1. Introduction
Over the last two decades, global oil production, oil consumption, and the risk of oil pollution have gradually increased [
1]. In 2022, global oil production amounted to 4.4 billion tons [
2]. During the extraction, transport, and use of oil, accidents occur frequently, causing severe and sometimes irreversible pollution of the environment and harm to humans [
3,
4].
There are various methods to remove oil spills from water surfaces. First, the pollution is usually contained by oil booms which prevent further spreading and the oil can then be burnt in situ. Furthermore, dispersants can be applied, which break up the oil film into fine droplets that are decomposed by bioremediation. It is also possible to use sorbents, by which the oil is absorbed and thus separated from the water. Various types of skimmers can be used to mechanically remove oil from the water surface. All methods have various deficiencies that make their use difficult and especially limit the removal of oil from inland waters.
For many technical applications, unexpected solutions come from the field of biology. Millions of years of evolution led to optimized surfaces of living organisms for their interaction with the environment [
5,
6]. Solutions-often rather unfamiliar to materials scientists and difficult to accept [
7]. Our long-time routine examination of around 20,000 different species showed that there is an almost infinite variety of structures and functionalities. Some species in particular stand out for their excellent water repellency. Further tests showed that these often have very good oil adsorption properties [
8,
9]. It was shown that, e.g., leaves of the floating fern
Salvinia molesta (
Figure 1 and
Figure 2), adsorb oil and are able to transport it on their surfaces. It was shown that these structures are also able to release the oil again, allowing it to be transported into a vessel and thus enable the separation and collection of oil [
8].
The investigation of these structured surfaces shows that the oil adsorption rate depends on various factors. For example, it depends on the viscosity of the oil, the hierarchical micro-architecture (e.g., size, shape, and distance of the microstructures (hairs)), and the wetting behavior of the surface. The observations, particularly in
Salvinia leaves, inspired to transfer the effect to technical surfaces for the separation of oil and water, which enables a non-toxic, easy-to-use, and versatile process for a sustainable oil spill clean-up without additional energy input and waste material. This enables a novel physical method to remove, e.g., oil films from water surfaces and allows the development of a device for bioinspired oil–water separation. We called the device that functions according to this principle a Bionic Oil Adsorber (BOA) [
8]. Here, we present further new results of the unexpected fast and efficient oil adsorption and transportation based on our research project carried out in 2019–2022 [
9], in which we investigated biological surfaces as well as different technical textiles including the result from a flow simulation project of the surfaces [
10].
In the two-dimensional simulation, the surface structure of
Salvinia was approximately modeled by cylinders of uniform diameter and spacing. This led to the conclusion that the material for the biomimetic textiles should be superhydrophobic and completely wettable with oil (contact angle to oil ≤ 20°). Additionally, the cylinder diameter and the ratio of cylinder distance to cylinder diameter should be adapted to the oil viscosity. These data serve as the basis for the development of a biologically inspired textile by which the oil–water separation can be transferred to engineering [
10]. We refer to it as BOA-Textile.
Apart from the BOA, there are various scientific approaches to developing an oil–water separation process by adsorption with superhydrophobic surfaces. In this publication, we present a technical implementation of the BOA and provide a brief overview of the structures and materials used in these processes.
2. Bionic Oil Adsorber
On the basis of the biological investigations and computer simulations described above, various textiles were selected with which the biological structure can be reproduced. A total of 54 different textiles were examined, with a selection of different two-dimensional (2D) and three-dimensional (3D) textiles, such as three spiral structures, two spacer textiles, and eight velours. Spiral structures have an open mesh structure with spirals of hydrolysis-resistant polyethylene terephthalate (PET) joined together on the left and right side. One spiral structure was finished with a nonwoven. The spacer textiles consist of two cover surfaces that are maintained at a distance by pile yarn. They are produced using the warp knitting process on a raschel machine. The velours include both woven structures and tufted materials. Beneath these core textiles, other textiles like nonwovens with needled fiber batts were investigated. Velour, in an optical manner, was comparable to the biological role model, but the density of the pile yarn was not as high as the density of hairs on the surface of Salvinia. The knitted spacer textiles with two main surfaces show a lot of fiber batts as contact surfaces.
Two-dimensional textiles were mainly considered to better understand the transport processes. With 3D textiles, the biological models can be reproduced better. Primarily polymers were used as raw materials, with a focus on polyesters and polyamides. The textiles were hydrophobized with a layered silicate in a dip-coating process. By investigating the adsorption speed and the possible volume flow for oils with viscosities of 4.5 cP to 196.7 cP (at 20 °C), it was found that the highest transport speeds and volume flows were achieved with knitted spacer fabrics. The maximum volume flow is 4 L per hour by use of 1 m2 functional textile and diesel as test medium. This textile can be produced industrially and is therefore easily scalable.
Subsequently, a device was developed with which the textile can be applied on bodies of water. For this purpose, the principle presented was analyzed and transferred to a floating oil collector [
8].
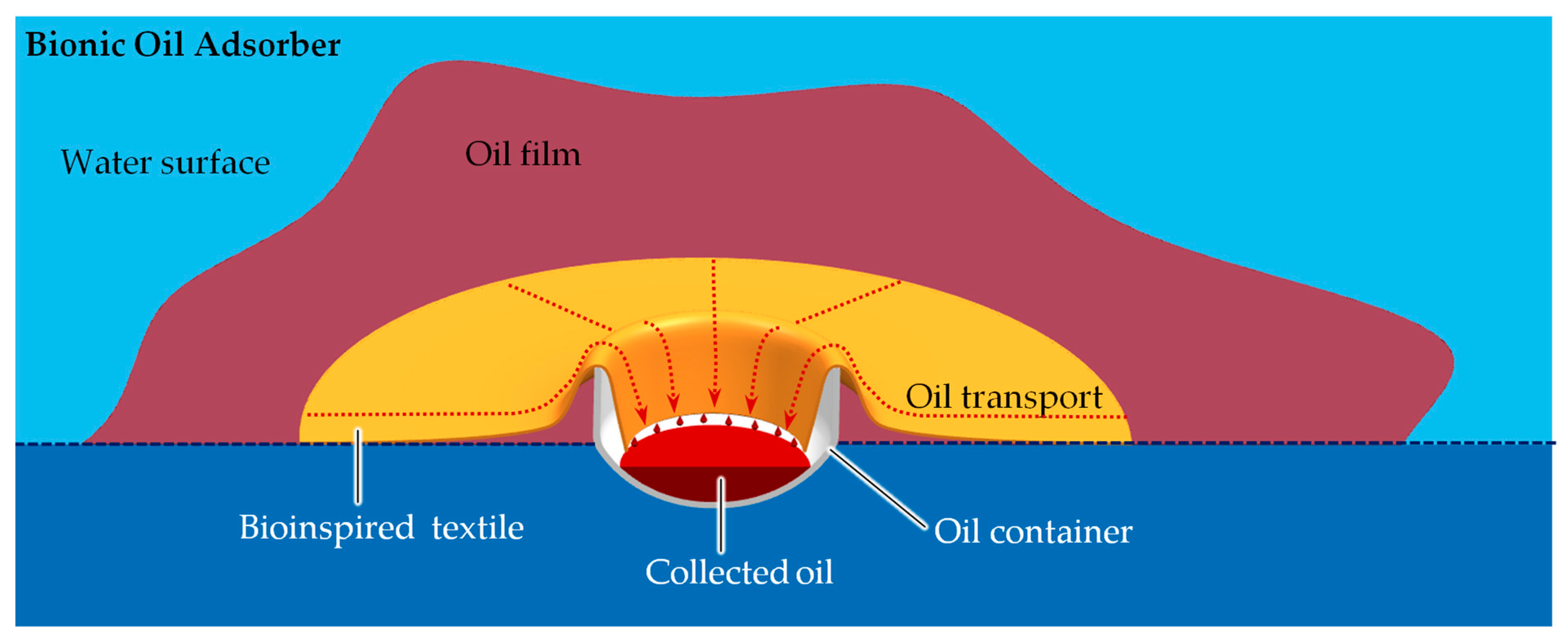
The textile adsorbs oil from the water surface and transports it into the oil container which can be emptied manually in certain time intervals. Depending on the size and type of the oil contamination, the BOA can be designed in different sizes so different collection volumes can be realized. The whole BOA apparatus consists of various components, the most important of which is the BOA textile (
Figure 3). It adsorbs the oil on the water surface and transports it within the plane of the textile. This allows the oil to flow into the internal part of the BOA, where it desorbs from the textile and is collected in a collection container. This container is removable and can be emptied manually if necessary.
The advantage of the principle is that no additional energy has to be applied to operate the BOA. The oil is separated from the surrounding water by the surface properties of the textile and transported through the textile driven solely by capillary forces, even against gravity. When it reaches the end of the textile in the collecting container, the oil desorbs without any further external influence due to the gravitational forces. At certain points of the textile, the transport takes place at different velocities. This is related to which forces counteract the capillary force
. These are mainly the frictional force
and the gravitational force
, the latter leading to faster transport at the point of desorption. Due to the changing effects of the forces the different force balances
until
develop at different points of the textile (
Figure 4). It can be observed that the oil does not immediately drip out of the textile when it reaches the textile edge inside the oil collecting container. Initially, an oil volume builds up inside the textile so that a liquid boundary can be seen above the textile edge (point 4 in
Figure 4). This creates a fluid static pressure. If this pressure is greater than the surface tension of the transported oil, a drop forms at the terminal edge of the textile inside the BOA device and the oil desorbs. From this point on, a steady dripping can be observed. During the use of the device, the BOA can float autonomously on any water surface like a buoy and only needs to be maintained at certain intervals, depending on the oil volume and the final dimensioning of the unit, i.e., the capacity.
The different force balances have to be considered in the construction of the final BOA. It is particularly relevant to keep the slope and rise height of the textile from the outside of the BOA into the internal container as low as possible (point 2 in
Figure 4).
Most transport processes of liquids in textiles are based on capillary forces. The flow of liquids through a porous system is considered as a network of many capillaries and can be described by the Lucas–Washburn equation (Equation (1)). The velocity is determined as the derivative of the rise height
h from the surface tension
, the capillary radius
r, the contact angle
θ, the viscosity of the liquid
η, the density of the liquid
and the gravity
g:
The rise height can also be described as a function of time by solving the differential equation (Equation (2)). Gravity can be neglected if the transport is horizontal, the height of climb
h, and the radius
r is very small:
If Equation (2) is used to calculate the transport velocity, it corresponds to the velocity of the visually visible flow edge of the oil in the BOA textile in horizontal orientation (point 1 in
Figure 4b and picture in
Figure 4c).
Preliminary tests have shown that oil can be successfully separated from water surfaces using a prototype of the device. Experiments in an application-related environment, like small harbors or ponds, are pending.
If the oil volume flows measured so far are scaled to the circumference of the textile of 80 cm provided in the application so far, approximately 3 L of diesel can be separated from water by one device of the Bionic Oil Adsorber per hour.
In total, the BOA device offers high added value in contrast to conventional cleaning methods for oil on inland waters. No additional energy requirements, such as with oil skimmers, are necessary, there is no secondary toxicity, such as with oil dispersants, there is high reusability of textiles and equipment, “no waste remains”, and it is inexpensive in terms of the amount of oil removed.
3. Other Technologies for Oil–Water Separation
The results regarding the novel bionic oil-adsorbing method presented here are very promising, especially since no additional energy is required and the method is not toxic to the environment. Beyond the BOA, there are other approaches, which were identified in a literature search. For this purpose, we considered the references that cite [
8] and, in addition, those found using the search term “oil water separation” on Google scholar (publication year < 2021). The presented and discussed adsorbing materials are foams, sponges, meshes, textiles, and nanoscaled structures.
3.1. Foams and Sponges
A frequently chosen approaches for the production of adsorbers are sponges and foams. For example, conventional foams are equipped with novel coatings to create a superhydrophobic surface within the sponge. For this purpose, Mu et al. coat a melamine foam with a siloxane coating, as this provides alkyl silane on the surface. This results in a water contact angle of 156° [
11].
Another approach is based on the use of sponges made of polyurethane (PU), whereby the surface is coated with a mixture of different nanoparticles (e.g., graphite powder, manganese, and iron oxide). Due to the special surface characteristics of individual pores, it is possible to absorb oil from the oil–water interface. After application, oil can be recovered and recycled. Due to the porosity of the sponge, a large volume of oil can be absorbed and stored. As a result, thirty times its own weight can be taken up due the cleaning method is environmentally friendly, the same as the BOA [
12]. PU foams can also be functionalized with silicon dioxide and activated carbon in such a way that oils of different viscosities can be adsorbed by water surfaces [
13].
By using perfluorotriethoxysilanes, Pang et al. were able to find another approach with which polyurethane foams can be hydrophobized. In a sol-gel process, a mechanically stable coating was produced, which even after 100 compressions showed a contact angle of 144°. The foam had an adsorption capacity of 20–40 times its own weight and the separation rate was 98.72% [
14].
The authors of another study [
15] also focused on the use of fluorine-based polymers for the production of a superhydrophobic foam. They used a thermally impacted nonsolvent-induced phase separation method to produce a three-dimensional porous monolith on polyvinylidene fluoride (PVDF). This had a water contact angle of 154° and an oil contact angle of 0°. The material has an absorption capacity of 21.3 g/g and can be reused up to five times. It is proposed as a material for large-scale oil spill cleanup.
Venkatesan et al. [
16] use bio-based materials by processing leather cuttings into activated carbon at 400 °C and coating a melamine sponge with it. The water contact angle of this material is approximately 158°, whereas without the coating it is approximately 152°. Thus, an adsorption capacity of 75.77 to 141.24 times its own weight could be achieved.
Particles can also be used to functionalize foams. For example, titanium dioxide and graphene oxide are processed into a foam in a hydrothermal treatment and freeze-drying method. In this way, a separation and adsorption efficiency of 98% was achieved [
17].
3.2. Meshes and Textiles
Another structure for oil–water separation is meshes and textile materials. These materials can be used to separate oil from water by pressing the mixture by means of pressure through the structures. For the production of superhydrophobic nets, copper, stainless steel, nickel, and titanium are often used as metallic materials [
18]. A copper mesh can be functionalized in a two-step soaking process so as to create a micro-nanostructure on the surface. This allows water contact angles of 158.7° to be achieved [
19]. In a further process, nanoparticles were bound to the mesh surface by etching with an ammonia-base titanium dioxide, which resulted in nanostructuring. The surface was then further functionalized with n-hexadecyltrimethoxysilanes (HDTMS) to achieve superhydrophobicity and superoleophilicity The application is planned for use with heavy oil [
20].
In addition to the hydrophobic surface chemistry, the production of porosity is also highly relevant for adsorption. For this purpose, a back-propagation neural network could be developed with which the porosity can be adapted to the application of stainless steel meshes [
21].
By functionalizing such stainless steel meshes with multi-walled carbon nanotubes through thermal chemical vapor deposition, a three-dimensional porous structure can be created. The water contact angles are between 140 and 150°, whereby oil completely wets the surface [
22].
Apart from synthetic materials, natural materials also show a pronounced hydrophobicity and oleophilicity. Hollow fibers of
Chorosia-fruits are cellulosic materials that naturally exhibit oil adsorption when untreated. Treatment with sodium chlorite (NaClO
2) can further increase oil absorption and achieve oil–water separation [
23].
3.3. Nanoscaled Structures
Nanofibers are also used to achieve a surface structured at the nanolevel. In the solution blow process, hydrophobic nanofiber membranes made of polybutylene succinate (PBS) can be produced with relatively high productivity (27.0 g/h per nozzle). With these membranes, an oil adsorption capacity of up to 38.5 g/g could be achieved. Since PBS is biodegradable, the membrane can be returned to the biodegradation process by hydrolysis [
24].
Currently, nanofibers are produced more frequently by electrospinning than by the solution blow process. This makes it possible, for example, to produce membranes based on polyacrylonitrile (PAN) for oil–water separation. However, Sun et al. [
25] use superhydrophilicity, not superhydrophobicity, for this purpose.Other authors were inspired by spider silk, which enabled them to produce a membrane with nanoscale pores (mean pore size 0.634 µm) on the base of PAN. This allows nanoemulsions to be separated by sieving. The membrane consists of two layers, with a nanofiber layer serving as a support layer and a layer of spindle-knotted structures [
26]. Multilayer membranes are also made of PVDF. For example, a functional layer of sodium alginate, chitosan, and iron is applied to a PVDF membrane by grafting with polyacrylic acid [
27].
Other methods and materials are also used to achieve structuring at the nanofiber level. For example, nanoparticles can be used to functionalize microfiltration by polyvinylidene fluoride membranes [
28]. Other research groups are trying to use fluorine-free materials: Gao et al. [
29] use an epoxidized natural rubber latex with polyvinyl alcohol to make a three-dimensional porous material with a nanostructure; Jha et al. [
30] prepare superhydrophobic nanotubes from polypyrrole; and Ubah et al. use
Moringa oleifera to functionalize zerovalent iron particles [
31].
4. Discussion
Research is currently being conducted on sponges, foams, nets, textiles, nanofibers, and nanoporous structures as solutions for adsorbing oil from water. In these projects, metals (e.g., stainless steel), polymers, ceramics, and natural fibers are used as adsorbing media (
Figure 5) [
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32].
Most research work is currently concerned with the development of oil-adsorbing sponges and foams. This may be due to the fact that these can be produced economically on an industrial scale if development is sufficiently advanced. Currently, many nanostructured surfaces can only be produced on a laboratory scale, which is why further material-specific process development is necessary. Due to this difficulty, other materials such as textiles still offer an attractive alternative for the production of adsorption materials for oil–water separation.
Fundamentally, it can be observed that all methods are based on a hierarchical structuring of the adsorption materials on the micro and nano levels. This is an attempt to create a Cassie–Baxter stage to achieve a stable and distinctive hydrophobicity.
Concerning materials, mainly metallic and polymeric materials are used, as this is an application area with high requirements, such as resistance to oils, salt water, and UV radiation. Due to these requirements, it is obvious to use perfluorinated materials. These are still widely used in industry for hydrophobization, but they have a bioaccumulative effect and are heavily regulated in the EU, for example [
33]. Therefore, research should pay attention to the use of other resistant materials. Considering bioeconomy and circular value creation, it is reasonable to increasingly use bio-based raw materials. There are already some attempts to use adsorption materials made of wood or biodegradable polymers [
34].
The wetting property of the adsorbent material is essential for the separation of oil and water. Almost all approaches found are based on superhydrophobicity. The highest water contact angles (WCA) are reported for melamine foams and copper meshes (approximately 158°). In comparison, the WCA of Salvinia molesta is even higher at 164°, which can be explained by its hierarchical structure. The WCA of the BOA textile coated with layered silicate is also slightly lower (approximated 152°). This indicates that the biological model has not yet been fully transferred and other technical solutions, such as those presented in the literature, can still be improved by bionic approaches. It may be worth optimizing the wetting properties to improve the separation rate.
The described materials for oil–water separation can be used for different separations depending on their ability to transport oils of different viscosities. For the BOA, oil viscosities from 4.5 cP to 196.7 cP (at 20 °C) were investigated and the BOA textile proved to be functional in this range. Other materials found in the literature are mostly tested with gasoline, diesel oil, kerosene, and soybean and other vegetable oils. These normally show a low viscosity around 5 cP at room temperature. It appears to be particularly challenging to separate highly viscous oils. High viscosities are found particularly in accidents involving crude and heavy oils.
5. Conclusions
As mentioned above, oil spill contamination on water are a major environmental problem worldwide. The technologies previously cited and described deal with the solution to this problem, and as shown, the approaches are very diverse. All methods based on adsorbent materials have in common that these materials achieve separation by wetting properties and achieve adsorption by a porous surface. The latter creates a capillary system that is filled by the oil. For hydrophobicity and porosity, it is favorable to use a multi-hierarchical surface. This is mainly achieved by a macrostructure, such as a mesh or foam, and a complementary coating, such as nanoparticles.
The novel method of the Bionic Oil Adsorber presented here is also based on a hierarchical structure, the BOA textile, which has been hydrophobized by a coating. But it fundamentally differs from all published approaches: no external energy needs to be supplied for operation, it can be used multiple times, and no toxic substances are introduced into the water body and the oil is sustainably collected in a removable collector. This makes it cost-effective and sustainable. In this publication, we were able to show quantitatively for the first time that the adsorbing and collecting potential is larger than expected, and that the BOA is an important addition to the existing approaches and a very promising technology for removing oil from waters in an environmentally friendly and cost-effective way in the future.
The BOA device can be used preventively and in acute emergencies. Its design can be adapted to the size of the oil spills. It is easy to handle, can be used independent, and does not need any energy supply. Thus, it offers an interesting alternative to existing oil–water separation processes.
Self-driven oil separation from water surfaces by biomimetic adsorbing and transporting materials probably differs from all the existing technologies by its fast, self-driven, automatic, and completely sustainable removal of the contaminant from a water surface.
6. Future Directions
All presented materials should not only be produced efficiently but also be used efficiently. To this end, it is desirable to make them reusable on the one hand and to use digital developments such as artificial intelligence on the other, in order to be able to use oil removal methods in a targeted manner [
35].
The BOA and other adsorbing materials contribute to the achievement of various Sustainable Development Goals (SDGs) of the UN. These are primarily Clean Water and Sanitation (Goal 6) and Life Below Water (Goal 14). Indirectly, it also contributes to Zero Hunger (Goal 2) and Life on Land (Goal 15), as clean water is a highly important component for food security and a healthy life on land for people and animals. However, in the production of the textile and the entire product, all other SDGs should also be considered. Especially in the textile process chain, there is often exploitation of workers, which should be avoided at all costs.
In addition, there are some research groups working on smart materials for oil–water separation. These are divided into stimulus-responsive and prewetting-induced types. The former can switch their wetting properties between superhydrophobic and superhydrophilic, whereas the latter can separate either oil or water from mixtures without external stimulus. Similar structures and materials are used for this as for the other developments presented [
36].
With regard to this future work, inspiring role models can be found in nature that enable the rapid development of new materials and processes. One of the best examples continues to be
Salvinia [
37]. Moreover, as early evolutionary structures, terrestrial cyanobacteria like
Hassallia byssoidea can offer exciting impulses for the design of surfaces that are fundamentally amenable to oil–water separation due to superhydrophobicity [
6].
Adsorbent textiles can also be used in other areas for oil–water separation. For example, emulsions in industrial applications or in the automotive sector could be purified so that they can either be reused or properly disposed of. As this is a very large field of application apart from oil spills in marine environments, the application of the developed adsorption materials should be tested in this field as well.
Oil films on water are a major global environmental threat. We could prove, that the novel biomimetic BOA technology for a self-driven separation and automatic collection of oil films including their complete removal from water is surprisingly highly efficient and sustainable. Moreover, it can possibly be applied in different related separation processes. The technology differs from all existing approaches that have been proposed to date. However, further work is necessary to adapt and scale up the collectors for different applications and scales: a promising task for future applied engineering projects.
Supplementary Materials
The following supporting information can be downloaded at:
https://www.mdpi.com/article/10.3390/separations10120592/s1, Video S1: Adsorption of an oil droplet by
Salvinia molesta; Video S2: Adsorption of an oil droplet by bioinspired textile; Video S3: Functional principle of Bionic Oil Adsorber.
Author Contributions
Conceptualization, W.B. and L.B.; methodology, W.B.; formal analysis, L.B.; investigation, L.B., W.B. and M.M.; data curation, L.B.; writing—original draft preparation, L.B.; writing—review and editing, W.B., M.M. and K.K.; visualization, L.B.; supervision, W.B., K.K. and T.G.; project administration, W.B. and L.B.; funding acquisition, W.B., M.M., K.K. and T.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the German Federal Foundation for the Environment (Deutsche Bundesstiftung Umwelt) with the title ‘Bionic Oil Adsorber (BOA)—Entwicklung eines physikalischen bionischen Verfahrens zur Entfernung von Ölverschmutzungen auf Wasser unter Einsatz superhydrophober Funktionstextilien’, grant number 34602/01-23.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to nondisclosure reasons.
Acknowledgments
We acknowledge our colleagues and students. We thank David Scheithe, ITA Aachen, for critically revising the English text.
Conflicts of Interest
Author Kai Klopp was employed by the company Heimbach GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Fingas, M. Introduction to Oil Spills and their Clean-up. In Handbook of Biodiesel and Petrodiesel Fuels, 1st ed.; Konur, O., Ed.; CRC Press: London, UK; Boca Raton, NY, USA, 2021; pp. 875–889. ISBN 9780367456252. [Google Scholar] [CrossRef]
- Statista. Global Oil Production 2022|Statista. Available online: https://www.statista.com/statistics/265229/global-oil-production-in-million-metric-tons/ (accessed on 16 August 2023).
- Helle, I.; Jolma, A.; Venesjärvi, R. Species and habitats in danger: Estimating the relative risk posed by oil spills in the northern Baltic Sea. Ecosphere 2016, 7, e01344. [Google Scholar] [CrossRef]
- Green, J.; Trett, M.W. The Fate and Effects of Oil in Freshwater; Springer: Dordrecht, The Netherlands, 1989; ISBN 978-94-010-6990-8. [Google Scholar] [CrossRef]
- Barthlott, W.; Mail, M.; Bhushan, B.; Koch, K. Plant Surfaces: Structures and Functions for Biomimetic Innovations. Nano-Micro Lett. 2017, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Barthlott, W.; Büdel, B.; Mail, M.; Neumann, K.M.; Bartels, D.; Fischer, E. Superhydrophobic Terrestrial Cyanobacteria and Land Plant Transition. Front. Plant Sci. 2022, 13, 880439. [Google Scholar] [CrossRef] [PubMed]
- Barthlott, W. Self-Cleaning Surfaces in Plants: The Discovery of the Lotus Effect as a Key Innovation for Biomimetic Technologies. In Handbook of Self-Cleaning Surfaces and Materials; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2023; pp. 359–369. [Google Scholar] [CrossRef]
- Barthlott, W.; Moosmann, M.; Noll, I.; Akdere, M.; Wagner, J.; Roling, N.; Koepchen-Thomä, L.; Azad, M.A.K.; Klopp, K.; Gries, T.; et al. Adsorption and superficial transport of oil on biological and bionic superhydrophobic surfaces: A novel technique for oil-water separation. Philos. Trans. A Math. Phys. Eng. Sci. 2020, 378, 20190447. [Google Scholar] [CrossRef] [PubMed]
- Barthlott, W.; Gries, T.; Klopp, K.; Ditsche, P.; Beek, L.; Akdere, M.; Mail, M. Entwicklung eines physikalischen bionischen Verfahrens zur Entfernung von Ölverschmutzungen auf Wasser unter Einsatz superhydrophober Fuktionstextilien: BOA (Bionic Oil Adsorber). Abschlussbericht über ein Forschungsprojekt gefördert unter dem Az 34602/01 von der Deutschen Bundesstiftung Umwelt. 2023. Available online: https://www.dbu.de/OPAC/ab/DBU-Abschlussbericht-AZ-34602_01-Hauptbericht.pdf (accessed on 25 August 2023).
- Wagner, J.; Akdere, M.; Gürbüz, K.; Beek, L.; Klopp, K.; Ditsche, P.; Mail, M.; Gries, T.; Barthlott, W. Oil adsorbing and transporting surfaces: A simulative determination of parameters for bionic functional textiles. Bioinspir. Biomim. 2023, 18, 36006. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Yue, X.; Hao, B.; Wang, R.; Ma, P.-C. Facile preparation of melamine foam with superhydrophobic performance and its system integration with prototype equipment for the clean-up of oil spills on water surface. Sci. Total Environ. 2022, 833, 155184. [Google Scholar] [CrossRef] [PubMed]
- Nandwana, V.; Ribet, S.M.; Reis, R.D.; Kuang, Y.; More, Y.; Dravid, V.P. OHM Sponge: A Versatile, Efficient, and Ecofriendly Environmental Remediation Platform. Ind. Eng. Chem. Res. 2020, 59, 10945–10954. [Google Scholar] [CrossRef]
- Malik, A.; Sajjad, S.; Leghari, S.A.K.; Naz, Y.; Masood, M.; Ahmad, I.; Uzair, B. Marvelous oleophillic adsorption ability of SiO2/activated carbon and GO composite nanostructure using polyurethane for rapid oil spill cleanup. Appl. Nanosci. 2021, 11, 1211–1223. [Google Scholar] [CrossRef]
- Pang, Y.; Yu, Z.; Chen, H.; Xiang, Q.; Wang, Q.; Xie, C.; Liu, Y. Superhydrophobic polyurethane sponge based on sepiolite for efficient oil/water separation. J. Hazard. Mater. 2022, 434, 128833. [Google Scholar] [CrossRef]
- Wang, B.; Wang, B.; Zhang, Y.; Ma, S.; Yang, X.; Feng, Y.; Liu, C.; Shen, C. Superhydrophobic porous polyvinylidene fluoride monolith with outstanding environmental suitability for high-efficient continuous oil/water separation under harsh conditions. J. Environ. Chem. Eng. 2022, 10, 107480. [Google Scholar] [CrossRef]
- Venkatesan, N.; Yuvaraj, P.; Fathima, N.N. Fabrication of non-fluorinated superhydrophobic and flame retardant porous material for efficient oil/water separation. Mater. Chem. Phys. 2022, 286, 126190. [Google Scholar] [CrossRef]
- Zhan, B.; Liu, Y.; Zhou, W.-T.; Li, S.-Y.; Chen, Z.-B.; Stegmaier, T.; Aliabadi, M.; Han, Z.-W.; Ren, L.-Q. Multifunctional 3D GO/g-C3N4/TiO2 foam for oil-water separation and dye adsorption. Appl. Surf. Sci. 2021, 541, 148638. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, Y.; Chen, M.; Xu, Z.; Li, L.; Zhou, Y. Metal mesh-based special wettability materials for oil-water separation: A review of the recent development. J. Pet. Sci. Eng. 2021, 205, 108889. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, P.; Gao, Y.; Yun, J. Fabrication of superhydrophobic copper meshes via simply soaking for oil/water separation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128648. [Google Scholar] [CrossRef]
- Liu, B.; Fu, Y.; Guo, Z. Superhydrophobic/Superoleophilic Copper Mesh for Heavy Oil-water Separation. Chem. Lett. 2022, 51, 796–798. [Google Scholar] [CrossRef]
- Wang, M.; Xu, J.; Ren, W.; Wang, J.; Zou, Z.; Wang, X. Laser Electrochemical Deposition Hybrid Preparation of an Oil–Water Separation Mesh with Controllable Pore Diameter Based on a BP Neural Network. Langmuir 2023, 39, 7281–7293. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Johnson, N.; Drelich, J.; Yap, Y.K. The performance of superhydrophobic and superoleophilic carbon nanotube meshes in water–oil filtration. Carbon 2011, 49, 669–676. [Google Scholar] [CrossRef]
- Hakeim, O.A.; Abdelghaffar, F.; El-Gabry, L.K. Investigation of Egyptian Chorisia spp. fiber as a natural sorbent for oil spill cleanup. Environ. Technol. Innov. 2022, 25, 102134. [Google Scholar] [CrossRef]
- Bang, J.; Park, S.; Hwang, S.-W.; Oh, J.-K.; Yeo, H.; Jin, H.-J.; Kwak, H.W. Biodegradable and Hydrophobic Nanofibrous Membranes Produced by Solution Blow Spinning for Efficient Oil/Water Separation. SSRN J. 2022, 312, 137240. [Google Scholar] [CrossRef]
- Sun, F.; Li, T.-T.; Ren, H.-T.; Shiu, B.-C.; Peng, H.-K.; Lin, J.-H.; Lou, C.-W. Multi-scaled, hierarchical nanofibrous membrane for oil/water separation and photocatalysis: Preparation, characterization and properties evaluation. Prog. Org. Coat. 2021, 152, 106125. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Xiong, Y.; Zheng, W.; Liu, W.; He, M.; Li, L.; Liu, J.; Lu, L.; Peng, K. Spider silk bioinspired superhydrophilic nanofibrous membrane for efficient oil/water separation of nanoemulsions. Sep. Purif. Technol. 2022, 280, 119824. [Google Scholar] [CrossRef]
- Feng, Q.; Zhan, Y.; Yang, W.; Dong, H.; Sun, A.; Liu, Y.; Wen, X.; Chiao, Y.-H.; Zhang, S. Layer-by-layer construction of super-hydrophilic and self-healing polyvinylidene fluoride composite membrane for efficient oil/water emulsion separation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127462. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.; Yan, L.; Bai, Y.; Li, S.; Sorokin, P.; Shao, L. Biomimetic nanoparticle-engineered superwettable membranes for efficient oil/water separation. J. Membr. Sci. 2021, 618, 118525. [Google Scholar] [CrossRef]
- Gao, Q.; Cheng, S.; Wang, X.; Tang, Y.; Yuan, Y.; Li, A.; Guan, S. Three-dimensional hierarchical nanostructured porous epoxidized natural rubber latex/poly(vinyl alcohol) material for oil/water separation. J. Appl. Polym. Sci. 2022, 139, e52825. [Google Scholar] [CrossRef]
- Jha, P.; Koiry, S.P.; Sridevi, C.; Putta, V.; Gupta, D.; Chauhan, A.K. A strategy towards the synthesis of superhydrophobic/superoleophilic non-fluorinated polypyrrole nanotubes for oil–water separation. RSC Adv. 2020, 10, 33747–33752. [Google Scholar] [CrossRef]
- Ubah, P.C.; Dashti, A.F.; Saaid, M.; Imam, S.S.; Adnan, R. Fabrication and response optimization of Moringa oleifera-functionalized nanosorbents for the removal of diesel range organics from contaminated water. Environ. Sci. Pollut. Res. 2023, 30, 4462–4484. [Google Scholar] [CrossRef]
- Gupta, R.K.; Dunderdale, G.J.; England, M.W.; Hozumi, A. Oil/water separation techniques: A review of recent progresses and future directions. J. Mater. Chem. A 2017, 5, 16025–16058. [Google Scholar] [CrossRef]
- European Chemicals Agency. Per- and Polyfluoroalkyl Substances (PFAS). Available online: https://echa.europa.eu/hot-topics/perfluoroalkyl-chemicals-pfas (accessed on 18 August 2023).
- Zhang, W.; Liu, Y.; Tao, F.; An, Y.; Zhong, Y.; Liu, Z.; Hu, Z.; Zhang, X.; Wang, X. An overview of biomass-based Oil/Water separation materials. Sep. Purif. Technol. 2023, 316, 123767. [Google Scholar] [CrossRef]
- Steffi, P.F.; Thirumalaiyammal, B.; Anburaj, R.; Mishel, P.F. Artificial Intelligence in Bioremediation Modelling and Clean-Up of Contaminated Sites: Recent Advances, Challenges and Opportunities. In Omics Insights in Environmental Bioremediation; Springer: Singapore, 2022; pp. 683–702. [Google Scholar] [CrossRef]
- Xiang, B.; Sun, Q.; Zhong, Q.; Mu, P.; Li, J. Current research situation and future prospect of superwetting smart oil/water separation materials. J. Mater. Chem. A 2022, 10, 20190–20217. [Google Scholar] [CrossRef]
- Bing, W.; Wang, H.; Tian, L.; Zhao, J.; Jin, H.; Du, W.; Ren, L. Small Structure, Large Effect: Functional Surfaces Inspired by Salvinia Leaves. Small Struct. 2021, 2, 2100079. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).