Advancements in Adsorption Techniques for Sustainable Water Purification: A Focus on Lead Removal
Abstract
:1. Introduction
2. Lead (Pb2+) Toxicity and Sources
3. Conventional and Emerging Methods for Lead treatment
4. Multiple Modification Strategies for Conventional and Emerging Adsorbents
5. Adsorption Mechanism and Alternative Remediator
5.1. Adsorption Isotherm
5.2. Factors Affecting Adsorption Mechanism
5.2.1. Effect of Hydrogen Ion Concentration
5.2.2. Adsorbate Concentration
5.2.3. Time of Interaction
5.2.4. Coexisting Ion
5.2.5. Type of Adsorbent
5.2.6. Temperature Effect
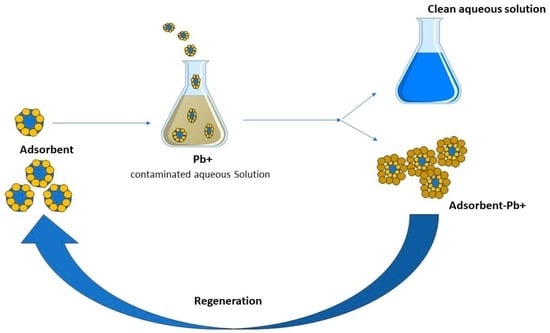
5.3. Regeneration/Recycling
6. Starch-Based Adsorbents
Starch Nanomaterial as Adsorbents for Lead
7. Challenges and Future Research Direction
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, R.; Ansari, K. Enhanced sequestration of methylene blue and crystal violet dye onto green synthesis of pectin modified hybrid (Pect/AILP-Kal) nanocomposite. Process Biochem. 2021, 111, 132–143. [Google Scholar] [CrossRef]
- Dinka, M.O. Safe Drinking Water: Concepts, Benefits, Principles and Standards. Water Chall. Urban. World 2018, 163, 163–174. [Google Scholar] [CrossRef]
- Iqbal, Z.; Tanweer, M.S.; Alam, M. Recent advances in adsorptive removal of wastewater pollutants by chemically modified metal oxides: A review. J. Water Process Eng. 2022, 46, 102641. [Google Scholar] [CrossRef]
- Hokkanen, S.; Bhatnagar, A.; Sillanpää, M. A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res. 2016, 91, 156–173. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, V.; Sharma, K.; Kumar, V.; Choudhary, S.; Mankotia, P.; Kumar, B.; Mishra, H.; Moulick, A.; Ekielski, A.; et al. A review of adsorbents for heavy metal decontamination: Growing approach to wastewater treatment. Materials 2021, 14, 4702. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.J.; Aziz, H.A.; Aziz, S.Q.; Abu Amr, S.S. An overview of electro-oxidation processes performance in stabilized landfill leachate treatment. Desalin. Water Treat. 2013, 51, 2170–2184. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Wilson, L.; Morin-crini, N.; Crini, G.; Lichtfouse, E.; Wilson, L. Conventional and non-conventional adsorbents for wastewater treatment To cite this version: HAL Id: Hal-02082916 Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2019, 17, 195–213. [Google Scholar] [CrossRef]
- Lead Removal from Water by Low Cost Adsorbents: A Review, 2AD. Available online: https://www.researchgate.net/publication/288942240_Lead_Removal_from_Water_by_Low_Cost_AdsorbentsA_Review (accessed on 18 October 2023).
- Kamal, B.; Rafey, A. A mini review of treatment methods for lead removal from wastewater. Int. J. Environ. Anal. Chem. 2023, 103, 5126–5141. [Google Scholar] [CrossRef]
- Kumar, V.; Dwivedi, S.K.; Oh, S. A critical review on lead removal from industrial wastewater: Recent advances and future outlook. J. Water Process Eng. 2022, 45, 102518. [Google Scholar] [CrossRef]
- Chowdhury, I.R.; Chowdhury, S.; Mazumder, M.A.J.; Al-Ahmed, A. Removal of Lead Ions (Pb2+) from Water and Wastewater: A Review on the Low-Cost Adsorbents; Springer International Publishing: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Li, N.; Tao, J.; Yan, B.; Cui, X.; Chen, G. Adsorption of Lead from Aqueous Solution by Biochar: A Review. Clean Technol. 2022, 4, 629–652. [Google Scholar] [CrossRef]
- Maftouh, A.; El Fatni, O.; Hajjaji, S. Comparative Review of Different Adsorption Techniques Used in Heavy Metals Comparative Review of Different Adsorption Techniques Used in Heavy Metals Removal in Water. Biointerface Res. Appl. Chem. 2023, 13, 397. [Google Scholar] [CrossRef]
- Topare, N.S.; Wadgaonkar, V.S. A review on application of low-cost adsorbents for heavy metals removal from wastewater. Mater. Today Proc. 2023, 77, 8–18. [Google Scholar] [CrossRef]
- Garvasis, J.; Prasad, A.R.; Shamsheera, K.O.; Nidheesh Roy, T.A.; Joseph, A. Weed to nano seeds: Ultrasonic assisted one-pot fabrication of superparamagnetic magnetite nano adsorbents from Siam weed flower extract for the removal of lead from water. J. Hazard. Mater. Adv. 2022, 8, 100163. [Google Scholar] [CrossRef]
- Lv, L.; Chen, N.; Feng, C.; Zhang, J.; Li, M. Heavy metal ions removal from aqueous solution by xanthate. RSC Adv. 2017, 7, 27992–28000. [Google Scholar] [CrossRef]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Clean Water Action. Available online: https://cleanwater.org/ (accessed on 26 June 2023).
- Cilliers, L.; Retief, F. Lead Poisoning and the Downfall of Rome. Hist. Toxicol. Environ. Health 2014, 1, 118–126. [Google Scholar] [CrossRef]
- Liu, B.; Khan, A.; Kim, K.H.; Kukkar, D.; Zhang, M. The adsorptive removal of lead ions in aquatic media: Performance comparison between advanced functional materials and conventional materials. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2441–2483. [Google Scholar] [CrossRef]
- Lead(II) Nitrate. 2023. Available online: https://www.chemeurope.com/en/encyclopedia/Lead%28II%29_nitrate.html (accessed on 30 June 2023).
- Lead(II) Chloride. 2023. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB0385798.htm (accessed on 10 May 2023).
- Gad, S.C.; Pham, T. Lead. In Encyclopedia of Toxicology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 61–65. [Google Scholar] [CrossRef]
- Lead(II) Acetate. 2023. Available online: https://www.acs.org/molecule-of-the-week/archive/l/lead-ii-acetate.html (accessed on 10 May 2023).
- Gulson, B.; Korsch, M.; Matisons, M.; Douglas, C.; Gillam, L.; McLaughlin, V. Windblown lead carbonate as the main source of lead in blood of children from a seaside community: An example of local birds as “canaries in the mine”. Environ. Health Perspect. 2009, 117, 148–154. [Google Scholar] [CrossRef]
- Buica, G.; Antonov, A.E.; Beiu, C.; Pasculescu, D.; Remus, D. Occupational health and safety management in construction sector—The cost of work accidents. Qual.-Access Success 2017, 18, 35–40. [Google Scholar]
- Acharya, J.; Sahu, J.N.; Mohanty, C.R.; Meikap, B.C. Removal of lead(II) from wastewater by activated carbon developed from Tamarind wood by zinc chloride activation. Chem. Eng. J. 2009, 149, 249–262. [Google Scholar] [CrossRef]
- Cechinel, M.A.P.; de Souza, A.A.U. Ulson De Souza, Study of lead (II) adsorption onto activated carbon originating from cow bone. J. Clean. Prod. 2014, 65, 342–349. [Google Scholar] [CrossRef]
- Qu, X.; Alvarez, P.J.; Li, Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013, 47, 3931–3946. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sharma, R.K.; Agrawal, M.; Marshall, F.M. Health risk assessment of heavy metals via dietary intake of foodstuffs from the wastewater irrigated site of a dry tropical area of India. Food Chem. Toxicol. 2010, 48, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, S.K.; Tung, T.M.; Yaseen, Z.M. Development of artificial intelligence for modeling wastewater heavy metal removal: State of the art, application assessment and possible future research. J. Clean. Prod. 2020, 250, 119473. [Google Scholar] [CrossRef]
- Bala, S.; Garg, D.; Thirumalesh, B.V.; Sharma, M.; Sridhar, K.; Inbaraj, B.S.; Tripathi, M. Recent Strategies for Bioremediation of Emerging Pollutants: A Review for a Green and Sustainable Environment. Toxics 2022, 10, 484. [Google Scholar] [CrossRef]
- Kazadi Mbamba, C.; Batstone, D.J.; Flores-Alsina, X.; Tait, S. A generalised chemical precipitation modelling approach in wastewater treatment applied to calcite. Water Res. 2015, 68, 342–353. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Starch, cellulose, pectin, gum, alginate, chitin and chitosan derived (nano)materials for sustainable water treatment: A review. Carbohydr. Polym. 2021, 251, 116986. [Google Scholar] [CrossRef]
- Crane, R.A.; Scott, T.B. Nanoscale zero-valent iron: Future prospects for an emerging water treatment technology. J. Hazard. Mater. 2012, 211–212, 112–125. [Google Scholar] [CrossRef]
- Wei, Y.; Wei, S.; Liu, C.; Chen, T.; Tang, Y.; Ma, J.; Yin, K.; Luo, S. Efficient removal of arsenic from groundwater using iron oxide nanoneedle array-decorated biochar fibers with high Fe utilization and fast adsorption kinetics. Water Res. 2019, 167, 115107. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Kamari, A.; Koay, Y.J. Equilibrium and kinetics studies of adsorption of copper (II) on chitosan and chitosan/PVA beads. Int. J. Biol. Macromol. 2004, 34, 155–161. [Google Scholar] [CrossRef]
- Bala, S.; Garg, D.; Thirumalesh, B.V.; Sharma, M.; Sridhar, K.; Inbaraj, B.S. High Adsorption of Benzoic Acid on Single Walled Carbon Nanotube Bundles. Sci. Rep. 2020, 10, 10013. [Google Scholar] [CrossRef]
- Meenakshi, G.; Roop, C.B. Activated Carbon Adsorption. 2005. Available online: https://books.google.ps/books?hl=en&lr=&id=VUluBwAAQBAJ&oi=fnd&pg=PP1&dq=type+of+inorganic+pollutant+that+is+adsorbed+in+activated+carbon&ots=gtQ9k0r-bD&sig=CEZgbRzXhR660_WDCVq6c6_5q4Q&redir_esc=y#v=onepage&q&f=false (accessed on 20 September 2023).
- Mohamed, E.F.; El-Mekawy, A.; Ahmed, S.A.S.; Fathy, N.A. High Adsorption Capacity of Ammonia Gas Pollutant Using Adsorbents of Carbon Composites. Arab. J. Sci. Eng. 2023, 1–11. [Google Scholar] [CrossRef]
- Williams, E.L.; Grosjean, D. Exposure of Deacidified and Untreated Paper to Ambient Levels of Sulfur Dioxide and Nitrogen Dioxide: Nature and Yields of Reaction Products. J. Am. Inst. Conserv. 1992, 31, 199–212. [Google Scholar] [CrossRef]
- Kiwaan, H.A.; Mohamed, F.S.; El-Ghamaz, N.A.; Beshry, N.M.; El-Bindary, A.A. Experimental and electrical studies of Na-X zeolite for the adsorption of different dyes. J. Mol. Liq. 2021, 332, 115877. [Google Scholar] [CrossRef]
- Velarde, L.; Nabavi, M.S.; Escalera, E.; Antti, M.L.; Akhtar, F. Adsorption of heavy metals on natural zeolites: A review. Chemosphere 2023, 328, 138508. [Google Scholar] [CrossRef]
- Zhan, L.; Zhao, L.; Wu, L.; You, Y.; Bate, B. A passive sink-zeolite permeable reactive barrier to control NH4+-N pollution plume within groundwater: Conceptual design and numerical modeling. Chemosphere 2023, 334, 138965. [Google Scholar] [CrossRef]
- Verma, M.; Kumar, A.; Lee, I.; Kumar, V.; Park, J.H.; Kim, H. Simultaneous capturing of mixed contaminants from wastewater using novel one-pot chitosan functionalized with EDTA and graphene oxide adsorbent. Environ. Pollut. 2022, 304, 119130. [Google Scholar] [CrossRef]
- Saheed, I.O.; Da Oh, W.; Suah, F.B.M. Chitosan modifications for adsorption of pollutants—A review. J. Hazard. Mater. 2021, 408, 124889. [Google Scholar] [CrossRef]
- Jabeen, A.; Bhatti, H.N.; Noreen, S.; Gaffar, A. Adsorptive removal of 2, 4, 6-trichloro-phenol from wastewater by mango seed shell and its magnetic composites: Batch and column study. Int. J. Environ. Anal. Chem. 2021, 103, 5639–5659. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E.; Montuelle, B.; Steinman, A.D. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. Int. Sch. Res. Netw. ISRN Ecol. 2011, 2011, 20. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Z.; Wu, H.; Pan, S.; Cheng, X.; Sun, Y.; Xu, Y. Effective and simultaneous removal of organic/inorganic arsenic using polymer-based hydrated iron oxide adsorbent: Capacity evaluation and mechanism. Sci. Total Environ. 2020, 742, 140508. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Ihsanullah, I.; Abdul Aziz, H.; Azmier Ahmad, M.; Al-Harthi, M.A. Sustainable wastewater treatment by biochar/layered double hydroxide composites: Progress, challenges, and outlook. Bioresour. Technol. 2021, 319, 124128. [Google Scholar] [CrossRef] [PubMed]
- Cairns, S.; Chaudhuri, S.; Sigmund, G.; Robertson, I.; Hawkins, N.; Dunlop, T.; Hofmann, T. Wood ash amended biochar for the removal of lead, copper, zinc and cadmium from aqueous solution. Environ. Technol. Innov. 2021, 24, 101961. [Google Scholar] [CrossRef]
- Barquilha, C.E.R.; Braga, M.C.B. Adsorption of organic and inorganic pollutants onto biochars: Challenges, operating conditions, and mechanisms. Bioresour. Technol. Rep. 2021, 15, 100728. [Google Scholar] [CrossRef]
- Abdelnaby, A.; Abdelaleem, N.M.; Elshewy, E.; Mansour, A.H.; Ibrahim, S.S. Application of Bentonite Clay, Date Pit, and Chitosan Nanoparticles as Promising Adsorbents to Sequester Toxic Lead and Cadmium from Milk. Biol. Trace Elem. Res. 2022, 201, 2650–2664. [Google Scholar] [CrossRef]
- Pandey, S. A comprehensive review on recent developments in bentonite-based materials used as adsorbents for wastewater treatment. J. Mol. Liq. 2017, 241, 1091–1113. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Misra, B.N. Grafting: A versatile means to modify polymers: Techniques, factors and applications. Prog. Polym. Sci. 2004, 29, 767–814. [Google Scholar] [CrossRef]
- Akhtar, J.; Aishah, N.; Amin, S.; Shahzad, K. A review on removal of pharmaceuticals from water by adsorption. Desalin. Water Treat. 2016, 57, 12842–12860. [Google Scholar] [CrossRef]
- Ifthikar, J.; Jiao, X.; NgamJiao, A.; Wang, T.; Khan, A.; Jawad, A.; Xue, Q.; Liu, L.; Chen, Z. Facile One-Pot Synthesis of Sustainable Carboxymethyl Chitosan—Sewage Sludge Biochar for Effective Heavy Metal Chelation and Regeneration. Bioresour. Technol. 2018, 262, 22–31. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Y.; Li, X.; Yang, M.; Wang, J.; Chen, Y. A promising and cost-effective biochar adsorbent derived from jujube pit for the removal of Pb(II) from aqueous solution. Sci. Rep. 2020, 10, 7473. [Google Scholar] [CrossRef]
- Randall, J.M.; Hautala, E.; McDonald, G. Binding of heavy metal ions by formaldehyde-polymerized peanut skins. J. Appl. Polym. Sci. 1978, 22, 379–387. [Google Scholar] [CrossRef]
- Kumari, S.; Rath, P.; Kumar, A.S.H.; Tiwari, T.N. Removal of hexavalent chromium using chitosan prepared from shrimp shells. Afr. J. Biotechnol. 2016, 15, 50–54. [Google Scholar] [CrossRef]
- Celik, A.; Demirbaş, A. Removal of heavy metal ions from aqueous solutions via adsorption onto modified lignin from pulping wastes. Energy Sour. 2005, 27, 1167–1177. [Google Scholar] [CrossRef]
- Xie, B.; Hou, Y.; Li, Y. Modified lignin nanosphere adsorbent for lead and copper ions. BioResources 2021, 16, 249–262. [Google Scholar] [CrossRef]
- Ge, H.; Huang, H.; Xu, M.; Chen, Q. Cellulose/poly(ethylene imine) composites as efficient and reusable adsorbents for heavy metal ions. Cellulose 2016, 23, 2527–2537. [Google Scholar] [CrossRef]
- Masri, M.S.; Friedman, M. Effect of chemical modification of wool on metal ion binding. J. Appl. Polym. Sci. 1974, 18, 2367–2377. [Google Scholar] [CrossRef]
- Vunain, E.; Mishra, A.K.; Mamba, B.B. Dendrimers, mesoporous silicas and chitosan-based nanosorbents for the removal of heavy-metal ions: A review. Int. J. Biol. Macromol. 2016, 86, 570–586. [Google Scholar] [CrossRef]
- Thiebault, T. Raw and modified clays and clay minerals for the removal of pharmaceutical products from aqueous solutions: State of the art and future perspectives. Crit. Rev. Environ. Sci. Technol. 2019, 50, 1451–1514. [Google Scholar] [CrossRef]
- Irannajad, M.; Kamran Haghighi, H. Removal of Heavy Metals from Polluted Solutions by Zeolitic Adsorbents: A Review. Environ. Process. 2021, 8, 7–35. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.; Adams, R.; Carnethon, M.; De Simone, G.; Bruce Ferguson, T.; Flegal, K.; Ford, E.; Furie, K.; Go, A.; Greenlund, K. CME Test Questions: June 2020. J. Vasc. Interv. Radiol. 2020, 31, 960. [Google Scholar] [CrossRef]
- Low, S.K.; Tan, M.C.; Chin, N.L. Effect of ultrasound pre-treatment on adsorbent in dye adsorption compared with ultrasound simultaneous adsorption. Ultrason. Sonochem. 2018, 48, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Daud, M.; McKay, G.; Shehzad, F.; Al-Harthi, M.A. Recent progress in layered double hydroxides (LDH)-containing hybrids as adsorbents for water remediation. Appl. Clay Sci. 2017, 143, 279–292. [Google Scholar] [CrossRef]
- Wang, J.; Gu, Z.; Zhang, J.; Chen, X.; Li, M.; Yu, Y.; Ge, M.; Li, X. Mesoporous structure TiO2/SiO2 composite for methylene blue adsorption and photodegradation. Micro Nano Lett. 2019, 14, 323–328. [Google Scholar] [CrossRef]
- Mahvi, A.H.; Balarak, D.; Bazrafshan, E. Remarkable reusability of magnetic Fe3O4-graphene oxide composite: A highly effective adsorbent for Cr(VI) ions. Int. J. Environ. Anal. Chem. 2023, 103, 3501–3521. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Wu, M.; Pang, Y.; Hao, Z.; Hu, M.; Qiu, R.; Chen, Z. Enhanced adsorption of tetracycline by an iron and manganese oxides loaded biochar: Kinetics, mechanism and column adsorption. Bioresour. Technol. 2021, 320, 124264. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, T.; Choudhury, B. Metal Oxides for Removal of Heavy Metal Ions; Springer: Berlin/Heidelberg, Germany, 2021; pp. 157–178. [Google Scholar] [CrossRef]
- Saleh, T.A.; Gupta, V.K. Column with CNT/magnesium oxide composite for lead(II) removal from water. Environ. Sci. Pollut. Res. 2012, 19, 1224–1228. [Google Scholar] [CrossRef]
- Ignatius, A.; Arunbabu, V.; Neethu, J.; Ramasamy, E.V. Rhizofiltration of lead using an aromatic medicinal plant Plectranthus amboinicus cultured in a hydroponic nutrient film technique (NFT) system. Environ. Sci. Pollut. Res. 2014, 21, 13007–13016. [Google Scholar] [CrossRef]
- Abegunde, S.M.; Idowu, K.S.; Adejuwon, O.M.; Adeyemi-Adejolu, T. A review on the influence of chemical modification on the performance of adsorbents. Resour. Environ. Sustain. 2020, 1, 100001. [Google Scholar] [CrossRef]
- Yu, D.; Wang, Y.; Wu, M.; Zhang, L.; Wang, L.; Ni, H. Surface functionalization of cellulose with hyperbranched polyamide for efficient adsorption of organic dyes and heavy metals. J. Clean. Prod. 2019, 232, 774–783. [Google Scholar] [CrossRef]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J. Enhanced removal of nitrate from water using surface modification of adsorbents—A review. J. Environ. Manag. 2013, 131, 363–374. [Google Scholar] [CrossRef]
- Baskar, A.V.; Bolan, N.; Hoang, S.A.; Sooriyakumar, P.; Kumar, M.; Singh, L.; Jasemizad, T.; Padhye, L.P.; Singh, G.; Vinu, A.; et al. Recovery, regeneration and sustainable management of spent adsorbents from wastewater treatment streams: A review. Sci. Total Environ. 2022, 822, 153555. [Google Scholar] [CrossRef] [PubMed]
- Anum, A.; Ibrahim, S.M.; Tahir, A.A.; Nazir, M.A.; Malik, M.; Shah, S.S.A.; Ehsan, A.; Wattoo, M.A.; Rehman, A. Construction of hybrid sulfur-doped MOF-235@g-C3N4 photocatalyst for the efficient removal of nicotine. Inorg. Chem. Commun. 2023, 157, 111268. [Google Scholar] [CrossRef]
- Din, M.A.U.; Shah, S.S.A.; Javed, M.S.; Sohail, M.; ur Rehman, A.; Nazir, M.A.; Assiri, M.A.; Najam, T.; Cheng, N. Synthesis of MXene-based single-atom catalysts for energy conversion applications. Chem. Eng. J. 2023, 474, 145700. [Google Scholar] [CrossRef]
- Hethnawi, A.; Nassar, N.N.; Manasrah, A.D.; Vitale, G. Polyethylenimine-Functionalized Pyroxene Nanoparticles Embedded on Diatomite for Adsorptive Removal of Dye from Textile Wastewater in a Fixed-Bed Column; Elsevier B.V.: Amsterdam, The Netherlands, 2017; Volume 320, ISBN 1403210977. [Google Scholar]
- Rodríguez, E.; Arqués, J.L.; Rodríguez, R.; Nuñez, M.; Medina, M.; Talarico, T.L.; Casas, I.A.; Chung, T.C.; Dobrogosz, W.J.; Axelsson, L.; et al. Adsorption Isotherms: Enlightenment of the Phenomenon of Adsorption. Intech 1989, 32, 137–144. Available online: https://www.intechopen.com/books/advanced-biometric-technologies/liveness-detection-in-biometrics (accessed on 7 August 2023).
- Younas, F.; Mustafa, A.; Farooqi, Z.U.R.; Wang, X.; Younas, S.; Mohy-Ud-din, W.; Hameed, M.A.; Abrar, M.M.; Maitlo, A.A.; Noreen, S.; et al. Current and Emerging Adsorbent Technologies for Wastewater Treatment: Trends, Limitations, and Environmental Implications. Water 2021, 13, 215. [Google Scholar] [CrossRef]
- Huang, S.H.; Chen, D.H. Rapid removal of heavy metal cations and anions from aqueous solutions by an amino-functionalized magnetic nano-adsorbent. J. Hazard. Mater. 2009, 163, 174–179. [Google Scholar] [CrossRef]
- Hassan, P.B.; Rasheed, R.O.; Zargoosh, K. Cadmium and Lead Removal from Aqueous Solution Using Magnetite Nanoparticles Biofabricated from Portulaca oleracea Leaf Extract. J. Nanomater. 2022, 2022, 1024554. [Google Scholar] [CrossRef]
- Yadav, M.K.; Saidulu, D.; Ghosal, P.S.; Mukherjee, A.; Gupta, A.K. A review on the management of arsenic-laden spent adsorbent: Insights of global practices, process criticality, and sustainable solutions. Environ. Technol. Innov. 2022, 27, 102500. [Google Scholar] [CrossRef]
- Alsawy, T.; Rashad, E.; El-Qelish, M.; Mohammed, R.H. A comprehensive review on the chemical regeneration of biochar adsorbent for sustainable wastewater treatment. Npj Clean Water 2022, 5, 29. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Pramanik, S.; Abdelgawad, M.A.; Abualsoud, B.M.; Kadi, A.; Ansari, M.J.; Deepak, A. Recent Advances of Chitosan Formulations in Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 10975. [Google Scholar] [CrossRef]
- Mahdavi, M.; Bin, M. Fabrication and Characterization of SiO2/(3-Aminopropyl) triethoxysilane-Coated Magnetite Nanoparticles for Lead (II) Removal from Aqueous Solution. J. Inorg. Organomet. Polym. Mater. 2013, 23, 599–607. [Google Scholar] [CrossRef]
- Fan, H.L.; Zhou, S.F.; Jiao, W.Z.; Qi, G.S.; Liu, Y.Z. Removal of heavy metal ions by magnetic chitosan nanoparticles prepared continuously via high-gravity reactive precipitation method. Carbohydr. Polym. 2017, 174, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Abood, M.I.; TYAl-Abdullah, Z. Synthesis of Ag-Nanoparticles and their Application in Treatment of Waste Water. In AIP Conference Proceedings; AIP Publishing: College Park, MD, USA, 2022; Volume 2398. [Google Scholar] [CrossRef]
- Chen, K.; He, J.; Li, Y.; Cai, X.; Zhang, K.; Liu, T.; Hu, Y.; Lin, D.; Kong, L.; Liu, J. Removal of cadmium and lead ions from water by sulfonated magnetic nanoparticle adsorbents. J. Colloid Interface Sci. 2017, 494, 307–316. [Google Scholar] [CrossRef]
- Talukder, M.E.; Pervez, M.N.; Jianming, W.; Stylios, G.K.; Hassan, M.M.; Song, H.; Naddeo, V.; Figoli, A. Ag nanoparticles immobilized sulfonated polyethersulfone/polyethersulfone electrospun nanofiber membrane for the removal of heavy metals. Sci. Rep. 2022, 12, 5814. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, Z.K.; Sarkar, F.K. Selective Removal of Lead (II) Ion from Wastewater Using Superparamagnetic Monodispersed Iron Oxide (Fe3O4) Nanoparticles as a Effective Adsorbent. Int. J. Nanosci. Nanotechnol. 2013, 9, 109–114. [Google Scholar]
- Jafari, M. Removal of Heavy Mineral from Mine Effluent with Amino Silica-Modified Magnetic Nanoparticles. Int. J. Mech. Eng. 2021, 6, 4253–4265. [Google Scholar]
- Al, S.S.; Merdas, S.M.; Guzar, S.H. [Poly(Thiourea-Formaldehyde)-Epoxy resin)] Nanomagnetic Full-IPN’s for Removal of Heavy Metals from Aqueous Solution: Synthesis and Characterization. J. Educ. Pure Sci.-Univ. Thi-Qar 2021, 11, 23–33. [Google Scholar]
- Kasirajan, R.; Bekele, A.; Girma, E. Adsorption of lead (Pb-II) using CaO-NPs synthesized by solgel process from hen eggshell: Response surface methodology for modeling, optimization and kinetic studies. S. Afr. J. Chem. Eng. 2022, 40, 209–229. [Google Scholar] [CrossRef]
- Irawan, C.; Nata, I.F.; Lee, C.K. Removal of Pb(II) and As(V) using magnetic nanoparticles coated montmorillonite via one-pot solvothermal reaction as adsorbent. J. Environ. Chem. Eng. 2019, 7, 103000. [Google Scholar] [CrossRef]
- Tiwari, S.; Hasan, A.; Pandey, L.M. A Novel Bio-Sorbent Comprising Encapsulated Agrobacterium fabrum (SLAJ731) and Iron Oxide Nanoparticles for Removal of Crude Oil Co-Contaminant, Lead Pb(II); Elsevier B.V.: Amsterdam, The Netherlands, 2017; Volume 5. [Google Scholar] [CrossRef]
- Ngoc, B.T.P.; Thi, N.H.T.; Dinh, T.S.; Le Tan, N.T.; Le, N.P.T.; Mai, T.P.; Nguyen, D.Q. Synthesis of Ferromagnetic Nanocomposites from Nanocrystalline Cellulose and Characterization as an Adsorbent to Remove Lead in the Water. Chem. Eng. Trans. 2022, 97, 19–24. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Aguilar, S.F.; Kurkuri, M.D.; Rodrigues, D.F.; Ray, R.L. Elevated Adsorption of Lead and Arsenic over Silver Nanoparticles Deposited on Poly(amidoamine) Grafted Carbon Nanotubes. Nanomaterials 2022, 12, 3852. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Wang, W.; Tan, F.; Luo, F.; Chen, J.; Qiao, X. Investigation on the efficiency and mechanism of Cd(II) and Pb(II) removal from aqueous solutions using MgO nanoparticles. J. Hazard. Mater. 2015, 299, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.; Pittman, C.U.; Mohan, D. Magnetic magnetite (Fe3O4) nanoparticle synthesis and applications for lead (Pb2+) and chromium (Cr6+) removal from water. J. Colloid Interface Sci. 2016, 468, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.Y.; Raj, T.N.V.; Archana, S.; Prasad, S.B.B.; Olivera, S.; Muralidhara, H.B. SnO2 nanoparticles as effective adsorbents for the removal of cadmium and lead from aqueous solution: Adsorption mechanism and kinetic studies. J. Water Process Eng. 2016, 13, 44–52. [Google Scholar] [CrossRef]
- Ghasemi, M.; Azimi-Amin, J. Effect of pH on Green Synthesis of Reduced Graphene Oxide Using Lemon Extract and Application of Fe3 O4/RGO nanocomposites for the removal of Pb (II) from aqueous solution. J. Water Environ. Nanotechnol. 2022, 7, 101–120. [Google Scholar] [CrossRef]
- Nor, N.M.; Atul, J.; Wazir, H. Pb2+ Removal by Using Activated Coconut Waste Modified with Different Metal Oxide Nanoparticles. ESTEEM Acad. J. 2022, 18, 1–10. [Google Scholar]
- Rezk, R.A.; Abdel-Salam, Z.; Abdel Ghany, N.A.; Abdelkreem, M.; Abdel-Harith, M. LIBS and pXRF validation for the removal of Pb by bio-CaCO3 nanoparticles from contaminated water. SN Appl. Sci. 2022, 4, 151. [Google Scholar] [CrossRef]
- Ge, H.; Hua, T.; Chen, X. Selective adsorption of lead on grafted and crosslinked chitosan nanoparticles prepared by using Pb2+ as template. J. Hazard. Mater. 2016, 308, 225–232. [Google Scholar] [CrossRef]
- Hashem, B.Y.; Alswat, A.A.; Ali, S.L.; Al-Odaini, N.A.; Alshorifi, F.T. Facile Synthesis of NiO-CuO/Activated Carbon Nanocomposites for Use in the Removal of Lead and Cadmium Ions from Water. ACS Omega 2022, 7, 47183–47191. [Google Scholar] [CrossRef]
- Taylor, P.; Saleh, T.A. Desalination and Water Treatment Nanocomposite of carbon nanotubes/silica nanoparticles and their use for adsorption of Pb (II): From surface properties to sorption mechanism. Desalin. Water Treat. 2016, 57, 10730–10744. [Google Scholar] [CrossRef]
- El Mouden, A.; El Messaoudi, N.; El Guerraf, A.; Bouich, A.; Mehmeti, V.; Lacherai, A.; Jada, A.; Sher, F. Multifunctional cobalt oxide nanocomposites for efficient removal of heavy metals from aqueous solutions. Chemosphere 2023, 317, 137922. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R. Fabrication of Polystyrene/AlOOH Hybrid Material for Pb(II) Decontamination from Wastewater: Isotherm, Kinetic, and Thermodynamic Studies. Colloids Interfaces 2022, 6, 72. [Google Scholar] [CrossRef]
- Jaber, L.; Ihsanullah, I.; Almanassra, I.W.; Backer, S.N.; Abushawish, A.; Khalil, A.K.A.; Alawadhi, H.; Shanableh, A.; Atieh, M.A. Adsorptive Removal of Lead and Chromate Ions from Water by Using Iron-Doped Granular Activated Carbon Obtained from Coconut Shells. Sustainability 2022, 14, 10877. [Google Scholar] [CrossRef]
- Manatunga, D.C.; De Silva, R.M.; De Silva, K.M.N.; Ratnaweera, R. Natural polysaccharides leading to super adsorbent hydroxyapatite nanoparticles for the removal of heavy metals and dyes from aqueous solutions. RSC Adv. 2016, 6, 105618–105630. [Google Scholar] [CrossRef]
- Tabasum, S.; Younas, M.; Zaeem, M.A.; Majeed, I.; Majeed, M.; Noreen, A.; Iqbal, M.N.; Zia, K.M. A review on blending of corn starch with natural and synthetic polymers, and inorganic nanoparticles with mathematical modeling. Int. J. Biol. Macromol. 2019, 122, 969–996. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.; Li, X.; Zhang, Y.; Chen, L.; Li, L.; Miao, S. Progress in tailoring starch intrinsic structures to improve its nutritional value. Food Hydrocoll. 2021, 113, 106447. [Google Scholar] [CrossRef]
- Apriyanto, A.; Compart, J.; Fettke, J. A review of starch, a unique biopolymer—Structure, metabolism and in planta modifications. Plant Sci. 2022, 318, 111223. [Google Scholar] [CrossRef]
- Das, A.; Sit, N. Modification of Taro Starch and Starch Nanoparticles by Various Physical Methods and their Characterization. Starch-Stärke 2021, 73, 2000227. [Google Scholar] [CrossRef]
- Haq, F.; Yu, H.; Wang, L.; Teng, L.; Haroon, M.; Khan, R.U.; Mehmood, S.; Bilal-Ul-Amin; Ullah, R.S.; Khan, A.; et al. Advances in chemical modifications of starches and their applications. Carbohydr. Res. 2019, 476, 12–35. [Google Scholar] [CrossRef]
- Zhang, B.; Cui, D.; Liu, M.; Gong, H.; Huang, Y.; Han, F. Corn porous starch: Preparation, characterization and adsorption property. Int. J. Biol. Macromol. 2012, 50, 250–256. [Google Scholar] [CrossRef]
- Naushad, M.; Ahamad, T.; Sharma, G.; Al-Muhtaseb, A.H.; Albadarin, A.B.; Alam, M.M.; ALOthman, Z.A.; Alshehri, S.M.; Ghfar, A.A. Synthesis and characterization of a new starch/SnO2 nanocomposite for efficient adsorption of toxic Hg2+ metal ion. Chem. Eng. J. 2016, 300, 306–316. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Hamadi, A.; Foroutan, R.; Peighambardoust, S.J.; Ramavandi, B. Decontamination of Cd2+ and Pb2+ from aqueous solution using a magnetic nanocomposite of eggshell/starch/Fe3O4. J. Water Process Eng. 2022, 48, 102911. [Google Scholar] [CrossRef]
- Chang, Y.; Yan, X.; Wang, Q.; Ren, L.; Tong, J.; Zhou, J. High efficiency and low cost preparation of size controlled starch nanoparticles through ultrasonic treatment and precipitation. Food Chem. 2017, 227, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, M.; Xiong, L.; Qiu, L.; Bian, X.; Sun, C.; Sun, Q. Oxidation modification of debranched starch for the preparation of starch nanoparticles with calcium ions. Food Hydrocoll. 2018, 85, 86–92. [Google Scholar] [CrossRef]
- Torres, F.G.; De-la-torre, G.E. Synthesis, characteristics, and applications of modified starch nanoparticles: A review. Int. J. Biol. Macromol. 2022, 194, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.; Deng, L.; Yin, J.; Yang, T.; Li, J.; He, W. Recent advances in starch-based magnetic adsorbents for the removal of contaminants from wastewater: A review. Int. J. Biol. Macromol. 2022, 218, 909–929. [Google Scholar] [CrossRef] [PubMed]
- Kemper, J.; Burkholder, A.; Jain, A.; Mustufa, T.; Wyrobek, K.; Burdette, C.; Song, D.; Okamura, A.; Fichtinger, G. Starch Nanoparticles: A Review. Med. Phys. 2005, 32, 2108. [Google Scholar] [CrossRef]
- Giller, K.E.; Witter, E.; Mcgrath, S.P. Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: A review. Soil Biol. Biochem. 1998, 30, 1389–1414. [Google Scholar] [CrossRef]





| Form | Chemical Formula | Characteristics | Generation Source | Industrial Remediation | Reference |
|---|---|---|---|---|---|
| Lead nitrate | (NO3)2 | A colourless crystal or white powder and is soluble in water | Used in pigments, as heat stabilizer in nylon and polyesters, in coatings of photo thermographic paper | Chemical precipitation, using lime, soda, or sodium sulphide precipitants | [21] |
| Lead chloride | PbCl2 | White crystals or powder, insoluble in cold water, soluble in hot water, low water solubility, odourless. | Occurs naturally as the mineral cotunnite, used in the synthesis of other lead compounds and is a precursor for many organometallic lead derivatives, lead-acid batteries, pigments. Used in making ceramics, infrared transmitting glass | Chemical precipitation using lime, soda, or sodium sulphide precipitants | [22] |
| Lead acetate | Pb (CH3COO)2 | White crystalline powder or solid sweet taste, soluble in water and glycerine | Production of dyes and mordants, also used in hair dyes, and as a fixative for some photographic processes | Chemical precipitation | [23,24] |
| Lead carbonate | PbCO3 | White solid, occurs naturally as the mineral cerussite | Used in the production of pigments, glass, and ceramics. | [25] |
| Conventional Technologies | ||||
|---|---|---|---|---|
| Category | Method | Description | Example | Ref. |
| Bioremediation | Bioremediation | Using microorganisms to break down contaminants | Bioremediation of petroleum hydrocarbon-contaminated groundwater using a mixed culture of microorganisms. | [32] |
| Chemical | Precipitation | Using (pH adjustment and flocculation) | Precipitation model is developed in the calcite precipitation via dynamic pH titration tests. | [33] |
| Ion Exchange | Using an ion exchange resin to remove contaminants from water | Removal of heavy metals from wastewater using ion exchange. | [5,32] | |
| Physical | Membrane Filtration | Using a membrane to remove contaminants from water. | Removal of microplastics from water using membrane filtration. | [32] |
| Reverse Osmosis | Using pressure to remove contaminants from water through a semi-permeable membrane | Removal of fluoride from drinking water using reverse osmosis. | [32] | |
| Emerging Technologies | ||||
| Nanoscale Materials | Nanoscale Materials | Using nanoscale materials to remediate contaminated water | Removal of arsenic from groundwater using iron oxide nanoparticles. | [36] |
| Phytoremediation | Phytoremediation | Using plants to remove contaminants from water | Removal of heavy metals from wastewater using water hyacinth. | [34] |
| Zero-Valent Iron | Zero-Valent Iron | Using zero-valent iron to remediate contaminated water | Removal of hexavalent chromium from groundwater using zero-valent iron. | [35] |
| Material | Application Method | Advantages | Disadvantages | Pollutant | Reference |
|---|---|---|---|---|---|
| 1. Activated Carbon | Adsorption column, batch mixing | High adsorption capacity, versatile, effective for a wide range of contaminants, commercially available | Expensive, can require regeneration or disposal after use, may require additional treatment for desorption of adsorbed contaminants |
| [38,39] |
| 2. Silica Gel | Adsorption column, packed bed | High surface area, stable, commercially available, | Limited selectivity for specific contaminants, may require frequent replacement or regeneration, can release dust if mishandled |
| [20,40,41] |
| 3. Zeolites | Fixed bed, packed columns | High selectivity for specific ions, ion exchange capabilities, stable, regenerable, commercially available | Limited capacity for certain contaminants, potential for clogging in fixed-bed systems, regeneration process may require additional chemicals. |
| [5,42,43,44] |
| 4. Chitosan | Application Method: Batch mixing, filtration, membrane adsorption | Natural, biodegradable, versatile, high metal ion adsorption capacity, effective for heavy metals and dyes | Limited stability in acidic conditions, limited regeneration capabilities, potential for gel formation in aqueous systems. |
| [5,45,46] |
| 5. Polymeric Resins | Column, batch mixing | High selectivity for specific ions, excellent ion exchange capabilities, regenerable, commercially available | Limited selectivity for specific contaminants, variable quality depending on feedstock, may require pre-treatment for efficient adsorption. |
| [47,48,49] |
| 6. Biochar | Fixed bed, soil amendment, filtration | Fixed bed, soil amendment, filtration | Limited selectivity for specific contaminants, variable quality depending on feedstock, may require pre-treatment for efficient adsorption. |
| [50,51,52] |
| 7. Natural Clays (e.g., Bentonite) | Mixing, packed bed, sedimentation | Abundant, cost-effective, natural, versatile, can remove various contaminants including heavy metals and organic compounds. | Limited adsorption capacity for some contaminants, potential for clogging, variable performance depending on clay type and composition. |
| [53,54] |
| No. | Material | Adsorption Capacity mg/g | Regeneration %/No. Cycle | Equilibrium Time (min) | Ref. |
|---|---|---|---|---|---|
| 1 | Bio-adsorbent modified with carboxy methyl chitosan (BMCMC) | 210 | NA | 60 | [57] |
| 2 | Jujube pit biochar (JPB) | 137.1 | 70%/5 | 30 | [58] |
| 3 | Formaldehyde-polymerized peanut skins (FPPS) | 217.6 | NA | NA | [59] |
| 4 | Andean Sacha inchi shell biomass (SISB) | 17.066 | NA | NA | [60] |
| 5 | Improved lignin material (ILM) | 17.5 | NA | 240 | [61] |
| 6 | Carboxymethyl lignin nanoparticles (CMLN) | 333.26 | NA | NA | [62] |
| 7 | Poly Ethelene imine-grafted cellulose (PEI) | 248.2 | >86%/5 | 4800 | [63] |
| No. | Adsorbent Material/Modification | Modified/ Unmodified | Adsorption Capacity (mg/g) | Regeneration | Adsorbate | Source of Wastewater | Initial Concentration of Contaminants | Operational Conditions | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1. | Lignin/eucalyptus lignin nanosphere | ECLNP’s | 126 | After 3 cycles reached around 94 | (Pb2+) + Cu2+ | NA | Pb2+ −20 mg/L Cu2+ −20 mg/L | pH Pb = 6 pH Cu = 5.5 °C = 30 | [62] |
| LNP’s | 10 | ||||||||
| 2. | Chitosan/carboxymethyl nanoparticles | XCMCP | 59.85 | After 7 cycles it changed from 32.1 to 29.7 | (Pb2+) | Electroplating, Mine, and battery production wastewater | NA | pH = 6 | [16] |
| Unmodified form | NA | ||||||||
| 3. | Cellulose/hyperbranched polyamide functionalized cellulose | HPFC | 138 Cu (II) | 85% of Cu (II) could be removed after 5 cycles. | Cu2+ ions | Textile wastewater | NA | pH < 8.33 298 k | [78] |
| Unmodified form | NA | ||||||||
| 4. | Biochar/modified with carboxy methyl chitosan | BMCMC Unmodified BC | 594.17 NA | Shows good reusability and stability | Pb2+ | Sewage sludge | 25 ml | pH Pb2+ = 5 | [57] |
| 5. | Cellulose/polyethylene imine | Cellulose/PEI Pure cellulose | 184.0 25.6 | 86% after 5 cycles | Heavy metals (Pb2+, Cu) | Sewage | 500 mg/L | pH Cu2+ = 2 pH Pb = 5 room temperature | [63] |
| Model | Plot |
|---|---|
| Langmuir | |
| Freundlich | |
BET Sips (Freundlich–Langmuir) | |
| Ads. Capacity (mg/g) | Regeneration Percent/Cycle | pH Media | Initial Conc. Lead (Pb2+) | Equilibrium Time (min) | Mechanism of Adsorption | Regeneration Method | Ref. | |
|---|---|---|---|---|---|---|---|---|
| 17.65 | 90% | pH 4 | 10 mg/L | NA | Chelation | Acid treatment | [91] |
| 79.29 | NA | pH 6 | 15,20 mg/L | 720 | Ion exchange and complexation | Na2EDTA | [92] |
| 108.93 | 99% | pH 7–10 | 10 mg/L | 1440 | Cation exchange | Acid treatment | [94] |
| 2614 | NA | pH 6–8 | NA | 10,800 | precipitation and adsorption MgO | NA | [104] |
| 53.11 | NA | pH 5 | 25–100 mg/L | NA | Magnetic separation | Acid treatment | [105] |
| 13 | NA | pH 6 | 5–30 ppm | NA | Chemisorption | NA | [112] |
| 55.24 | 90%/5 | pH 8.5 | 50–350 mg/L | 140 | Spontaneous adsorption | NaOH | [113] |
| 241.6 | NA | pH 5.5–6 | 200 ppm | 50 | Multilayer adsorption | NA | [102] |
| 182.78 | 99.9%/4 | pH 7 | 100 ppm | 30 | Multilayer adsorption | NA | [111] |
| 23.61 | NA | pH 6 | 20–100 mg/L | NA | Chemisorption and ion exchange | Acid treatment | [114] |
| 18.7 | 99% | pH 8 | 40 mg/L | 15 | Intraparticle diffusion and the boundary layer effect. | 4 cycles without losing adsorption capacity | [103] |
| 25.65 | 96.6%/ | pH 8 | 10 mg/L | 60 | Chemisorption | NA | [93] |
| 315.42 | 92%/5 | pH > 4 | 50–500 mg/L | 10 | Spontaneous, feasible, and endothermic under the applied conditions | Ethanol and deionized water | [15] |
| 11.9 | 96.3% | pH 6 | 100 mg/L | NA | Physical and chemical adsorption | Acid treatment | [115] |
| 35.45 | 90–100% | pH > 5 | 20 ppm | 55–60 | Chemisorption | NA | [97] |
| 108.23 | NA | pH 5 | 10–15 mg/L | NA | Intraparticle diffusion | NA | [87] |
| 4.98 | NA | NA | 100 mg/L | 30 | Chemisorption | NA | [108] |
| 4.98 | NA | NA | 100 mg/L | 30 | Chemisorption | NA | [108] |
| 4.96 | NA | NA | 100 mg/L | 30 | Chemisorption | NA | [108] |
| 2.564 | NA | pH 6 | 1, 10.20, 30 ppm | 1500 | Monolayer sorption | NA | [98] |
| 108.5 | NA | pH 6 | 1500 mg/L | 20 | Ion exchange | NA | [109] |
| 370.37 | 49.4%/10 | pH 7.2 | 250–450 mg/L | 30 | Chemical surface adsorption, | Acid treatment | [95] |
| 107.52 | 81.1/5 | pH 5 | 25–200 mg/L | 60 | cation–π interactions | Acid treatment | [107] |
| 175.44 | NA | pH 6.94 | 75.46 ppm | 135 | valence force or electron exchange | NA | [99] |
| 625 | NA | pH 5.5 | 2500–6000 ppm | 90 | Multilayer sorption | NA | [116] |
| 909 | NA | pH 5.5 | 2500–60,000 ppm | 45 | Multilayer sorption | NA | [116] |
| 38.15 | 7% decrease after 4 | pH 6.5 | NA | 480 | Electrostatic interaction | [100] | |
| 197.02 | NA | pH 5.5 | 1000–2900 mg/L | 240 | Intraparticle diffusion | External magnetic field | [101] |
| 1265.8 | 30%/3 | pH 7 | 100–400 ppm | 90 | Ionization | Acid treatment | [106] |
| 734.3 | 72%/5 | pH 5 | 0.015 mol/L | 240 | Physical and chemical adsorption | Acid treatment | [110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badran, A.M.; Utra, U.; Yussof, N.S.; Bashir, M.J.K. Advancements in Adsorption Techniques for Sustainable Water Purification: A Focus on Lead Removal. Separations 2023, 10, 565. https://doi.org/10.3390/separations10110565
Badran AM, Utra U, Yussof NS, Bashir MJK. Advancements in Adsorption Techniques for Sustainable Water Purification: A Focus on Lead Removal. Separations. 2023; 10(11):565. https://doi.org/10.3390/separations10110565
Chicago/Turabian StyleBadran, Amal M., Uthumporn Utra, Nor Shariffa Yussof, and Mohammed J. K. Bashir. 2023. "Advancements in Adsorption Techniques for Sustainable Water Purification: A Focus on Lead Removal" Separations 10, no. 11: 565. https://doi.org/10.3390/separations10110565
APA StyleBadran, A. M., Utra, U., Yussof, N. S., & Bashir, M. J. K. (2023). Advancements in Adsorption Techniques for Sustainable Water Purification: A Focus on Lead Removal. Separations, 10(11), 565. https://doi.org/10.3390/separations10110565