Effect of Extraction Methods on Essential Oil Composition: A Case Study of Irish Bog Myrtle-Myrica gale L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals and Reagents
2.1.2. Myrica gale Plant Material
2.2. Methods
2.2.1. Microwave-Assisted Hydrodistillation
2.2.2. Clevenger Hydrodistillation
2.2.3. Gas Chromatography–Mass Spectrometry (GC-MS)
2.2.4. Data Analysis and Component Identification
2.2.5. Multivariate Data Analysis and Molecular Networking
3. Results and Discussion
3.1. Chemical Profiles of Essential Oils and Volatiles from Plant Tissues
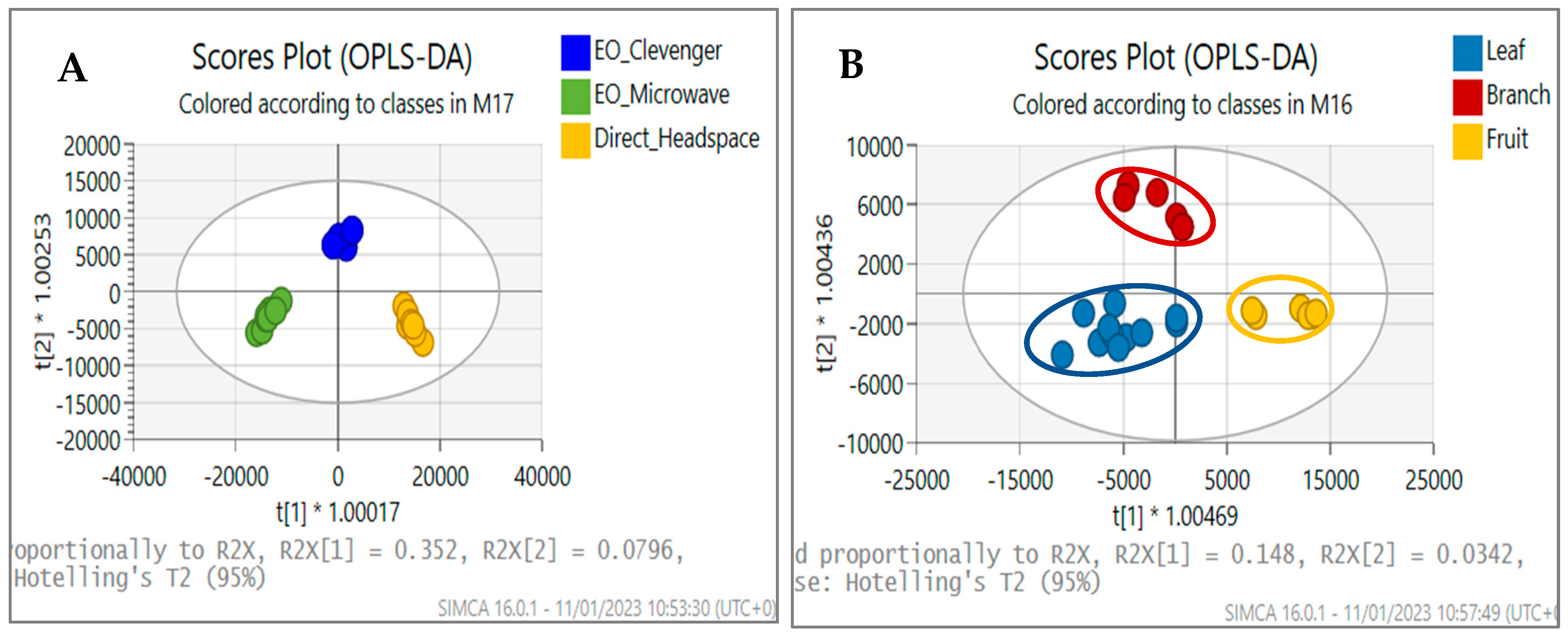
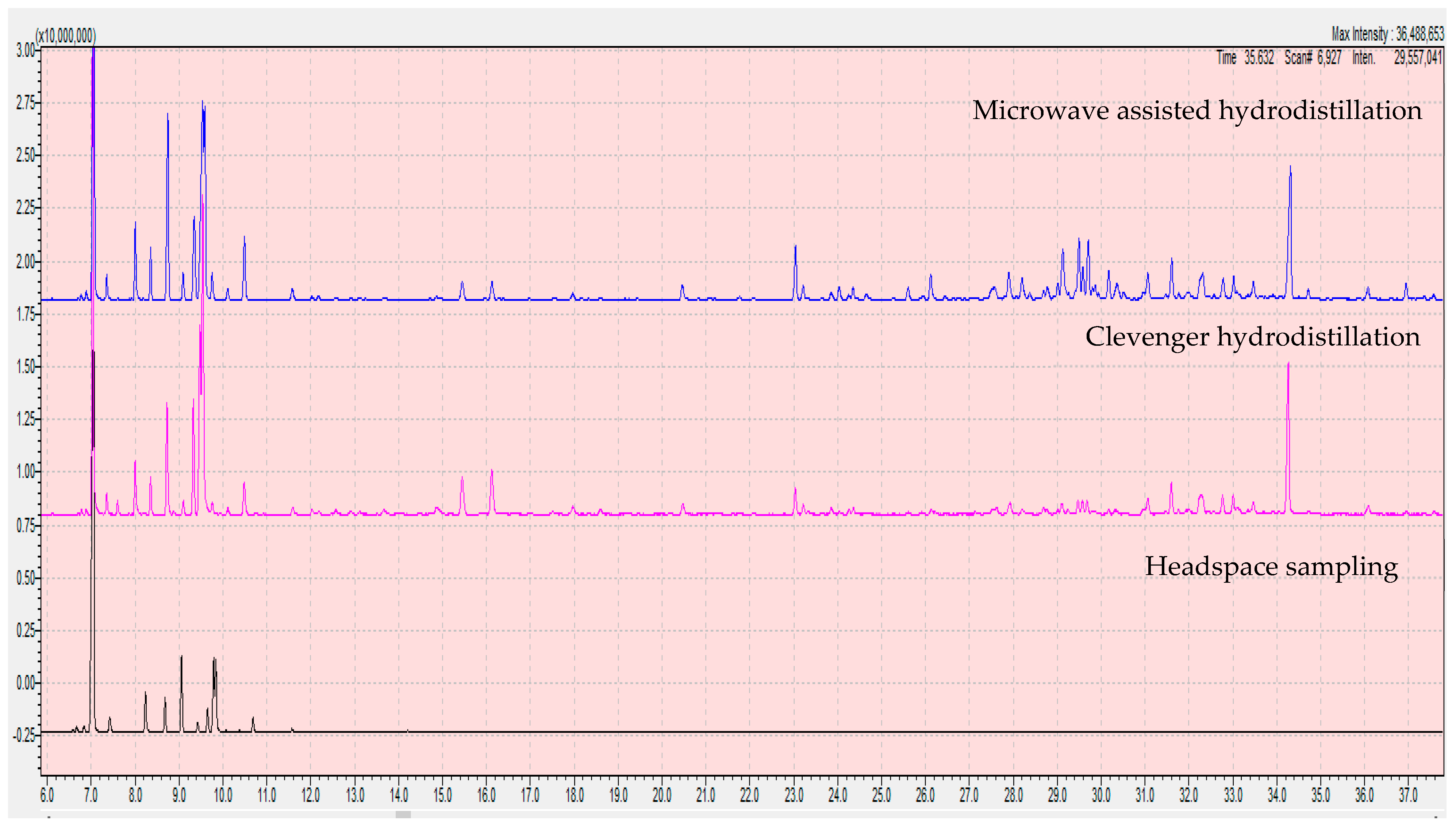
3.2. Comparison of Extraction Methods
3.3. Comparison of Plant Tissues and Collection Season
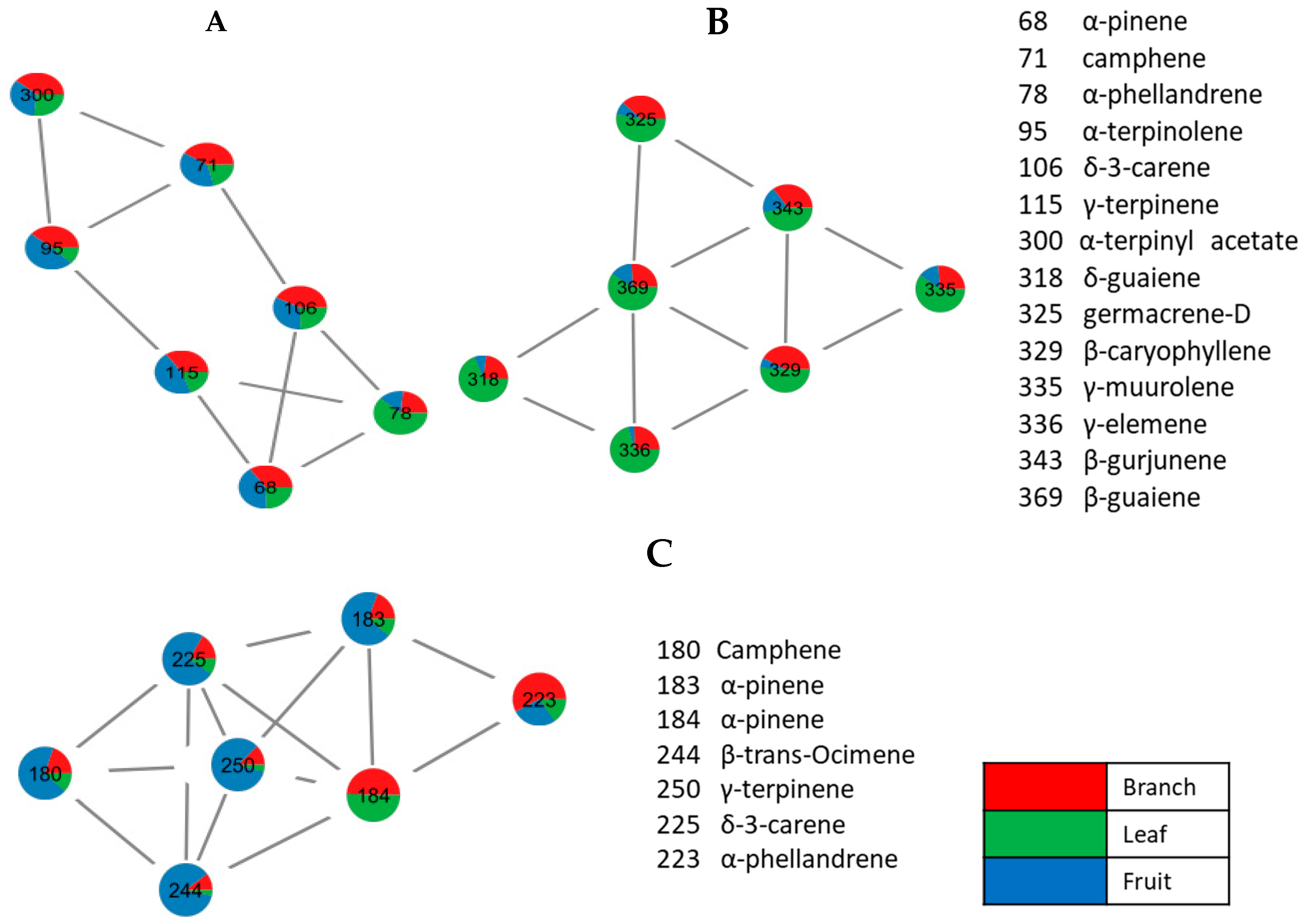
3.4. Molecular Networking
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fay, M.F. Ireland’s Generous Nature by Peter Wyse Jackson; Missouri Botanical Garden Press: St. Louis, MO, USA, 2014; pp. 19–410. [Google Scholar]
- Hart, H.C. Flora of the County Donegal: List of the Flowering Plants and Ferns with Their Localities and Distribution; Sealy, Bryers and Walker: Dublin, Ireland, 1898. [Google Scholar]
- Williams, N. Díolaim Luibheanna; Sáirséal-Ó Marcaigh: Dublin, Ireland, 1993; p. 195. [Google Scholar]
- Allen, D.E.; Hatfield, G. Medicinal Plants in Folk Tradition: An Ethnobotany of Britain & Ireland; Timber Press: Portland, OR, USA, 2004. [Google Scholar]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Oluwaseun Ademiluyi, A.; et al. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential Oils as Ecofriendly Biopesticides? Challenges and Constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Al-Maharik, N.; Jaradat, N.; Hawash, M.; Al-Lahham, S.; Qadi, M.; Shoman, I.; Jaber, S.; Rahem, R.A.; Hussein, F.; Issa, L. Chemical Composition, Antioxidant, Antimicrobial and Anti-Proliferative Activities of Essential Oils of Rosmarinus officinalis from five Different Sites in Palestine. Separations 2022, 9, 339. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Tandon, S. 7 Distillation Technology for Essential Oils. In Extraction Technologies for Medicinal and Aromatic Plants; International Center for Science and High Technology: Trieste, Italy, 2008; p. 115. [Google Scholar]
- Ložienė, K.; Labokas, J.; Vaičiulytė, V.; Švedienė, J.; Raudonienė, V.; Paškevičius, A.; Šveistytė, L.; Apšegaitė, V. Chemical composition and antimicrobial activity of fruit essential oils of Myrica gale, a neglected non-wood forest product. Balt. For. 2020, 26, 1–8. [Google Scholar] [CrossRef]
- Carlton, R.R.; Waterman, P.G.; Gray, A.I. Variation of leaf gland volatile oil within a population of sweet gale (Myrica gale) (Myricaceae). Chemoecology 1992, 3, 45–54. [Google Scholar] [CrossRef]
- Skene, K.R.; Sprent, J.I.; Raven, J.A.; Herdman, L. Myrica gale L. J. Ecol. 2000, 88, 1079–1094. [Google Scholar] [CrossRef]
- Wawrzynczak, K.; Sadowska, B.; Więckowska-Szakiel, M.; Kalemba, D. Composition and Antimicrobial Activity of Myrica gale L. Leaf and Flower Essential Oils and Hydrolates. Rec. Nat. Prod. 2021, 15, 35–45. [Google Scholar] [CrossRef]
- Jaenson, T.G.; Pålsson, K.; Borg-Karlson, A.K. Evaluation of extracts and oils of tick-repellent plants from Sweden. Med. Vet. Entomol. 2005, 19, 345–352. [Google Scholar] [CrossRef]
- Azwanida, N.N. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med. Aromat. Plants 2015, 4, 1–6. [Google Scholar]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Q.; Kong, D.-X.; Wu, H. Analysis and evaluation of essential oil components of cinnamon barks using GC–MS and FTIR spectroscopy. Ind. Crop. Prod. 2013, 41, 269–278. [Google Scholar] [CrossRef]
- Luque de Castro, M.D.; Jimenez-Carmona, M.M.; Fernandez-Perez, V. Towards more rational techniques for the isolation of valuable essential oils from plants. TrAC Trends Anal. Chem. 1999, 18, 708–716. [Google Scholar] [CrossRef]
- Wawrzyńczak, K.; Jakiel, A.; Kalemba, D. Composition of leaf and flower essential oil of Myrica gale L. Biotechnol. Food Sci. 2019, 83, 87–96. [Google Scholar]
- Sylvestre, M.; Legault, J.; Dufour, D.; Pichette, A. Chemical composition and anticancer activity of leaf essential oil of Myrica gale L. Phytomedicine 2005, 12, 299–304. [Google Scholar] [CrossRef]
- Hung, N.H.; Quan, P.M.; Satyal, P.; Dai, D.N.; Van Hoa, V.; Huy, N.G.; Giang, L.D.; Ha, N.T.; Huong, L.T.; Hien, V.T.; et al. Acetylcholinesterase Inhibitory Activities of Essential Oils from Vietnamese Traditional Medicinal Plants. Molecules 2022, 27, 7092. [Google Scholar] [CrossRef]
- Miyazawa, M.; Yamafuji, C. Inhibition of Acetylcholinesterase Activity by Bicyclic Monoterpenoids. J. Agric. Food Chem. 2005, 53, 1765–1768. [Google Scholar] [CrossRef] [PubMed]
- Perry, N.S.; Houghton, P.J.; Theobald, A.; Jenner, P.; Perry, E.K. In-vitro inhibition of human erythrocyte acetyl-cholinesterase by Salvia lavandulaefolia essential oil and constituent terpenes. J. Pharm. Pharmacol. 2000, 52, 895–902. [Google Scholar] [CrossRef]
- Seo, S.-M.; Jung, C.-S.; Kang, J.; Lee, H.-R.; Kim, S.-W.; Hyun, J.; Park, I.-K. Larvicidal and Acetylcholinesterase Inhibitory Activities of Apiaceae Plant Essential Oils and Their Constituents against Aedes albopictus and Formulation Development. J. Agric. Food Chem. 2015, 63, 9977–9986. [Google Scholar] [CrossRef] [PubMed]
- Pigott, M.; Nagar, S.; Woulfe, I.; Scalabrino, G.; Sheridan, H. Unlocking Nature’s Pharmacy: Composition and bioactivity of essential oil of bog-myrtle (Myrica gale) grown on Irish boglands. Planta Med. 2022, 88, 236. [Google Scholar]
- Triba, M.N.; Le Moyec, L.; Amathieu, R.; Goossens, C.; Bouchemal, N.; Nahon, P.; Rutledge, D.N.; Savarin, P. PLS/OPLS models in metabolomics: The impact of permutation of dataset rows on the K-fold cross-validation quality parameters. Mol. Biosyst. 2014, 11, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Sithersingh, M.J.; Snow, N.H. Chapter 9—Headspace gas chromatography. In Handbooks in Separation Science; Elsevier: Amsterdam, The Netherlands, 2021; Volume 2, pp. 251–265. [Google Scholar]
- Bousbia, N.; Abert Vian, M.; Ferhat, M.A.; Petitcolas, E.; Meklati, B.Y.; Chemat, F. Comparison of two isolation methods for essential oil for rosemary leaves. Hydrodistillation and micro hydrodiffusion and gravity. Food Chem. 2009, 114, 355–362. [Google Scholar] [CrossRef]
- Handa, S.S. An Overview of Extraction Techniques for Medicinal and Aromatic Plants. Extr. Tech. Med. Aromat. Plants 2008, 1, 21–54. [Google Scholar]
- Trautman, E.P.; Crawford, J.M. Linking biosynthetic gene clusters to their metabolites via pathway-targeted molecular networking. Curr. Top. Med. Chem. 2016, 16, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
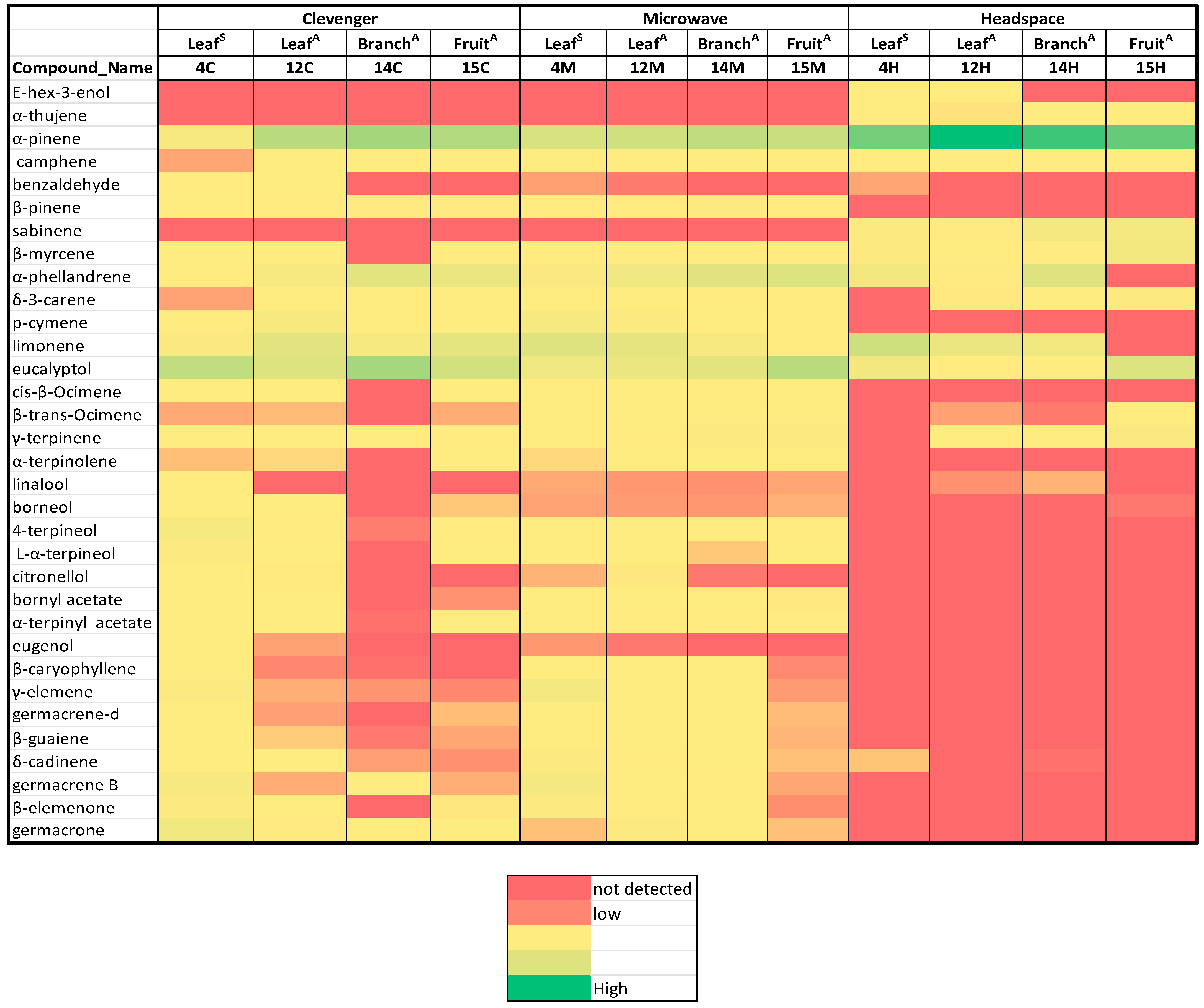
| Sample ID | Collection Date | Collection Season | Plant Part | Extraction Method | Essential Oil Code or Headspace Result Code |
|---|---|---|---|---|---|
| NTP0371 | 27 June 2022 | Summer | Leaves | MAH | 4M |
| NTP0371 | 27 June 2022 | Summer | Leaves | CH | 4C |
| NTP0371 | 27 June 2022 | Summer | Leaves | Direct (headspace) | 4H |
| NTP0379A | 3 October 2022 | Autumn | Leaves | MAH | 12M |
| NTP0379A | 3 October 2022 | Autumn | Leaves | CH | 12C |
| NTP0379A | 3 October 2022 | Autumn | Leaves | Direct (headspace) | 12H |
| NTP0379C | 3 October 2022 | Autumn | Branches | MAH | 14M |
| NTP0379C | 3 October 2022 | Autumn | Branches | CH | 14C |
| NTP0379C | 3 October 2022 | Autumn | Branches | Direct (headspace) | 14H |
| NTP0380 | 21 October 2022 | Autumn | Fruit | MAH | 15M |
| NTP0380 | 21 October 2022 | Autumn | Fruit | CH | 15C |
| NTP0380 | 21 October 2022 | Autumn | Fruit | Direct (headspace) | 15H |
| Clevenger | Microwave | Headspace | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaf S | Leaf A | Branch A | Fruit A | Leaf S | Leaf A | Branch A | Fruit A | Leaf S | Leaf A | Branch A | Fruit A | ||||
| S.No | Compound Name | RI* | 4C | 12C | 14C | 15C | 4M | 12M | 14M | 15M | 4H | 12H | 14H | 15H | Compound Type |
| 1 | E-hex-3-enol | 853 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 1.07 | 0.64 | n.d. | n.d. | OC |
| 2 | hexanol | 869 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | OC |
| 3 | α-thujene | 927 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 1.48 | 0.47 | 0.52 | 0.96 | MH |
| 4 | α-pinene | 937 | 6.04 | 28.16 | 35.03 | 30.69 | 17.37 | 19.93 | 25.47 | 22.82 | 48.56 | 70.45 | 60.80 | 52.96 | MH |
| 5 | camphene | 946 | 0.24 | 1.14 | 1.06 | 1.29 | 0.69 | 1.04 | 1.62 | 1.22 | 1.35 | 2.03 | 2.47 | 3.36 | MH |
| 6 | benzaldehyde | 957 | 2.09 | 0.61 | n.d. | n.d. | 0.21 | 0.07 | n.d. | n.d. | 0.23 | n.d. | n.d. | n.d. | OC |
| 7 | β-pinene | 974 | 1.04 | 2.86 | 3.67 | 3.81 | 2.78 | 3.26 | 5.13 | 3.19 | n.d. | n.d. | n.d. | n.d. | MH |
| 8 | sabinene | 976 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 5.17 | 3.96 | 7.17 | 7.49 | MH |
| 9 | β-myrcene | 987 | 1.67 | 1.81 | n.d. | 2.91 | 2.45 | 2.13 | 2.84 | 3.32 | 4.34 | 1.98 | 3.10 | 8.42 | MH |
| 10 | α-phellandrene | 1004 | 2.33 | 6.00 | 13.95 | 10.74 | 5.47 | 9.05 | 14.55 | 15.61 | 8.39 | 3.87 | 15.05 | n.d. | MH |
| 11 | δ-3-carene | 1015 | 0.23 | 0.73 | 0.95 | 1.89 | 0.54 | 1.22 | 2.58 | 2.81 | n.d. | 0.50 | 0.56 | 4.37 | MH |
| 12 | p-cymene | 1022 | 3.22 | 6.17 | 1.14 | 1.74 | 6.62 | 4.93 | 1.28 | 2.42 | n.d. | n.d. | n.d. | n.d. | MH |
| 13 | limonene | 1026 | 5.88 | 14.09 | 6.37 | 12.67 | 14.90 | 12.97 | 6.19 | 2.27 | 20.73 | 10.79 | 8.67 | n.d. | MH |
| 14 | eucalyptol | 1030 | 25.32 | 16.05 | 33.80 | 19.36 | 9.36 | 10.72 | 13.09 | 28.02 | 8.12 | 3.51 | 0.61 | 16.01 | OM |
| 15 | cis-β-ocimene | 1034 | 0.52 | 0.61 | n.d. | 0.59 | 1.26 | 1.21 | 2.67 | 1.51 | n.d. | n.d. | n.d. | n.d. | MH |
| 16 | β-trans-ocimene | 1047 | 0.25 | 0.32 | n.d. | 0.26 | 0.56 | 0.53 | 0.83 | 0.60 | n.d. | 0.22 | 0.06 | 0.89 | MH |
| 17 | γ-terpinene | 1055 | 1.01 | 2.03 | 0.55 | 3.32 | 1.70 | 3.29 | 4.17 | 4.75 | n.d. | 1.31 | 0.58 | 5.38 | MH |
| 18 | acetophenone | 1067 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | OC |
| 19 | α-terpinolene | 1087 | 0.34 | 0.43 | n.d. | 0.91 | 0.44 | 0.68 | 1.28 | 1.30 | n.d. | n.d. | n.d. | n.d. | MH |
| 20 | linalool | 1099 | 1.20 | n.d. | n.d. | n.d. | 0.25 | 0.18 | 0.15 | 0.23 | n.d. | 0.15 | 0.30 | n.d. | OM |
| 21 | nonanal | 1105 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | tr | tr | OC |
| 22 | allo-ocimene | 1130 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | tr | n.d. | n.d. | tr | MH |
| 23 | borneol | 1161 | 1.87 | 0.60 | n.d. | 0.37 | 0.23 | 0.20 | 0.18 | 0.28 | n.d. | n.d. | n.d. | 0.05 | OM |
| 24 | 4-terpineol | 1173 | 6.72 | 3.17 | 0.08 | 2.51 | 1.35 | 1.36 | 1.51 | 2.89 | n.d. | n.d. | n.d. | n.d. | OM |
| 25 | α-terpineol | 1188 | 4.58 | 1.90 | n.d. | 1.34 | 0.72 | 0.60 | 0.37 | 0.85 | n.d. | n.d. | n.d. | n.d. | OM |
| 26 | methyl salicylate | 1192 | 0.06 | 0.12 | n.d. | n.d. | 0.10 | 0.09 | tr | n.d. | 0.11 | n.d. | n.d. | n.d. | OC |
| 27 | α-fenchyl acetate | 1220 | 0.08 | n.d. | n.d. | n.d. | tr | 0.10 | 0.11 | 0.07 | n.d. | n.d. | n.d. | n.d. | OM |
| 28 | citronellol | 1229 | 0.94 | 0.51 | n.d. | n.d. | 0.29 | 0.49 | 0.05 | n.d. | n.d. | n.d. | n.d. | n.d. | OM |
| 29 | bornyl acetate | 1287 | 0.67 | 0.80 | n.d. | 0.16 | 0.57 | 1.05 | 1.05 | 0.50 | n.d. | n.d. | n.d. | n.d. | OM |
| 30 | 2-undecanone | 1293 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.16 | n.d. | n.d. | n.d. | n.d. | OC |
| 31 | methyl geranate | 1326 | n.d. | n.d. | n.d. | n.d. | tr | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | OM |
| 32 | carveol acetate | 1338 | tr | tr | n.d. | tr | tr | tr | tr | tr | n.d. | n.d. | n.d. | n.d. | OM |
| 33 | α-terpinyl acetate | 1349 | 1.51 | 1.80 | tr | 1.75 | 1.58 | 3.26 | 3.38 | 2.53 | n.d. | n.d. | n.d. | n.d. | OM |
| 34 | citronellol acetate | 1354 | 0.13 | 0.61 | n.d. | 0.61 | 0.28 | 0.82 | 0.27 | 0.15 | n.d. | n.d. | n.d. | n.d. | OM |
| 35 | eugenol | 1356 | 1.84 | 0.22 | n.d. | n.d. | 0.18 | 0.05 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | OC |
| 36 | neryl acetate | 1365 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | OM |
| 37 | α-copaene | 1375 | 0.17 | 0.20 | 0.06 | n.d. | 0.68 | 0.83 | 0.26 | 0.08 | n.d. | n.d. | n.d. | n.d. | SH |
| 38 | methyl cinnamate | 1381 | 0.67 | 0.34 | n.d. | n.d. | 0.21 | 0.25 | 0.15 | 0.09 | n.d. | n.d. | n.d. | n.d. | OC |
| 39 | geranyl acetate | 1383 | tr | 0.32 | n.d. | n.d. | 0.15 | 0.66 | 0.25 | n.d. | n.d. | n.d. | n.d. | n.d. | OM |
| 40 | tetradecene | 1394 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | tr | n.d. | n.d. | n.d. | OC |
| 41 | E-β-damascone | 1413 | tr | tr | n.d. | n.d. | tr | tr | tr | n.d. | n.d. | n.d. | n.d. | n.d. | OC |
| 42 | β-caryophyllene | 1418 | 0.74 | 0.12 | tr | n.d. | 1.45 | 0.76 | 0.77 | 0.13 | n.d. | n.d. | n.d. | n.d. | SH |
| 43 | γ-elemene | 1433 | 4.95 | 0.26 | 0.17 | 0.12 | 7.43 | 1.55 | 1.40 | 0.19 | n.d. | n.d. | n.d. | n.d. | SH |
| 44 | β-gurjunene | 1443 | 0.15 | 0.05 | n.d. | n.d. | 0.21 | 0.21 | 0.14 | 0.07 | n.d. | n.d. | n.d. | n.d. | SH |
| 45 | humulene | 1453 | tr | tr | n.d. | tr | tr | tr | tr | tr | n.d. | n.d. | n.d. | n.d. | SH |
| 46 | 2,6-di-tert-butyl-1,4-benzoquinone | 1465 | 0.52 | tr | n.d. | tr | tr | tr | tr | tr | n.d. | n.d. | n.d. | n.d. | OC |
| 47 | β-selinene | 1476 | 0.20 | 0.08 | n.d. | 0.08 | 0.48 | 0.66 | 0.13 | 0.09 | n.d. | n.d. | n.d. | n.d. | SH |
| 48 | γ-muurolene | 1480 | n.d. | n.d. | n.d. | n.d. | 0.06 | 0.06 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | SH |
| 49 | germacrene-D | 1485 | 1.27 | 0.21 | n.d. | 0.33 | 1.64 | 1.91 | 0.82 | 0.32 | n.d. | n.d. | n.d. | n.d. | SH |
| 50 | β-guaiene | 1494 | 0.82 | 0.39 | 0.06 | 0.24 | 1.89 | 1.43 | 0.61 | 0.30 | n.d. | n.d. | n.d. | n.d. | SH |
| 51 | α-muurolene | 1499 | 0.10 | 0.08 | n.d. | n.d. | 0.27 | 0.34 | 0.12 | n.d. | n.d. | n.d. | n.d. | n.d. | SH |
| 52 | δ-guaiene | 1507 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.06 | n.d. | n.d. | n.d. | n.d. | n.d. | SH |
| 53 | δ-cadinene | 1524 | 1.41 | 0.73 | 0.21 | 0.15 | 4.12 | 3.59 | 1.52 | 0.34 | 0.36 | n.d. | tr | n.d. | SH |
| 54 | germacrene B | 1557 | 5.94 | 0.26 | 0.57 | 0.27 | 6.74 | 1.67 | 2.41 | 0.24 | n.d. | n.d. | n.d. | n.d. | SH |
| 55 | β-elemenone | 1604 | 4.54 | 2.05 | n.d. | 0.50 | 4.28 | 2.29 | 0.82 | 0.14 | n.d. | n.d. | n.d. | n.d. | OS |
| 56 | germacrone | 1696 | 8.62 | 4.08 | 1.93 | 1.33 | 0.34 | 4.40 | 1.59 | 0.34 | n.d. | n.d. | n.d. | n.d. | OS |
| 57 | octyl octanoate | 1778 | n.d. | tr | tr | n.d. | tr | tr | tr | n.d. | n.d. | n.d. | n.d. | n.d. | OC |
| 58 | 6,10,14-trimethyl-pentadecan-2-one | 1844 | tr | tr | tr | n.d. | tr | 0.07 | tr | n.d. | n.d. | n.d. | n.d. | n.d. | OS |
| Monoterpene hydrocarbons | 22.77 | 64.35 | 62.70 | 70.81 | 54.78 | 60.25 | 68.63 | 61.82 | 90.01 | 95.58 | 98.98 | 83.83 | |||
| Oxygenated monoterpenes | 43.02 | 25.44 | 33.91 | 26.09 | 14.66 | 18.77 | 20.17 | 35.53 | 8.12 | 3.65 | 0.91 | 16.06 | |||
| Sesquiterpene hydrocarbons | 15.76 | 2.38 | 1.10 | 1.18 | 24.99 | 13.01 | 8.24 | 1.75 | 0.36 | 0.00 | 0.03 | 0.00 | |||
| Oxygenated sesquiterpenes | 13.16 | 6.13 | 1.93 | 1.82 | 4.63 | 6.77 | 2.41 | 0.48 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| Other compounds | 5.21 | 1.62 | 0.00 | 0.00 | 0.85 | 1.13 | 0.42 | 0.25 | 1.42 | 0.64 | 0.00 | 0.00 | |||
| Total | 99.92 | 99.92 | 99.64 | 99.90 | 99.90 | 99.93 | 99.86 | 99.84 | 99.92 | 99.87 | 99.92 | 99.89 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagar, S.; Pigott, M.; Whyms, S.; Berlemont, A.; Sheridan, H. Effect of Extraction Methods on Essential Oil Composition: A Case Study of Irish Bog Myrtle-Myrica gale L. Separations 2023, 10, 128. https://doi.org/10.3390/separations10020128
Nagar S, Pigott M, Whyms S, Berlemont A, Sheridan H. Effect of Extraction Methods on Essential Oil Composition: A Case Study of Irish Bog Myrtle-Myrica gale L. Separations. 2023; 10(2):128. https://doi.org/10.3390/separations10020128
Chicago/Turabian StyleNagar, Shipra, Maria Pigott, Sophie Whyms, Apolline Berlemont, and Helen Sheridan. 2023. "Effect of Extraction Methods on Essential Oil Composition: A Case Study of Irish Bog Myrtle-Myrica gale L." Separations 10, no. 2: 128. https://doi.org/10.3390/separations10020128
APA StyleNagar, S., Pigott, M., Whyms, S., Berlemont, A., & Sheridan, H. (2023). Effect of Extraction Methods on Essential Oil Composition: A Case Study of Irish Bog Myrtle-Myrica gale L. Separations, 10(2), 128. https://doi.org/10.3390/separations10020128







