Comprehensive Chemical Characterization of Qingkailing Capsules by Ultra-High-Performance Liquid Chromatography Combined with Fourier Transform Ion Cyclotron Resonance Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Qingkailing Capsules Sample
2.3. UHPLC-FT-ICR-MS Analysis Conditions
3. Results and Discussion
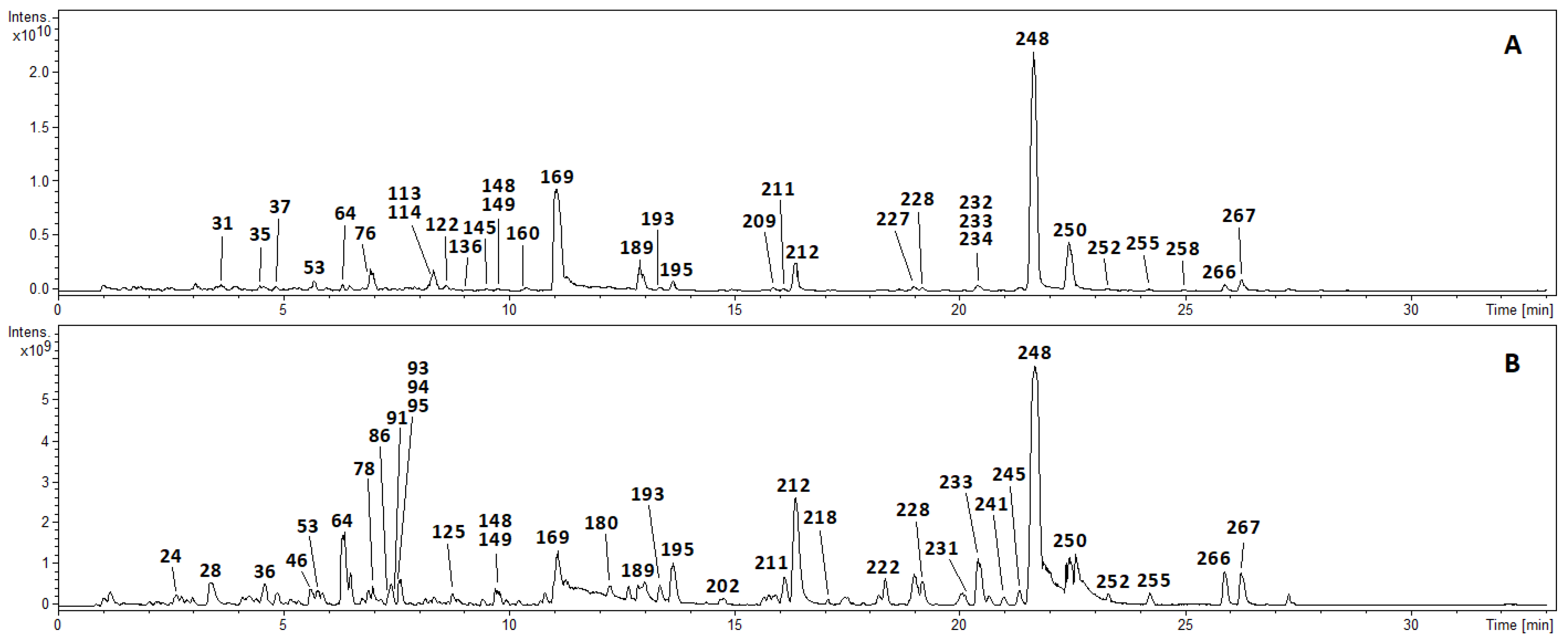
3.1. Chemical Profiling of Qingkailing Capsules by UHPLC-FT-ICR-MS
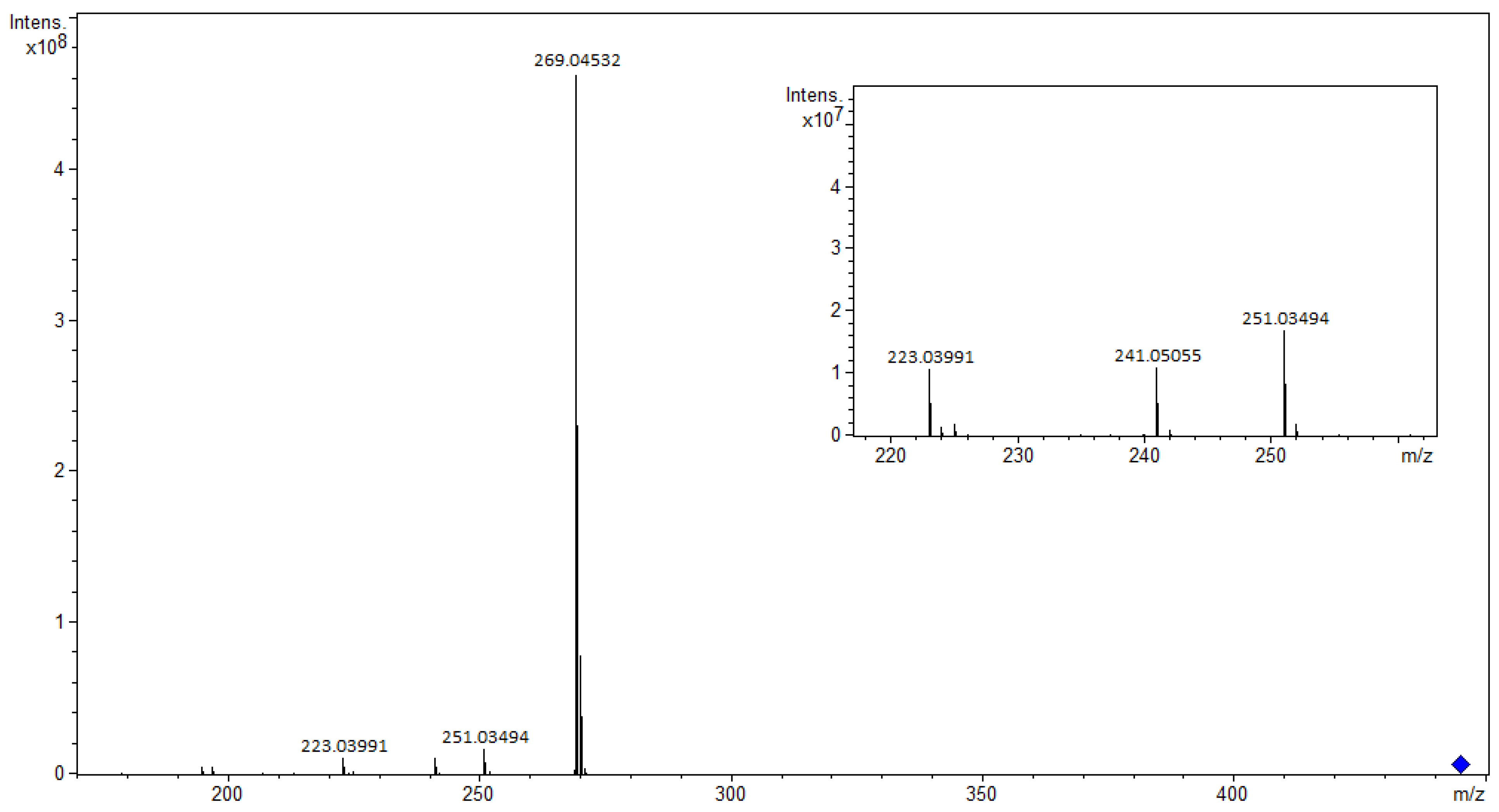
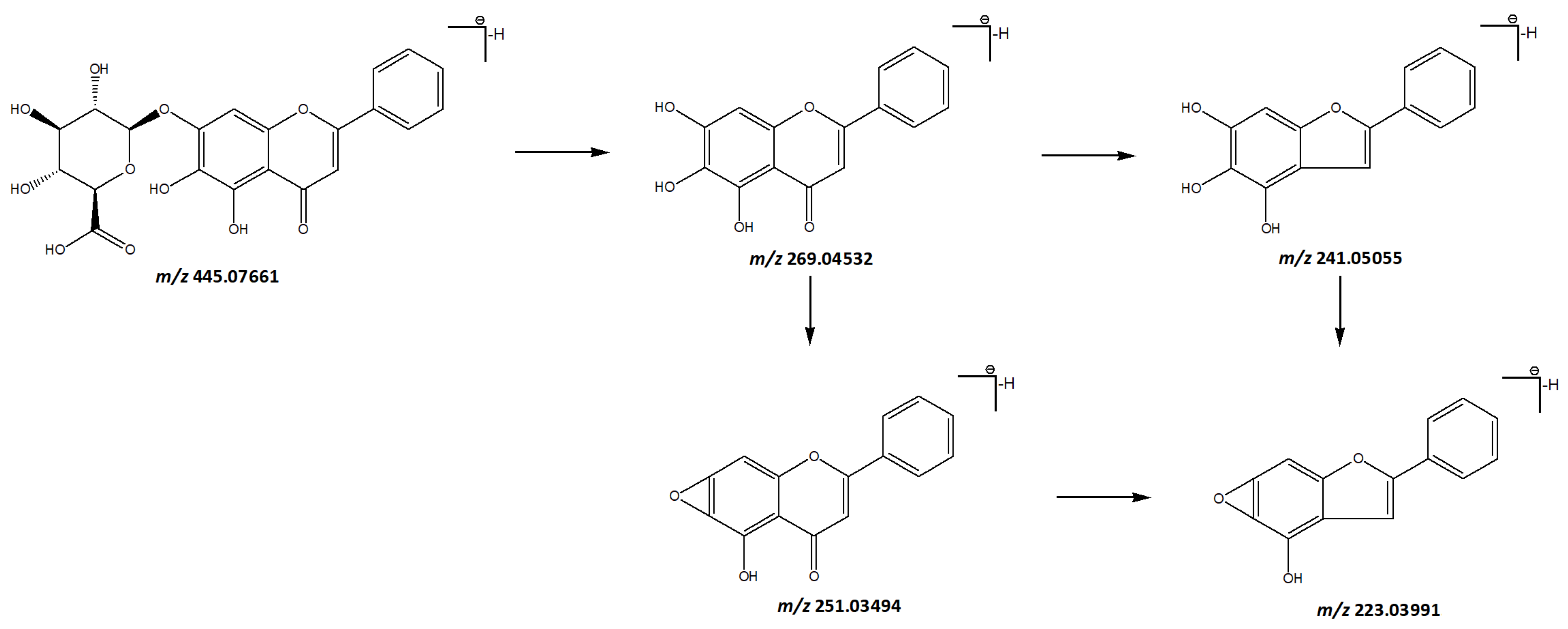
3.1.1. Flavonoids and Their Glycosides
3.1.2. Organic Acids
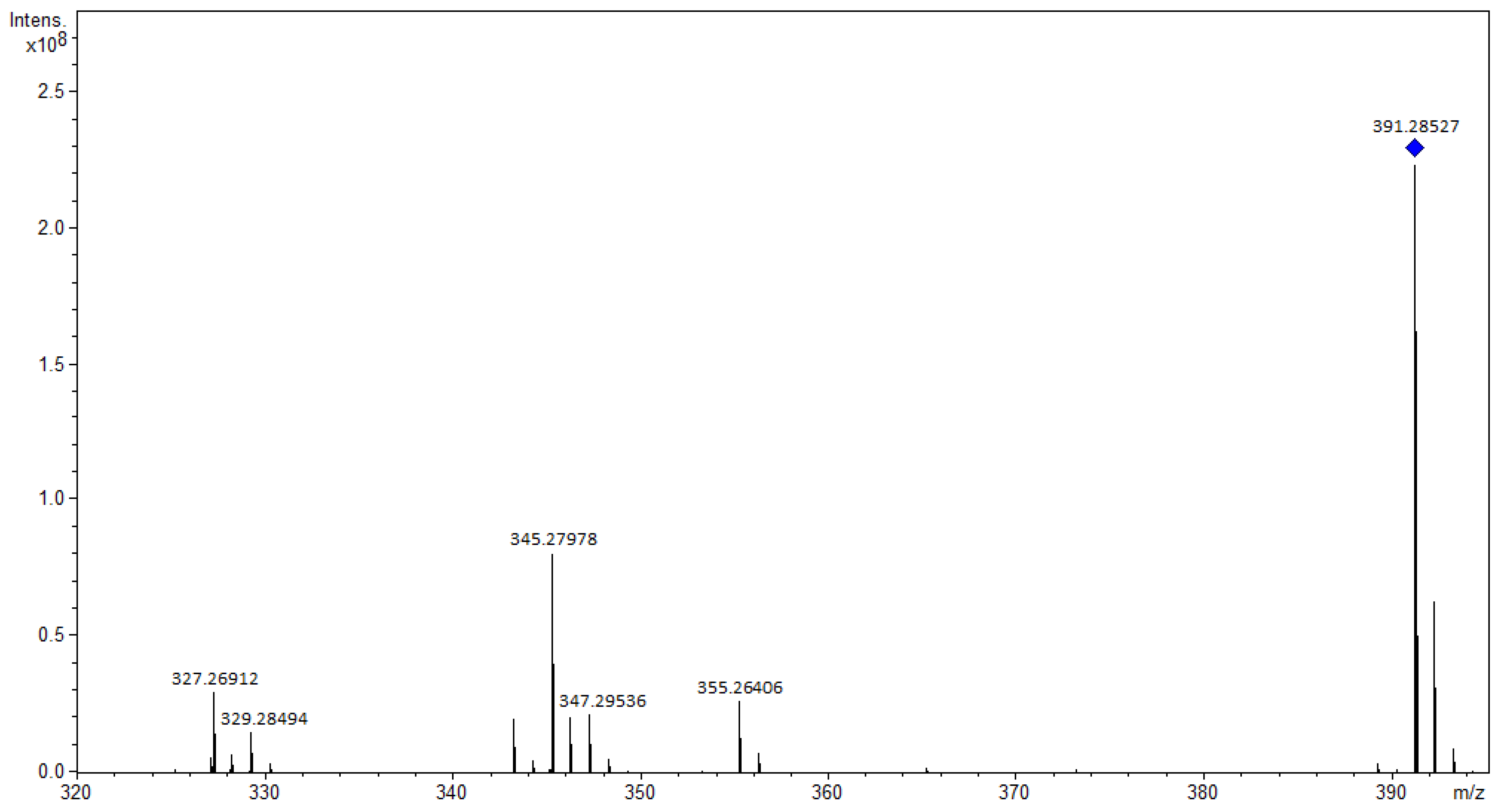
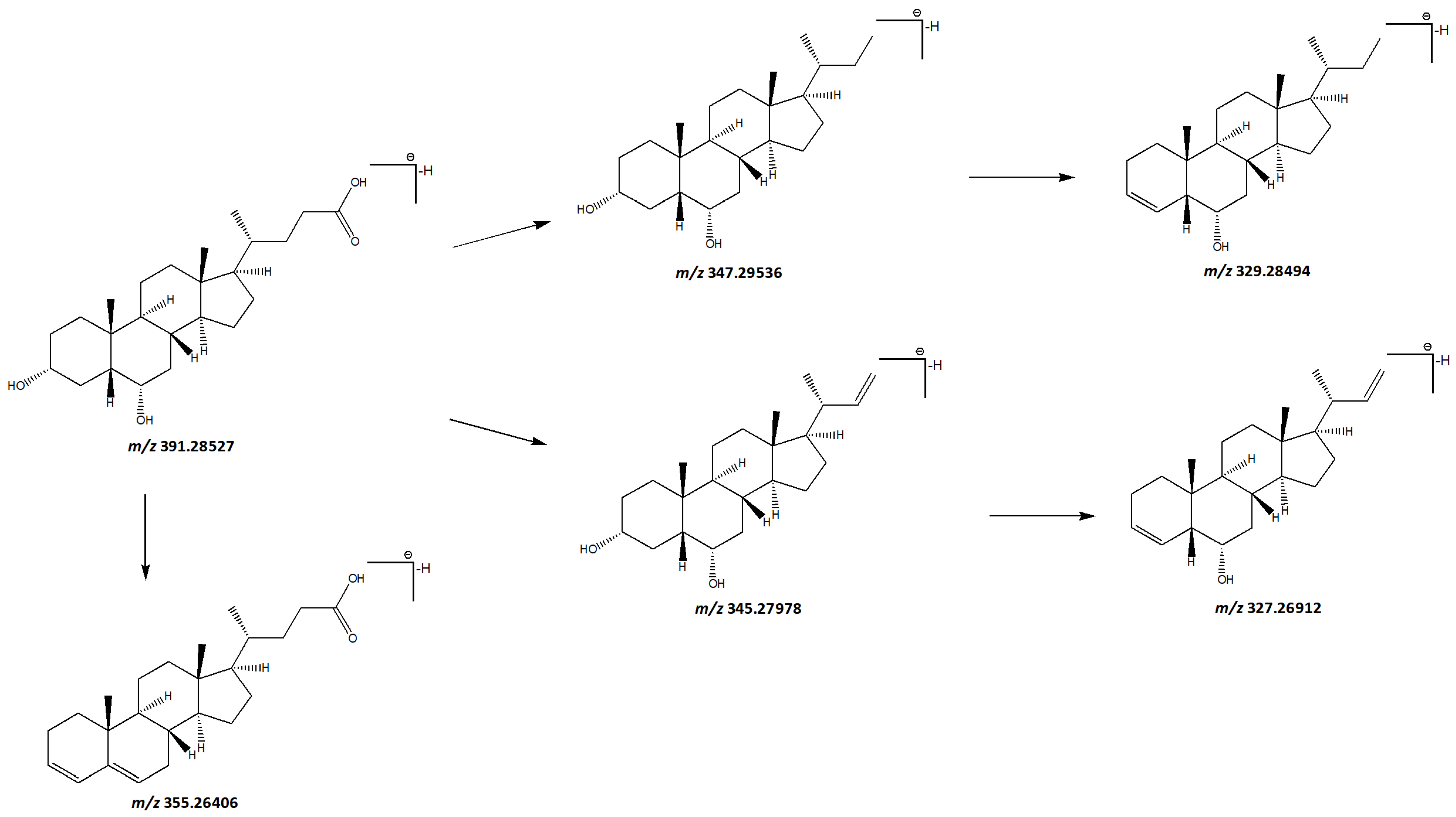
3.1.3. Terpenoids
3.1.4. Steroids
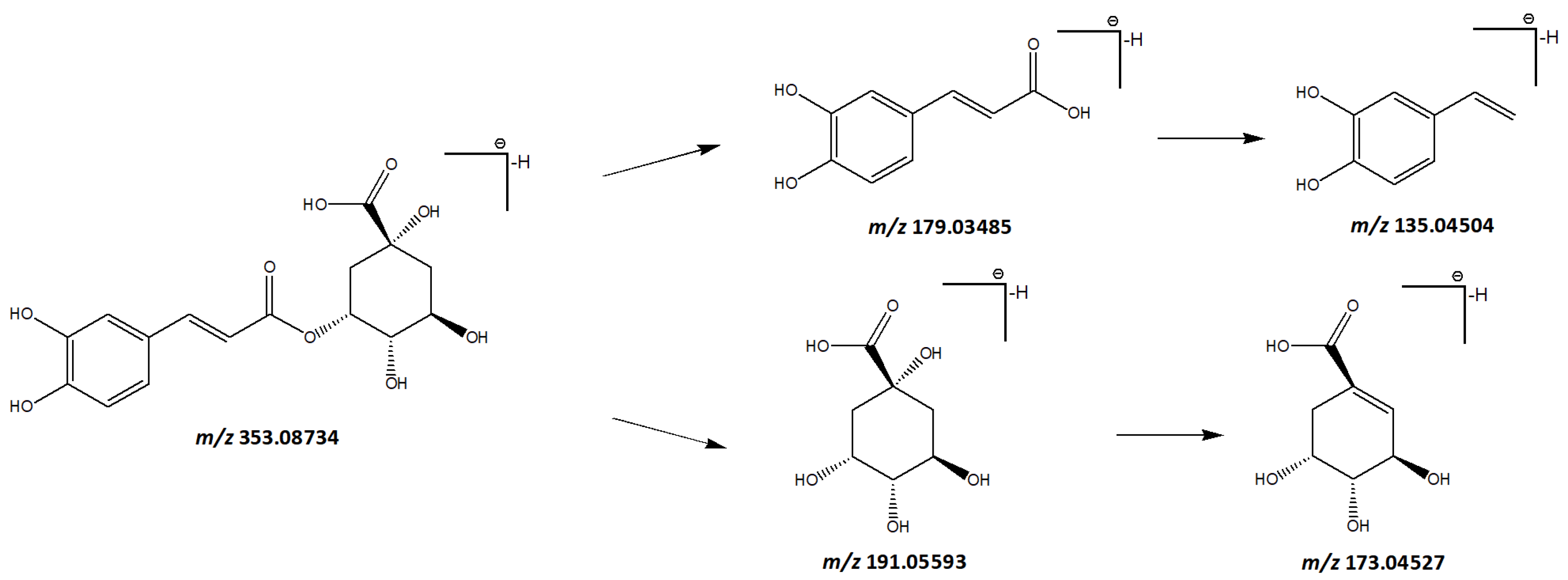
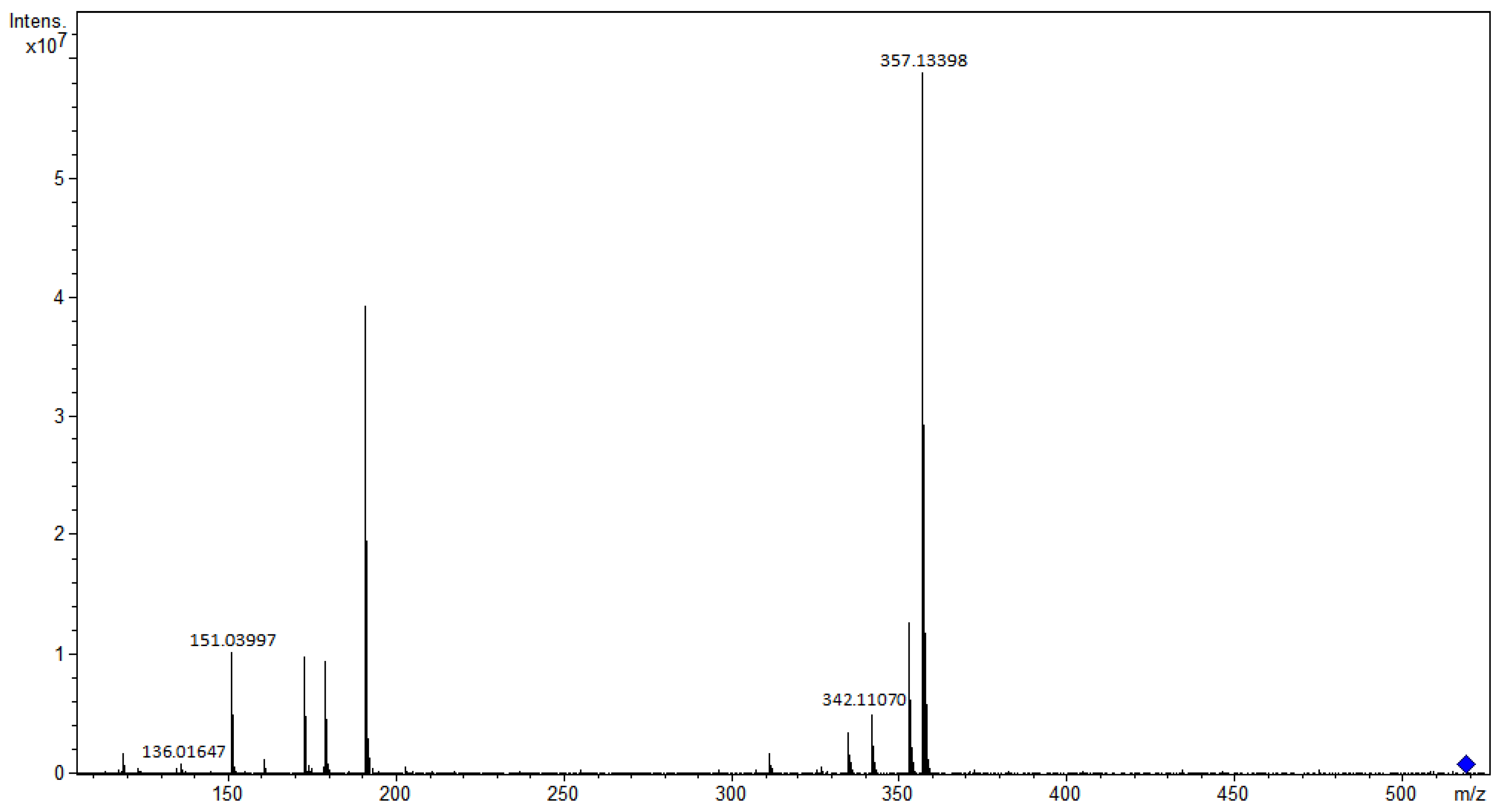
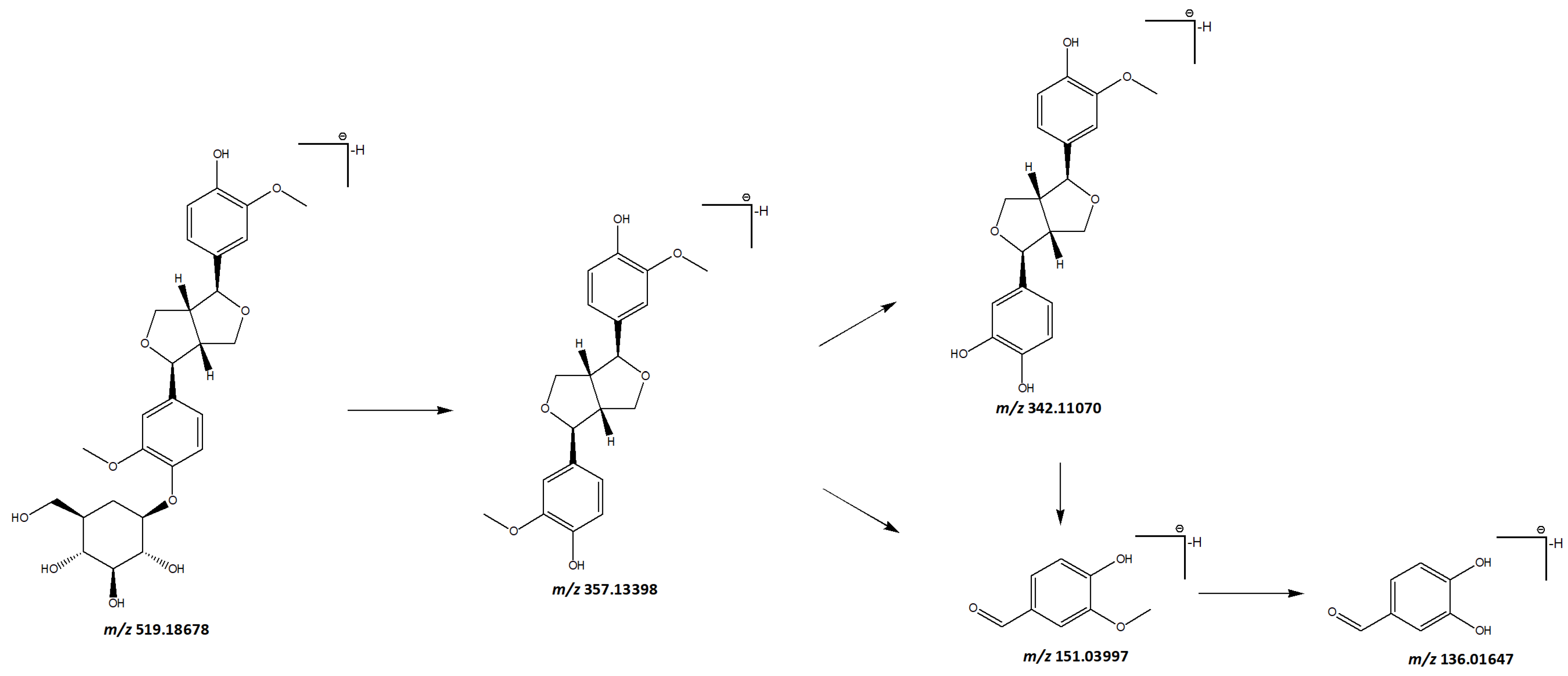
3.1.5. Phenylpropanoids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, J.; Zhu, Z.P.; Gao, T.H.; Chen, Y.; Yang, Q.S.; Fu, C.M.; Zhu, Y.N.; Wang, F.; Liao, W. Isatidis Radix and Isatidis Folium: A systematic review on ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2022, 283, 114648. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.K.; Li, W.; Fu, C.M.; Song, Y.; Fu, Q. Lonicerae japonicae flos and Lonicerae flos: A systematic review of ethnopharmacology, phytochemistry and pharmacology. Phytochem. Rev. 2019, 22, 1–61. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zeng, S.; Chen, L.; Sun, Q.; Liu, M.L.; Yang, H.; Ren, S.; Ming, T.Q.; Meng, X.L.; Xu, H.B. Updated pharmacological effects of Lonicerae japonicae flos, with a focus on its potential efficacy on coronavirus disease-2019 (COVID-19). Curr. Opin. Pharmacol. 2021, 60, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Y.; Wang, Z.; Qu, X. Effect of some active Chinese herbal fraction on brain tissue proteomic profile of ischemic mouse. Chin. J. Integr. Tradit. West Med. 2006, 26, 526–528. [Google Scholar]
- Liu, D.; Dai, T.T.; Zha, R.B.; Qu, P.; Li, Y.; Du, H.; Zhao, S.Y.; Song, L.; Zhang, H.; Sun, J.M. Study on sedative and hypnotic effects of Concha Margaritifera and its different processed products on the content of 5-HT in the brain of mice. Jilin J. Tradit. Chin. Med. 2014, 34, 61–63. [Google Scholar]
- Li, Y.; Sun, J.M.; Zhang, J.; Li, J.F.; Liu, W.C.; Zhang, H. Anti-depressant effect of Margaritifera concha on mice. Jilin J. Tradit. Chin. Med. 2014, 34, 388–389+392. [Google Scholar]
- Liu, R.; Wang, M.; Duan, J.A. Antipyretic and antioxidant activities of the aqueous extract of Cornu bubali (water buffalo horn). Am. J. Chin. Med. 2010, 38, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.Z.; Qin, S.S.; Han, J.Y.; Meng, J.; Liang, A.H. A review of the ethnopharmacology, phytochemistry, pharmacology and toxicology of Fructus Gardeniae (Zhi-zi). J. Ethnopharmacol. 2022, 289, 114984. [Google Scholar] [CrossRef]
- Fickert, P.; Zollner, G.; Fuchsbichler, A.; Stumptner, C.; Pojer, C.; Zenz, R.; Lammert, F.; Stieger, B.; Meier, P.J.; Zatloukal, K.; et al. Effects of ursodeoxycholic and cholic acid feeding on hepatocellular transporter expression in mouse liver. Gastroenterology 2001, 121, 170–183. [Google Scholar] [CrossRef]
- Kong, W.; Jin, C.; Xiao, X.; Zhao, Y.; Li, Z.; Zhang, P.; Liu, W.; Li, X.F. Comparative study of effects of two bile acid derivatives on Staphylococcus aureus by multiple analytical methods. J. Hazard. Mater. 2010, 179, 742–747. [Google Scholar] [CrossRef]
- Watanabe, S.; Fujita, K. Dietary hyodeoxycholic acid exerts hypolipidemic effects by reducing farnesoid X receptor antagonist bile acids in mouse enterohepatic tissues. Lipids 2014, 49, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Sehayek, E.; Ono, J.G.; Duncan, E.M.; Batta, A.K.; Salen, G.; Shefer, S.; Neguyen, L.B.; Yang, K.; Lipkin, M.; Breslow, J.L. Hyodeoxycholic acid efficiently suppresses atherosclerosis formation and plasma cholesterol levels in mice. J. Lipid. Res. 2001, 42, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Bai, Y.Y.; Wang, G.R.; Su, Y.S.; Tao, Y.L.; Wang, L.P.; Yang, L.; Wu, H.; Huang, F.; Shi, H.L.; et al. Hyodeoxycholic acid inhibits lipopolysaccharide-induced microglia inflammatory responses through regulating TGR5/AKT/NF-κB signaling pathway. J. Psychopharmacol. 2022, 36, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Song, K.; Zhang, H.L.; Yuan, M.C.; An, N.; Wei, Y.F.; Wang, L.Q.; Sun, Y.K.; Xing, Y.W.; Gao, Y.H. Anti-inflammatory and immunomodulatory effects of baicalin in cerebrovascular and neurological disorders. Brain Res. Bull. 2020, 164, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.H.; Yu, M.; Shi, M.Y.; Bo, P.; Gu, X.W.; Zhang, Z.W. Baicalin and its aglycone: A novel approach for treatment of metabolic disorders. Pharmacol. Rep. 2020, 72, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Zhang, Q.; Zhang, H.X.; Dong, L.Y.; Lu, J.Q.; Qiao, Y.J. Rapid identification of 14 bile acids contained in Qingkailing injection by HPLC-ESI-MS/MS. China J. Chin. Mater. Med. 2013, 38, 990–994. [Google Scholar]
- Zhang, H.Y.; Hu, P.; Luo, G.A.; Liang, Q.L.; Wang, Y.L.; Yan, S.K.; Wang, Y.M. Screening and identification of multi-component in Qingkailing injection using combination of liquid chromatography/time-of-flight mass spectrometry and liquid chromatography/ion trap mass spectrometry. Anal. Chim. Acta 2006, 577, 190–200. [Google Scholar] [CrossRef]
- Guo, M.X.; Zhang, L.; Liu, H.Y.; Qin, L.L.; Zhang, Z.X.; Bai, X.; Gao, X.Y. A metabolomic strategy to screen the prototype components and metabolites of Qingkailing injection in rat urine by high-performance liquid chromatography with tandem mass spectrometry. J. Sep. Sci. 2014, 37, 2844–2850. [Google Scholar] [CrossRef]
- Nikolaev, E.N.; Kostyukevich, Y.I.; Vladimirov, G.N. Fourier transform ion cyclotron resonance (FT ICR) mass spectrometry: Theory and simulations. Mass. Spectrom. Rev. 2016, 35, 219–258. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, H.H.; Jing, J.X.; Liu, T.; Wang, R.J.; Di, F.Y.; Han, F.; Zhao, Y.L.; Yu, Z.G. Rapid characterization of chemical constituents of Gansuibanxia decoction by UHPLC-FT-ICR-MS analysis. J. Pharm. Biomed. Anal. 2020, 179, 113029. [Google Scholar] [CrossRef]
- Liu, T.; Cui, Y.; Tian, X.M.; Li, S.H.; Han, F.; Ji, B.; Zhao, Y.L.; Yu, Z.G. Detection of chemical constituents in Gegenqinlian decoction by ultra-high performance liquid chromatography coupled with Fourier transform ion cyclotron resonance mass spectrometry. Anal. Methods 2017, 9, 5890–5902. [Google Scholar] [CrossRef]
- Feng, W.; Dong, Q.J.; Liu, M.Y.; Li, S.; Liu, T.; Wang, X.G.; Niu, L.Y. Screening and identification of multiple constituents and their metabolites of Zhi-zi-chi decoction in rat urine and bile by ultra-high-performance liquid chromatography quadrupole time-of-flight mass spectrometry. Biomed. Chromatogr. 2017, 31, e3978. [Google Scholar] [CrossRef] [PubMed]
- Li, T.P.; Zhang, M.L.; Tan, Z.E.; Miao, J.H.; He, Y.M.; Zhang, A.H.; Ou, M.; Huang, D.N.; Wu, F.F.; Wang, X.J. Rapid characterization of the constituents in Jigucao capsule using ultra high performance liquid chromatography with quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 2022, 45, 677–696. [Google Scholar] [CrossRef]
- Wang, L.; Liu, S.; Zhang, X.J.; Xing, J.P.; Liu, Z.Q.; Song, F.R. A strategy for identification and structural characterization of compounds from Gardenia jasminoides by integrating macroporous resin column chromatography and liquid chromatography-tandem mass spectrometry combined with ion-mobility spectrometry. J. Chromatogr. A 2016, 1452, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.W.; Li, Z.X.; Hu, P.; Feng, Q.; Xue, R.; Hu, Y.Y.; Huang, C.G. A practical method for the rapid detection and structural characterization of major constituents from traditional Chinese medical formulas: Analysis of multiple constituents in Yinchenhao Decoction. Anal. Methods 2015, 7, 4678–4690. [Google Scholar] [CrossRef]
- Li, R.R.; Cui, Y.F.; Zheng, X.F.; Qin, X.M.; Cao, J.J.; Li, Z.Y. Characterization of chemical components in the Guanxinning injection by liquid chromatography-mass spectrometry. J. Mass Spectrom. 2020, 55, e4662. [Google Scholar] [CrossRef]
- Xia, M.Q.; Yao, M.; Li, J.M.; Zhang, J.J.; Yu, Y.Y.; Yang, S.L.; Zhong, G.Y.; Pei, N.; Ouyang, H.; Feng, Y.L. Characterization of Chemical Constituents of Oxytropis microphylla (Pall.) DC. by Ultra-High-Performance Liquid Chromatography Coupled with Quadrupole-Time-of Flight Tandem Mass Spectrometry. Separations 2022, 9, 297. [Google Scholar] [CrossRef]
- Sun, X.J.; Li, P.; Qu, X.Y.; Liu, W.G. Isovitexin alleviates acute gouty arthritis in rats by inhibiting inflammation via the TLR4/MyD88/NF-κB pathway. Pharm. Biol. 2021, 59, 1326–1333. [Google Scholar] [CrossRef]
- Valentová, K.; Vrba, J.; Bancířová, M.; Ulrichová, J.; Křen, V. Isoquercitrin: Pharmacology, toxicology, and metabolism. Food Chem. Toxicol. 2014, 68, 267–282. [Google Scholar] [CrossRef]
- Prakash, A.N.; Prasad, N.; Puppala, E.R.; Panda, S.R.; Jain, S.; Ravichandiran, V.; Singh, M.; Naidu, V.G.M. Loganic acid protects against ulcerative colitis by inhibiting TLR4/NF-κB mediated inflammation and activating the SIRT1/Nrf2 anti-oxidant responses in-vitro and in-vivo. Int. Immunopharmacol. 2023, 122, 110585. [Google Scholar] [CrossRef]
- Lee, H.; Lee, D.H.; Oh, J.H.; Chung, J.H. Skullcapflavone II Suppresses TNF-α/IFN-γ-Induced TARC, MDC, and CTSS Production in HaCaT Cells. Int. J. Mol. Sci. 2021, 22, 6428. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; Xia, F.F.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.H.; Hung, T.W.; Wang, C.C.; Wu, S.W.; Yang, T.W.; Yang, C.Y.; Tseng, T.H.; Wang, C.J. Neochlorogenic Acid Attenuates Hepatic Lipid Accumulation and Inflammation via Regulating miR-34a In Vitro. Int. J. Mol. Sci. 2021, 22, 13163. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.L.; Yu, L.; Zhang, S.D.; Ping, K.; Ni, H.Y.; Qin, X.Y.; Zhao, C.J.; Wang, W.; Efferth, T.; Fu, Y.J. Cryptochlorogenic acid attenuates LPS-induced inflammatory response and oxidative stress via upregulation of the Nrf2/HO-1 signaling pathway in RAW 264.7 macrophages. Int. Immunopharmacol. 2020, 83, 106436. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Landa, J.F.; German-Ponciano, L.J.; Puga-Olguín, A.; Olmos-Vázquez, O.J. Pharmacological, Neurochemical, and Behavioral Mechanisms Underlying the Anxiolytic- and Antidepressant-like Effects of Flavonoid Chrysin. Molecules 2022, 27, 3551. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Zhang, J.Y.; Zhao, M.H.; Yan, M.; Zhao, Q.C.; Wu, Q.; Jin, H.; Shi, G.B. The analgesic activity and possible mechanisms of deacetyl asperulosidic acid methyl ester from Ji shi teng in mice. Pharmacol. Biochem. Behav. 2012, 102, 585–592. [Google Scholar] [CrossRef]
- Liu, L.P.; Wu, Q.; Chen, Y.P.; Gu, G.X.; Gao, R.N.; Peng, B.; Wang, Y.; Li, A.B.; Guo, J.P.; Xu, X.R.; et al. Updated Pharmacological Effects, Molecular Mechanisms, and Therapeutic Potential of Natural Product Geniposide. Molecules 2022, 27, 3319. [Google Scholar] [CrossRef]
- Zhang, H.H.; Gu, H.J.; Jia, Q.H.; Zhao, Y.Q.; Li, H.Q.; Shen, S.R.; Liu, X.; Wang, G.S.; Shi, Q.M. Syringin protects against colitis by ameliorating inflammation. Arch Biochem. Biophys. 2020, 680, 108242. [Google Scholar] [CrossRef]
- Youssef, F.S.; Ashour, M.L.; El-Beshbishy, H.A.; Ahmed Hamza, A.; Singab, A.N.B.; Wink, M. Pinoresinol-4-O-β-D-glucopyranoside: A lignan from prunes (Prunus domestica) attenuates oxidative stress, hyperglycaemia and hepatic toxicity in vitro and in vivo. J. Pharm. Pharmacol. 2020, 72, 1830–1839. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Lin, S. Comprehensive Chemical Characterization of Qingkailing Capsules by Ultra-High-Performance Liquid Chromatography Combined with Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Separations 2023, 10, 588. https://doi.org/10.3390/separations10120588
Liu T, Lin S. Comprehensive Chemical Characterization of Qingkailing Capsules by Ultra-High-Performance Liquid Chromatography Combined with Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Separations. 2023; 10(12):588. https://doi.org/10.3390/separations10120588
Chicago/Turabian StyleLiu, Ting, and Shu Lin. 2023. "Comprehensive Chemical Characterization of Qingkailing Capsules by Ultra-High-Performance Liquid Chromatography Combined with Fourier Transform Ion Cyclotron Resonance Mass Spectrometry" Separations 10, no. 12: 588. https://doi.org/10.3390/separations10120588
APA StyleLiu, T., & Lin, S. (2023). Comprehensive Chemical Characterization of Qingkailing Capsules by Ultra-High-Performance Liquid Chromatography Combined with Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Separations, 10(12), 588. https://doi.org/10.3390/separations10120588





