 | 21.89 | ||||
| Adsorbent | Method | PAHs | LOD a | LDR b | RSD c (%) | Time (min) | Dosage (mg) | Ref. |
|---|---|---|---|---|---|---|---|---|
| G/Fe3O4@PT d | MSPE-GC/FID | 5 | 0.01–0.02 | 0.03–80 | 4.3–6.3 | 10 | 20 | [30] |
| CNFs-Fe3O4 e | MSPE-GC/FID | 3 | 0.01–0.03 | 0.08–100 | 3.2–11.2 | 15 | 10 | [31] |
| M-C18 f | MSPE-GC/MS | 16 | 0.8–36 | 10–800 | 2–10 | 6 | 50 | [32] |
| Fe3O4-ppy g | MSPE-GC/MS | 10 | (0.38 − 5.01) × 103 | 0.01–2 | 13.3 | 3 | 20 | [33] |
| MNPs-N6 h | MSPE-UPLC i | 4 | 0.05–0.58 | 1–50 | 6.9 | 32 | 40 | [34] |
| OPA/MMNPs j | MSPE-GC/MS | 16 | 0.01–0.07 | 0.2–100 | 17.6 | 2 | 50 | [13] |
| 3DG/Fe3O4 | MSPE-GC/FID/MS | 13 | 0.01–0.07 | 0.03–100 | 2–7 | 4 | 10 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sereshti, H.; Karimi, M.; Karami, S.; Mahpishanian, S.; Esmaeili Bidhendi, M.; Rezania, S.; Mojiri, A.; Kamyab, H.; Rashidi Nodeh, H. Analysis of Polycyclic Aromatic Hydrocarbons Using Magnetic Three-Dimensional Graphene Solid-Phase Extraction Coupled with Gas Chromatography–Mass Spectrometry. Separations 2023, 10, 564. https://doi.org/10.3390/separations10110564
Sereshti H, Karimi M, Karami S, Mahpishanian S, Esmaeili Bidhendi M, Rezania S, Mojiri A, Kamyab H, Rashidi Nodeh H. Analysis of Polycyclic Aromatic Hydrocarbons Using Magnetic Three-Dimensional Graphene Solid-Phase Extraction Coupled with Gas Chromatography–Mass Spectrometry. Separations. 2023; 10(11):564. https://doi.org/10.3390/separations10110564
Chicago/Turabian StyleSereshti, Hassan, Mahsa Karimi, Sajad Karami, Shokouh Mahpishanian, Mehdi Esmaeili Bidhendi, Shahabaldin Rezania, Amin Mojiri, Hesam Kamyab, and Hamid Rashidi Nodeh. 2023. "Analysis of Polycyclic Aromatic Hydrocarbons Using Magnetic Three-Dimensional Graphene Solid-Phase Extraction Coupled with Gas Chromatography–Mass Spectrometry" Separations 10, no. 11: 564. https://doi.org/10.3390/separations10110564
APA StyleSereshti, H., Karimi, M., Karami, S., Mahpishanian, S., Esmaeili Bidhendi, M., Rezania, S., Mojiri, A., Kamyab, H., & Rashidi Nodeh, H. (2023). Analysis of Polycyclic Aromatic Hydrocarbons Using Magnetic Three-Dimensional Graphene Solid-Phase Extraction Coupled with Gas Chromatography–Mass Spectrometry. Separations, 10(11), 564. https://doi.org/10.3390/separations10110564









