Cytotoxic, Scolicidal, and Insecticidal Activities of Lavandula stoechas Essential Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Essential Oil Extraction
2.3. Cytotoxic Activity of L. stoechas
Cell Culture
2.4. Cytotoxicity Assessment by MTT Assay (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide Tetrazolium)
2.5. Scolicidal Activity
Collection of Protoscoleces from Hydatid Cysts and the Viability Test
2.6. Determination of In Vitro Scolicidal Activity
2.7. Acaricidal activity of L. steochas against Larvae and Adult of R. annulatus
2.8. Adult Immersion Test of R. annulatus
2.9. Larvicidal Activity against R. annulatus
2.10. Repellent Activity against R. annulatus
2.11. Insecticidal Activity against Musca domestica
Rearing of Housefly Colony
2.12. Larvicidal Bioassay against Musca domestica
2.13. Pupicidal Bioassay against Musca domestica
2.14. Statistics
3. Results
3.1. Chemical Composition of the Essential Oil
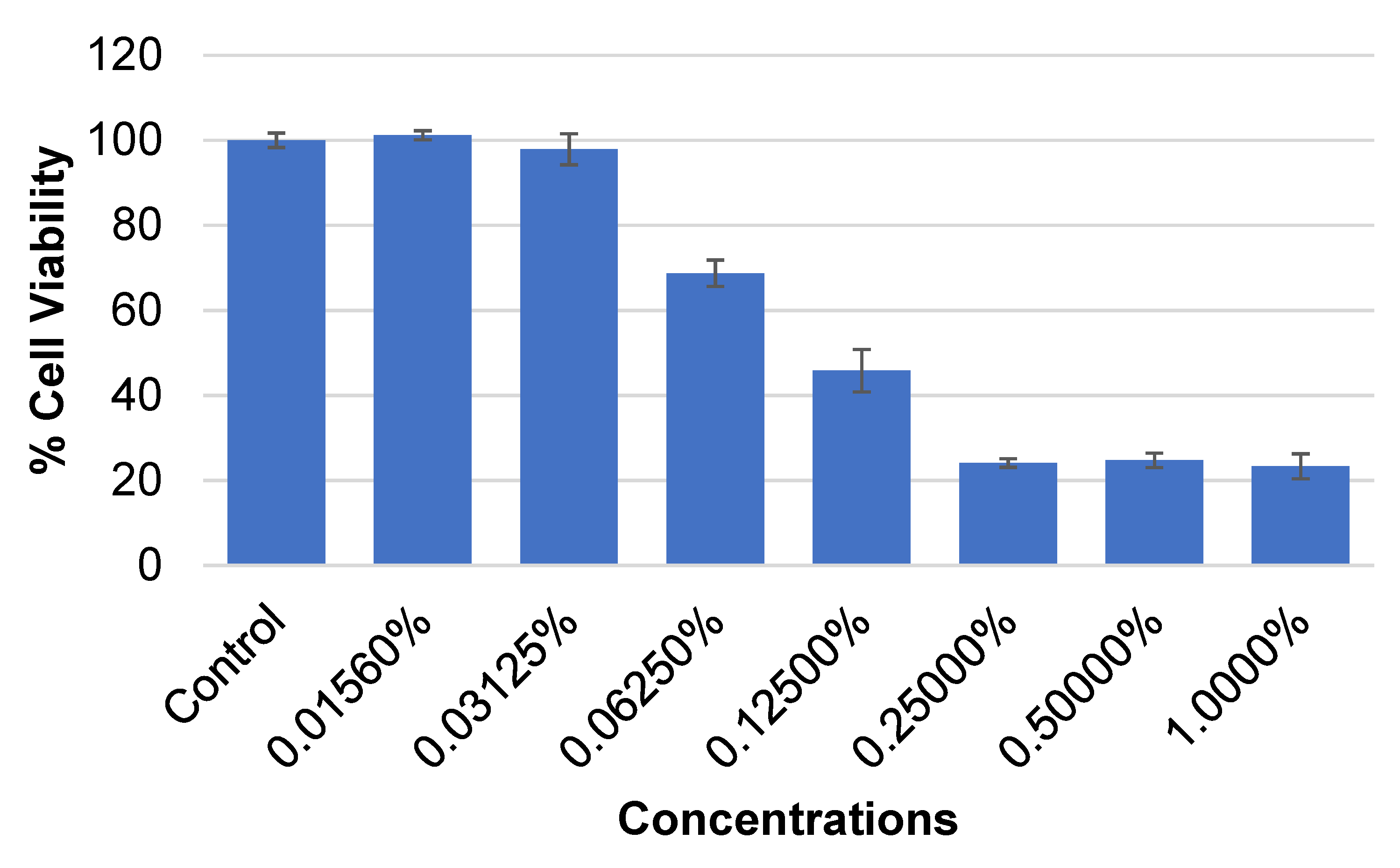
3.2. Cytotoxicity Assessment
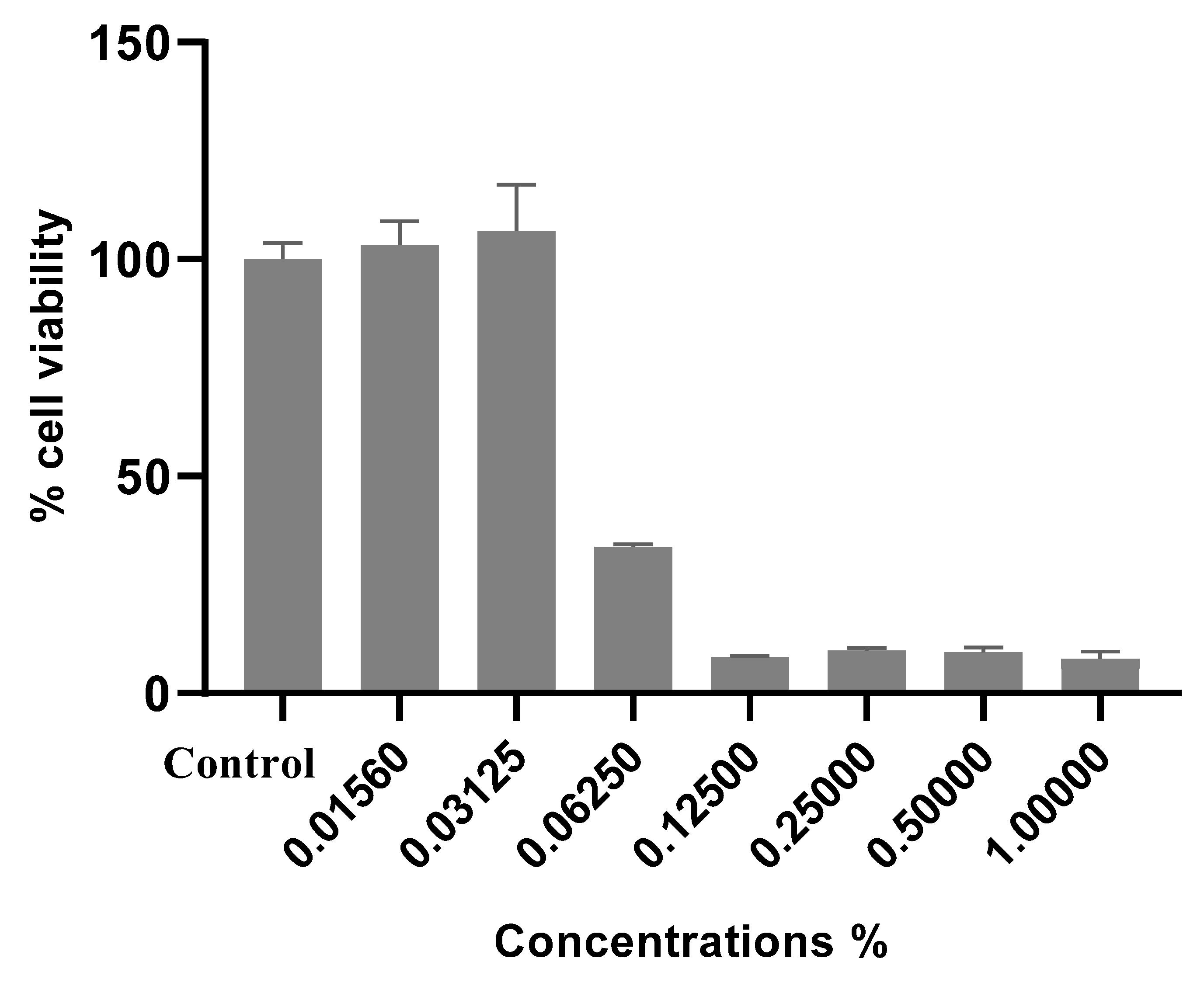
3.3. In Vitro Scolicidal Activity
3.4. Acaricidal Activity L. stoechas EO against Adult and Larvae of R. annulatus Ticks
3.5. Insecticidal Effect of L. stoechas against Larvae and Pupae of Musca domestica
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Panigrahi, P.N.; Gupta, A.R.; Behera, S.K.; Panda, B.S.K.; Patra, R.C.; Mohanty, B.N.; Sahoo, G.R. Evaluation of gastrointestinal helminths in canine population of Bhubaneswar, Odisha, India: A public health appraisal. Vet. World 2014, 7, 295–298. [Google Scholar] [CrossRef]
- Abdel-Baki, A.S.; Almalki, E.; Mansour, L.; Al-Quarishy, S. In vitro scolicidal effects of Salvadora persica root extract against protoscolices of Echinococcus granulosus. Korean J. Parasitol. 2016, 54, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Maurice, M.N.; Huseein, E.A.M.; Monib, M.E.M.M.; Alsharif, F.M.; Namazi, N.I.; Ahmad, A.A. Evaluation of the scolicidal activities of eugenol essential oil and its nanoemulsion against protoscoleces of hydatid cysts. PLoS One 2021, 16, e0259290. [Google Scholar] [CrossRef] [PubMed]
- Rokni, M. Echinococcosis/hydatidosis in Iran. Iran J. Parasitol. 2009, 4, 1e16. [Google Scholar]
- Norouzi, R.; Ataei, A.; Hejazy, M.; Noreddin, A.; El Zowalaty, M.E. Scolicidal Effects of Nanoparticles Against Hydatid Cyst Protoscolices in vitro. Int. J. Nanomed. 2020, 15, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Shakibaiea, M.; Khalaf, A.K.; Rashidipour, M.; Mahmoudvand, H. Effects of green synthesized zinc nanoparticles alone and along with albendazole against hydatid cyst protoscoleces. Ann. Med. Surg. 2022, 78, 10. [Google Scholar] [CrossRef]
- Junghanss, T.; Da Silva, A.M.; Horton, J.; Chiodini, P.L.; Brunetti, E. Clinical management of cystic echinococcosis: State of the art, problems, and perspectives. Am. J. Trop. Med. Hyg. 2008, 79, 301e311. [Google Scholar] [CrossRef]
- Al-Harbi, N.A.; Al Attar, N.M.; Hikal, D.M.; Mohamed, S.E.; Abdel Latef, A.A.H.; Ibrahim, A.A.; Abdein, M.A. Evaluation of Insecticidal Effects of Plants Essential Oils Extracted from Basil, Black Seeds and Lavender against Sitophilus oryzae. Plants 2021, 10, 829. [Google Scholar] [CrossRef]
- Anthony, J.P.; Fyfe, L.; Smith, H. Plant active components–a resource for antiparasitic agents? Trends Parasitol. 2005, 21, 462–468. [Google Scholar] [CrossRef]
- Brunetti, E.; Kern, P.; Vuitton, D.A. Writing Panel for the WHO-IW-GE. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010, 114, 1–16. [Google Scholar] [CrossRef]
- Almalki, E.; Al-Shaebi, E.M.; Al-Quarishy, S.; El-Matbouli, M.; Abdel-Baki, A.S. In vitro effectiveness of Curcuma longa and Zingiber officinale extracts on Echinococcus protoscoleces. Saudi J. Biol. Sci. 2017, 24, 90–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moazeni, M.; Saharkhiz, M.J.; Hosseini, A.A. In vitro lethal effect of ajowan (Trachyspermum ammi L.) essential oil on hydatid cyst protoscoleces. Vet. Parasitol. 2012, 187, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Moazeni, M.; Nazer, A. In vitro effectiveness of garlic (Allium sati-vum) extract on scolices of hydatid cyst. World J. Surg. 2010, 34, 2677–2681. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Abbas, R.Z.; Khan, J.A.; Iqbal, Z.; Bhatti, M.M.H.; Sindhu, Z.u.D.; Zia, M.A. Integrated strategies for the control and prevention of dengue vectors with particular reference to Aedes aegypti. Pak. Vet. J. 2014, 34, 1–10. [Google Scholar]
- Yadav, P.K.; Rafiqi, S.M.; Panigrahi, P.N.; Kumar, D.; Kumar, R.; Kumar, S. Recent trends in control of ectoparasites: A review. J. Entomol. Zool. Stud. 2017, 5, 808–813. [Google Scholar]
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129 (Suppl. 1), S3–S14. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Q.; Liu, J.Q.; Xu, B.L.; Lv, S.; Xia, S.; Zhou, X.N. Tick-borne pathogens and associated co-infections in ticks collected from domestic animals in central China. Parasit Vectors 2014, 7, 1–8. [Google Scholar] [CrossRef]
- Demessie, Y.; Derso, S. Tick borne hemoparasitic diseases of ruminants: A Review. Adv. Biol. Res. 2015, 9, 210–224. [Google Scholar]
- Opara, M.N.; Santali, A.; Mohammed, B.R.; Jegede, O.C. Prevalence of haemoparasites of small ruminants in Lafia Nassarawa State: A Guinea Savannah Zone of Nigeria. J. Vet. Adv. 2016, 6, 1251–1257. [Google Scholar]
- Kamaraj, C.; Rajakumar, G.; Rahuman, A.A.; Velayutham, K.; Bagavan, A.; Zahir, A.A.; Elango, G. Feeding deterrent activity of synthesized silver nanoparticles using Manilkara zapota leaf extract against the house fly, Musca domestica (Diptera: Muscidae). Parasitol. Res. 2012, 111, 2439–2448. [Google Scholar] [CrossRef]
- Khan, H.A.; Akram, W.; Arshad, M.; Hafeez, F. Toxicity and resistance of field collected Musca domestica (Diptera: Muscidae) against insect growth regulator insecticides. Parasitol. Res. 2016, 115, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Morey, R.A.; Khandagle, A.J. Bioefficacy of essential oils of medicinal plants against housefly, Musca domestica L. Parasitol. Res. 2012, 111, 1799–1805. [Google Scholar] [CrossRef] [PubMed]
- Sinthusiri, J.; Soonwera, M. Oviposition deterrent and ovicidal activities of seven herbal essential oils against female adults of housefly, Musca domestica L. Parasitol. Res. 2014, 113, 3015–3022. [Google Scholar] [CrossRef]
- Foil, L.D.; Coleman, P.; Fragoso-Sanchez, H.E.; Garcia-Vazquez, Z.; Guerrero, F.D.; Jonsson, N.N.; Langstaff, I.G.; Li, A.Y.; Machila, N.; Miller, R.J.; et al. Factors that influence the prevalence of acaricide resistance and tick-borne diseases. Vet. Parasitol. 2004, 125, 163–181. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.R.; Azeem, M.; Khalil, N.S.; Sakr, H.H.; Shaden, A.; Khalifa, M.; Awang, K.; Saeed, A.; Mohamed AFarag, M.A.; Al Ajmi, M.F.; et al. Essential oils of aromatic Egyptian plants repel nymphs of the tick Ixodes ricinus (Acari: Ixodidae). Exp. Appl. Acarol. 2017, 73, 139–157. [Google Scholar] [CrossRef] [Green Version]
- Showler, A.T. Botanically based repellent and insecticidal effects against horn flies and stable flies (Diptera: Muscidae). J. Integr. Pest. Manag. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Khan, M.N.; Sajid, M.S.; Rizwan, H.M.; Qudoos, A.; Abbas, R.Z.; Riaz, M.; Khan, M.K. Comparative efficacy of six anthelmintic treatments against natural infection of fasciola species in sheep. Pak. Vet. J. 2017, 37, 65–68. [Google Scholar]
- Liaqat, I.; Pervaiz, Q.; Bukhsh, S.J.; Ahmed, S.I.; Jahan, N. (2016) Investigation of bactericidal effects of medicinal plant extracts on clinical isolates and monitoring their biofilm forming potential. Pak. Vet. J. 2016, 36, 159–164. [Google Scholar]
- Chintalchere, J.M.; Dar, M.A.; Pandit, R.S. Biocontrol efficacy of bay essential oil against housefly, Musca domestica (Diptera: Muscidae). J. Basic Appl. Zool. 2020, 81, 1–12. [Google Scholar] [CrossRef]
- El-Akhal, F.; El Ouali Lalami, A.; Ez Zoubi, Y.; Greche, H.; Guemmouh, R. Chemical composition and larvicidal activity of essential oil of Origanum majorana (Lamiaceae) cultivated in Morocco against Culex pipiens (Diptera:Culicidae). Asian Pac. J. Trop. Biomed. 2014, 4, 746–750. [Google Scholar] [CrossRef]
- Conti, B.; Angelo, C.; Alessandra, B.; Francesca, G.; Luisa, P. Essential oil composition and larvicidal activity of six Mediterranean aromatic plants against the mosquito Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 2010, 107, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.G. The Genus Lavandula in Arabia and Tropical NE Africa. Notes R. Bot. Gard. Edinb. 1985, 42, 503–528. [Google Scholar]
- Badreddine, B.S.; Olfa, E.; Samir, D.; Hnia, C.; Lahbib, B.J.M. Chemical composition of Rosmarinus and Lavandula essential oils and their insecticidal effects on Orgyia trigotephras (Lepidoptera, Lymantriidae). Asian Pac. Trop. Med. 2015, 8, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Gamez, M.J.; Jimenez, J.; Risco, S.; Zarzuelo, A. Hypoglycemic activity in various species of the genus Lavandula: Lavandula stoechas L. and Lavandula multifida L. Pharmazie 1987, 10, 706–707. [Google Scholar]
- Cavanagh, H.M.A.; Wilkinson, J.M. Biological Activities of Lavender essential oil. Phytother. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef]
- Dadalioglu, I.; Evrendilek, G.A. Chemical compositions and antibacterial effects of essential oils of turkish oregano (Origanum minutiflorum), bay laurel (Laurus nobilis), spanish lavender (Lavandula stoechas), and fennel (Foeniculum vulgare) on common foodborne pathogens. J. Agri. Food Chem. 2004, 52, 8255–8260. [Google Scholar] [CrossRef]
- Benabdelkader, T.; Zitouni, A.; Guitton, Y.; Jullien, F.; Maitre, D.; Casabianca, H.; Legendre, L.; Kameli, A. Essential oils from wild populations of Algerian Lavandula stoechas L.: Composition, chemical variability, and in vitro biological properties. Chem. Biodivers. 2011, 8, 937–953. [Google Scholar] [CrossRef]
- Angioni, A.; Barra, A.; Coroneo, V.; Desi, S.; Cabras, P. Chemical composition, seasonal variability and antifungal activity of Lavandula stoechas L. ssp. Stoechas essential oils from stem/leaves and flowers. J. Agri. Food. Chem. 2006, 54, 4364–4370. [Google Scholar]
- Messaoud, C.; Chongrani, H.; Boussaid, M. Chemical composition and antioxidant activities of essential oils and methanol extracts of three wild Lavandula, L. species. Nat. Prod. Res. 2012, 26, 1976–1984. [Google Scholar] [CrossRef]
- Ezzoubi, Y.; Bousta, D.; Lachkar, M.; Farah, A. Antioxidant and anti-inflammatory properties of ethanolic extract of Lavandula stoechas L. from Taounate region in Morocco. Int. J. Phytopharm. 2014, 5, 21–26. [Google Scholar]
- Sokmen, A.; Abdel-Baki, A.A.S.; Al-Malki, E.S.; Al-Quraishy, S.; Abdel-Haleem, H.M. Constituents of essential oil of Origanum minutiflorum and its in vitro antioxidant, scolicidal and anticancer activities. J. King Saud Univ. Sci. 2020, 32, 2377–2382. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Siddiqui, M.A.; Singh, G.; Kashyap, M.P.; Khanna, V.K.; Yadav, S.; Chandra, D.; Pant, A.B. Influence of cytotoxic doses of 4-hydroxynonenal on selected neurotransmitter receptors in PC-12 cells. Toxicol. Vitr. 2008, 22, 1681–1688. [Google Scholar] [CrossRef]
- Haghani, A.; Roozitalab, A.; Safi, S.N. Low scolicidal effect of Ocimum bacilicum and Allium cepa on protoccoleces of hydatid cyst: An in vitro study. Comp. Clin. Pathol. 2014, 23, 847–853. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Quı´ez, J.; Sa´nchez Acedo, C. Species composition, distribution, and ecological preferences of the ticks of grazing sheep in north-central Spain. Med. Vet. Entomol. 2004, 18, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Drummond, R.; Ernst, S.; Trevino, J.; Gladney, W.; Graham, O. (1973) Boophilus annulatus and B. microplus: Laboratory tests of insecticides. J. Econ. Entomol. 1973, 66, 130–133. [Google Scholar] [CrossRef] [PubMed]
- FAO. Resistance Management and Integrated Parasite Control in Ruminants Guidelines, Module Ticks: Acaricide Resistance: Diagnosis, Management and Prevention; Food and Agriculture Organization, Animal Production and Health Division: Rome, Italy, 2004. [Google Scholar]
- Klafke, G.M.; Thomas, D.B.; Miller, R.J.; Pérez de León, A.A. Efficacy of a water-based botanical acaricide formulation applied in portable spray box against the southern cattle tick, Rhipicephalus (Boophilus) microplus (Acari: Ixodidae), infesting cattle. Ticks Tick Borne Dis. 2021, 12, 101721. [Google Scholar] [CrossRef] [PubMed]
- Matos, R.S.; Daemon, E.; de Oliveira Monteiro, C.M.; Sampieri, B.R.; Marchesini, P.B.C.; Delmonte, C. (2019) Thymol action on cells and tissues of the synganglia and salivary glands of Rhipicephalus sanguineus sensu lato females (Acari: Ixodidae). Ticks Tick Borne Dis. 2019, 10, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Wanzala, W.; Sika, N.; Gule, S.; Hassanali, A. Attractive and repellent host odours guide ticks to their respective feeding sites. Chemoecology 2004, 14, 229–232. [Google Scholar] [CrossRef]
- Jesikha, M. Control of Musca domestica using wastes from Citrus sinensis peel and Mangifera indica seed. Biol. Environ. Sci. 2014, 1, 17–26. [Google Scholar]
- Abdel-Baki, A.S.; Aboelhadid, S.M.; Sokmen, A.; Al-Quraishy, S.; Hassan, A.O.; Kamel, A.A. (2021) Larvicidal and pupicidal activities of Foeniculum vulgare essential oil, trans-anethole and fenchone against house fly Musca domestica and their inhibitory effect on acetylcholinestrase. Entomol. Res. 2021, 51, 568–577. [Google Scholar] [CrossRef]
- Busvine, J.R. A Critical Review of the Techniques for Testing Insecticides; CABI: London, UK, 1971; p. 345. [Google Scholar]
- Palacios, S.M.; Bertoni, A.; Rossi, Y.; Santander, R.; Urzua, A. Efficacy of essential oils from edible plants as insecticides against the house fly, Musca domestica L. Molecules 2009, 14, 1938–1947. [Google Scholar] [CrossRef] [PubMed]
- Sinthusiri, J.; Soonwera, M. Effect of Herbal Essential Oils against Larvae, Pupae and Adults of House Fly (Musca domestica L.: Diptera). In Proceedings of the 16th Asian Agricultural Symposium and 1st International Symposium on Agricultural Technology, Bangkok, Thailand, 25–27 August 2010; pp. 639–642. [Google Scholar]
- Kumar, P.; Mishra, S.; Malik, A.; Satya, S. Repellent, larvicidal and pupicidal properties of essential oils and their formulations against the housefly, Musca domestica. Med. Vet. Entomol. 2011, 25, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Finney, D.J. Probit Analysis: A Statistical Treatment of the Sigmoid Response Curve, 2nd ed.; Cambridge University Press: Cambridge, UK, 1952. [Google Scholar]
- Ellse, L.; Wall, R. The use of essential oils in veterinary ectoparasite control: A review. Med. Vet. Entomol. 2014, 28, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Aslam, A.; Shahzad, M.I.; Parveen, S.; Ashraf, H.; Naz, N.; Zehra, S.S.; Kamran, Z.; Qayyum, A.; Mukhtar, M. Evaluation of antiviral potential of different Cholistani plants against infectious bursal disease and infectious bronchitis virus. Pak. Vet. J. 2016, 36, 302–306. [Google Scholar]
- Radsetoulalova, I.; Hubert, J.; Lichovnikova, M. Acaricidal activity of plant essential oils against poultry red mite (Dermanyssus gallinae). MendelNet 2017, 24, 260–265. [Google Scholar]
- Aboelhadid, S.M.; Arafa, W.M.; Abdel-Baki, A.A.S.; Sokmen, A.; Al-Quraishy, S.; Hassan, A.O.; Kamel, A.A. Acaricidal activity of Foeniculum vulgare against Rhipicephalus annulatus is mainly dependent on its constituent from trans-anethone. PLoS ONE 2021, 16, e0260172. [Google Scholar] [CrossRef]
- Esmacily, M.; Bandani, A.; Zibaee, I.; Sharijian, J.; Zare, S. Sublethal effects of Artemisia annua L and Rosmatinus officinalis L, essential oils on life table parameters of Tetranychus urticae (Acari: Tetranychidae). Persian J. Acarol. 2017, 6, 39–52. [Google Scholar]
- Arabi, F.; Moharramipour, S.; Sefidkon, F. Chemical composition and insecticidal activity of essential oil from Perovskia abrotanoides (Lamiaceae) against Sitophilus oryzae (Coleoptera: Curculionidae) and Tribolium castaneum (Coleoptera: Tenebrionidae). Int. J. Trop. Insect Sci. 2008, 28, 144–150. [Google Scholar] [CrossRef]
- Yeh, R.Y.; Shiu, Y.L.; Shei, S.C.; Cheng, S.C.; Huang, S.Y.; Lin, J.C.; Liu, C.H. Evaluation of the antibacterial activity of leaf and twig extracts of stout camphor tree, Cinnamomum kanehirae, and the effects on immunity and disease resistance of white shrimp, Litopenaeus vannamei. Fish Shellfish. Immunol. 2009, 27, 26–32. [Google Scholar] [CrossRef]
- Govindarajan, M. Larvicidal and repellent properties of some essential oils against Culex tritaeniorhynchus Giles and Anopheles subpictus Grassi (Diptera: Culicidae). Asian Pac. J. Trop. Med. 2011, 4, 106–111. [Google Scholar] [CrossRef]
- Slimane, B.B.; Ezzine, O.; Dhahri, S.; Ben Jamaa, M.L. Essential oils from two Eucalyptus from Tunisia and their insecticidal action on Orgyia trigotephras (Lepidotera, Lymantriidae). Biol. Res. 2014, 47, 29. [Google Scholar] [CrossRef] [PubMed]
- El Ouali Lalami, A.; El-Akhal, F.; El Amri, N.; Maniar, S.; Faraj, C. State resistance of the mosquito Culex pipiens towards temephos central Morocco. Bull. Soc. Pathol. Exot. 2014, 107, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Toker, R.; Gölükcü; M; Tokgöz, H. Effects of distillation times on essential oil compositions of Origanum minutiflorum O. Schwarz Et. and P.H Davis. J. Essent. Oil Res. 2017, 29, 30–335. [Google Scholar] [CrossRef]
- Çelik, T.; Aslantürk, Ö. Cytotoxic and genotoxic effects of Lavandula stoechas aqueous extracts. Biologia 2007, 62, 292–296. [Google Scholar] [CrossRef]
- Siddiqui, M.; Siddiqui, H.H.; Mishra, A.; Usmani, A. Evaluation of Cytotoxic Activity of Lavandula stoechas Aerial Parts Fractions against HepG2 Cell Lines. Curr. Bioact. Compd. 2020, 16, 1281–1289. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Sudha, T.; Darwish, N.H.E.; Chader, H.; Belkadi, A.; Rajabi, M.; Houche, A.; Benkebailli, F.; Oudjida, F.; Mousa, S.A. A New Eucalyptol-Rich Lavender (Lavandula stoechas L.) Essential Oil: Emerging Potential for Therapy against Inflammation and Cancer. Molecules 2020, 25, 3671. [Google Scholar] [CrossRef]
- Djebir, S.; Ksouri, S.; Trigui, M.; Tounsi, S.; Boumaaza, A.; Hadef, Y.; Benakhla, A. Chemical Composition and Acaricidal Activity of the Essential Oils of Some Plant Species of Lamiaceae and Myrtaceae against the Vector of Tropical Bovine Theileriosis: Hyalomma scupense (syn. Hyalomma detritum). BioMed Res. Int. 2019, 2019, 7805467. [Google Scholar] [CrossRef]
- Pirali-Kheirabadi, K.; Teixeira da Silva, J.A. Lavandula angustifolia essential oil as a novel and promising natural candidate for tick (Rhipicephalus (Boophilus) annulatus) control. Exp. Parasitol. 2010, 126, 184–186. [Google Scholar] [CrossRef]
- Julio, L.F.; Díaz, C.E.; Aissani, N.; Valcarcel, F.; Burillo, J.; Olmeda, S.; González-Coloma, A. Ixodicidal compounds from pre-domesticated ndula luisieri. Ind. Crops Prod. 2017, 110, 83–87. [Google Scholar] [CrossRef]
- Sertkaya, E.; Kaya, K.; Soylu, S. Acaricidal activities of the essential oils from several medicinal plants against the carmine spider mite (Tetranychus cinnabarinus Boisd.) (Acarina: Tetranychidae). Ind. Crops Prod. 2010, 31, 107–112. [Google Scholar] [CrossRef]
- Mkolo, M.; Magano, S. Repellent effects of the essential oil of Lavendula angustifolia against adults of Hyalomma marginatum rufipes. J. South Afr. Vet. Assoc. 2007, 78, 149–152. [Google Scholar] [CrossRef]
- Jaenson, T.G.T.; Garboui, S.; Palsson, K. Repellency of oils of lemon eucalyptus, geranium, and lavender and the mosquito repellent MyggA natural to Ixodes ricinus (Acari: Ixodidae) in the laboratory and field. J. Med. Entomol. 2006, 43, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Traboulsi, A.F.; Taoubi, K.; El-Haj, S.; Bessiere, J.M.; Rammal, S. Insecticidal properties of essential plant oils against the mosquito Culex pipiens molestus (Diptera:Culicidae). Pest. Manag. Sci. 2002, 58, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Bosly, B. Evaluation of insecticidal activities of Mentha piperita and Lavandula angustifolia essential oils against house fly, Musca domestica L. (Diptera: Muscidae). J. Entomol. Nematol. 2013, 5, 50–54. [Google Scholar] [CrossRef]
- Cossetin, L.F.; Santi, E.M.T.; Cossetin, J.F.; Dillmann, J.B.; Baldissera, M.D.; Garlet, Q.I.; de Souza, T.P.; Loebens, L.; Heinzmann, B.M.; Machado, M.M.; et al. In vitro Safety and Efficacy of Lavender Essential Oil (Lamiales: Lamiaceae) as an Insecticide Against Houseflies (Diptera: Muscidae) and Blowflies (Diptera: Calliphoridae). J. Econ. Entomol. 2018, 111, 1974–1982. [Google Scholar] [CrossRef]
- Khater, H.F.; Khater, D.F. The insecticidal activity of four medicinal plants against the blowfly Lucilia sericata (Diptera: Calliphoridae). Int. J. Dermatol. 2009, 48, 492–497. [Google Scholar] [CrossRef]
- Sajfrtova, M.; Sovova, H.; Karban, J.; Rochova, K.; Pavela, R.; Barnet, M. Effect of separation method on chemical composition and insecticidal activity of Lamiaceae isolates. Ind. Crops Prod. 2013, 47, 69–77. [Google Scholar] [CrossRef]
- Selles, S.M.A.; Kouidri, M.; González, M.G.; González, J.; Sánchez, M.; González-Coloma, A.; Sanchis, J.; Elhachimi, L.; Olmeda, A.S.; Tercero, J.M.; et al. Acaricidal and Repellent Effects of Essential Oils against Ticks: A. Review. Pathogens 2021, 10, 1379. [Google Scholar] [CrossRef]
- George, D.R.; Sparagano, O.A.E.; Port, G.; Okello, E.; Shiel, R.S.; Guy, J.H. Repellence of plant essential oils to Dermanyssus gallinae and toxicity to the non-target invertebrate Tenebrio molitor. Vet. Parasitol. 2009, 162, 129–134. [Google Scholar] [CrossRef]
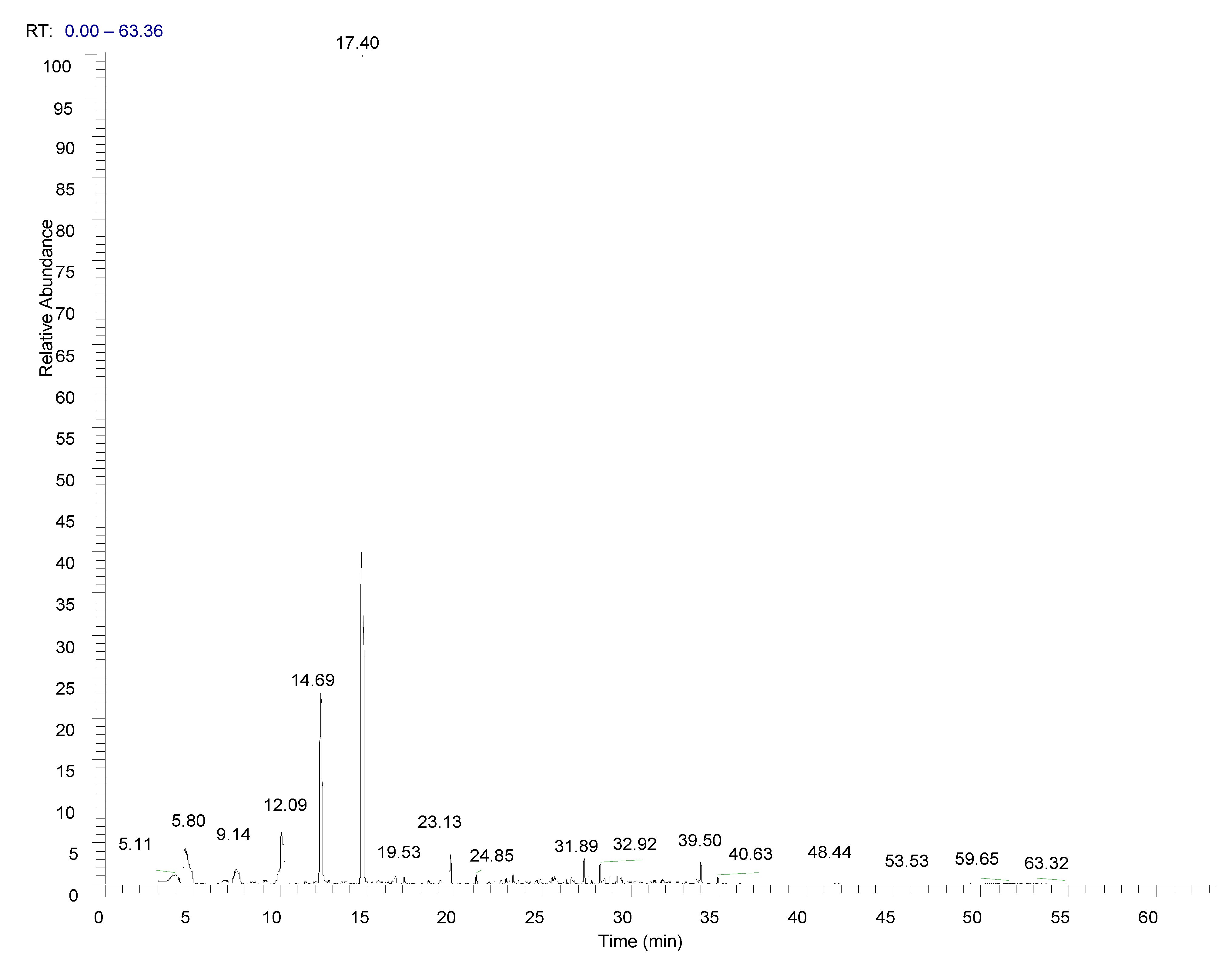

| Peak No | RT (min) | R.I.ex | R.I.lt | COMPOUND | % | |
|---|---|---|---|---|---|---|
| 1 | 8.33 | 930 | 939 | α-Pinene | 0.55 | MH |
| 2 | 9.14 | 945 | 954 | Camphene | 2.04 | MH |
| 3 | 9.36 | 960 | 960 | Thuja-2,4(10)-diene | 0.68 | MH |
| 4 | 10.38 | 987 | 990 | β-Myrcene | 0.22 | MH |
| 5 | 11.04 | 998 | 1130 | 2,6-Dimethyl-1,3,5,7-octatetraene Unknown & | 0.53 | other |
| 6 | 11.80 | 1021 | 1024 | p-cymene | 0.19 | MH |
| 7 | 11.99 | 1024 | 1029 | Limonene | 1.13 | MH |
| 8 | 12.09 | 1029 | 1031 | Eucalyptol | 6.93 | OM |
| 9 | 13.14 | 1057 | 1059 | γ-Terpinene | Trace * | MH |
| 11 | 13.68 | 1069 | 1072 | cis-Linalool oxide (furanoid) | Trace * | OM |
| 12 | 14.3 | 1083 | 1086 | trans-Linalool oxide (furanoid) | Trace * | OM |
| 13 | 14.69 | 1088 | 1086 | Fenchone | 18.15 | OM |
| 14 | 15.99 | 1122 | 1116 (endo) or 1121 (exo) | Fenchol ** | Trace * | OM |
| 15 | 16.29 | 1129 | 1126 | α-Campholene aldehyde | Trace * | OM |
| 16 | 17.40 | 1151 | 1146 | Camphor | 58.38 | OM |
| 17 | 18.46 | 1175 | 1169 | Borneol | 0.12 | OM |
| 18 | 18.69 | 1181 | 1177 | Terpinen-4-ol | Trace * | OM |
| 19 | 19.38 | 1195 | 1188 | α-Terpineol | 0.13 | OM |
| 21 | 19.53 | 1197 | 1195 | Myrtenal | 0.45 | OM |
| 22 | 20.10 | 1210 | 1205 | Verbenone | 0.33 | OM |
| 23 | 1214 | 1220 | Fenchyl acetate ** | Trace * | Mester | |
| 24 | 21.71 | 1248 | 1243 | Carvone | 0.17 | OM |
| 25 | 23.13 | 1279 | 1288 | Bornyl acetate | 1.40 | Mester |
| 26 | 24.81 | 1318 | 1326 | Myrtenyl acetate | 0.40 | Mester |
| 27 | 25.66 | 1338 | 1348 | α-Cubebene | Trace * | HS |
| 28 | 26.79 | 1363 | 1376 | Isoledene? | 0.23 | HS |
| 29 | 27.00 | 1368 | 1376 | α-Copaene | 0.10 | HS |
| 30 | 27.23 | 1373 | 1544 | Isolongifolene-4,5,9,10-dehydro?? Unknown & | 0.37 | HS |
| 31 | 31.07 | 1465 | 1476 | Cadina-1(6),4-diene, trans | 0.21 | HS |
| 32 | 31.84 | 1484 | 1496 | Viridiflorene (syn. Ledene) | 0.93 | HS |
| 33 | 32.15 | 1490 | 1500 | α-Muurolene | 0.41 | HS |
| 34 | 32.35 | 1496 | 1517 | α-dehydro Himachalene? | Trace * | HS |
| 35 | 32.92 | 1509 | 1523 | δ-Cadinene | 0.74 | HS |
| 36 | 33.19 | 1516 | 1529 | cis-Calamenene | 0.21 | HS |
| 37 | 33.59 | 1527 | 1534 | trans-Cadina-1,4-diene | 0.24 | HS |
| 38 | 34.04 | 1538 | 1545 | α-Calacorene | 0.35 | HS |
| 39 | 36.99 | 1615 | 1623 | α-Corocalene | 0.10 | HS |
| 40 | 39.21 | 1673 | 1676 | Cadalene | 0.10 | HS |
| Traces * | 0.36 | |||||
| Total (without traces = 0.36%) | 95.79 |
| Concentrations (%) | Mortality Rates after Exposure (%) (Mean ± SE) | ||
|---|---|---|---|
| 1 min | 3 min | 5 min | |
| 0.025% | 33.66 ± 1.52 c | 50.4 ± 1.24 c | 88.07 ± 1.33 b |
| 0.05 % | 41.07 ± 1.32 b | 72.47 ± 1.76 b | 98.17 ± 1.04 a |
| 0.1 % | 74.7 ± 1.99 a | 95.5 ± 1.19 a | 100.00 ± 0.00 a |
| Control | 3.03 ± 0.29 d | 4.1 ± 0.32 d | 5.2 ± 0.23 c |
| Concentrations % | Mortality % M ± SE | Egg Production Index (EPI) | LC50 (95% CL) |
LC90 (95% CL) |
χ2 (df = 3) | p |
|---|---|---|---|---|---|---|
| 0.625 | 0.00 ± 0.00 e | 35.83 ± 0.60 b | 2.34 (2.13–2.57) | 5.00 (4.39–5.89) | 3.861 | 0.277 |
| 1.25 | 16.66 ± 3.33 d | 31.80 ± 1.80 c | ||||
| 2.5 | 56.67 ± 6.67 c | 26.00 ± 0.58 d | ||||
| 5 | 86.66 ± 3.33 b | 24.33 ± 0.67 d | ||||
| 10 | 100.00 ± 0.00 a | 0.00 ± 0.00 e | ||||
| Deltamethrin 2 uL/mL | 26.67 ± 3.33 c | 23.33 ± 0.88 d | - | - | - | - |
| Ethyl alcohol 70% | 0.00 ± 0.00 e | 43.00 ± 1.00 a | - | - | - | - |
| Concentrations % | Mortality % M ± SE | LC50 (95% CL) | LC90 (95% CL) | χ2 (df = 3) | p |
|---|---|---|---|---|---|
| 0.625 | 6.67 ± 1.67 f | 3.82 (3.32–4.45) | 15.53 (11.94–22.07) | 5.43 | 0.143 |
| 1.25 | 15.00 ± 2.89 e | ||||
| 2.5 | 35.00 ± 2.88 c | ||||
| 5 | 51.66 ± 1.67 b | ||||
| 10 | 86.67 ± 1.66 a | ||||
| Deltamethrin 2 uL/mL | 22.66 ± 2.66 d | - | - | - | - |
| Ethyl alcohol 70% | 5.33 ± 1.66 f | - | - | - | - |
| Concentrations % | Mortality % (Mean ± SE) | LC50 (95% CL) | LC90 (95% CL) | χ2 (df = 3) | p |
|---|---|---|---|---|---|
| 0.625 | 6.67 ± 3.33 d | 1.79 (1.62–1.98) | 4.29 (3.72–5.13) | 0.845 | 0.839 |
| 1.25 | 30.00 ± 5.77 c | ||||
| 2.5 | 66.66 ± 3.33 b | ||||
| 5 | 93.33 ± 3.33 a | ||||
| 10 | 100.00 ± 0.00 a | ||||
| Deltamethrin 2 uL/mL | 0.00 ± 0.00 d | - | - | - | - |
| Ethyl alcohol 70% | 0.00 ± 0.00 d | - | - | - | - |
| Concentrations % | PIR % (Mean ± SE) | LC50 (95% CL) | LC90 (95% CL) | χ2 (df = 3) | p |
|---|---|---|---|---|---|
| 0.625 | 13.33 ± 3.33 d | 1.51 (1.35–1.68) | 3.94 (3.38–4.78) | 1.667 | 0.644 |
| 1.25 | 36.66 ± 6.67 c | ||||
| 2.5 | 76.67 ± 3.33 b | ||||
| 5 | 93.33 ± 6.66 a | ||||
| 10 | 100.00 ± 0.00 a | ||||
| Deltamethrin 2 uL/mL | 0.00 ± 0.00 e | - | - | - | - |
| Ethyl alcohol 70% | 0.00 ± 0.00 e | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Baki, A.-A.S.; Aboelhadid, S.M.; Al-Quraishy, S.; Hassan, A.O.; Daferera, D.; Sokmen, A.; Kamel, A.A. Cytotoxic, Scolicidal, and Insecticidal Activities of Lavandula stoechas Essential Oil. Separations 2023, 10, 100. https://doi.org/10.3390/separations10020100
Abdel-Baki A-AS, Aboelhadid SM, Al-Quraishy S, Hassan AO, Daferera D, Sokmen A, Kamel AA. Cytotoxic, Scolicidal, and Insecticidal Activities of Lavandula stoechas Essential Oil. Separations. 2023; 10(2):100. https://doi.org/10.3390/separations10020100
Chicago/Turabian StyleAbdel-Baki, Abdel-Azeem S., Shawky M. Aboelhadid, Saleh Al-Quraishy, Ahmed O. Hassan, Dimitra Daferera, Atalay Sokmen, and Asmaa A. Kamel. 2023. "Cytotoxic, Scolicidal, and Insecticidal Activities of Lavandula stoechas Essential Oil" Separations 10, no. 2: 100. https://doi.org/10.3390/separations10020100
APA StyleAbdel-Baki, A.-A. S., Aboelhadid, S. M., Al-Quraishy, S., Hassan, A. O., Daferera, D., Sokmen, A., & Kamel, A. A. (2023). Cytotoxic, Scolicidal, and Insecticidal Activities of Lavandula stoechas Essential Oil. Separations, 10(2), 100. https://doi.org/10.3390/separations10020100







