Effect of Hydrothermal Conditions on Kenaf-Based Carbon Quantum Dots Properties and Photocatalytic Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Kenaf-Based CQDs
2.3. Physicochemical Characterization
2.4. Quantum Yield Calculation
2.5. Photocatalytic Degradation Setup
2.6. Reaction Kinetics Analysis
3. Results and Discussion
3.1. Chemical Stability Analysis
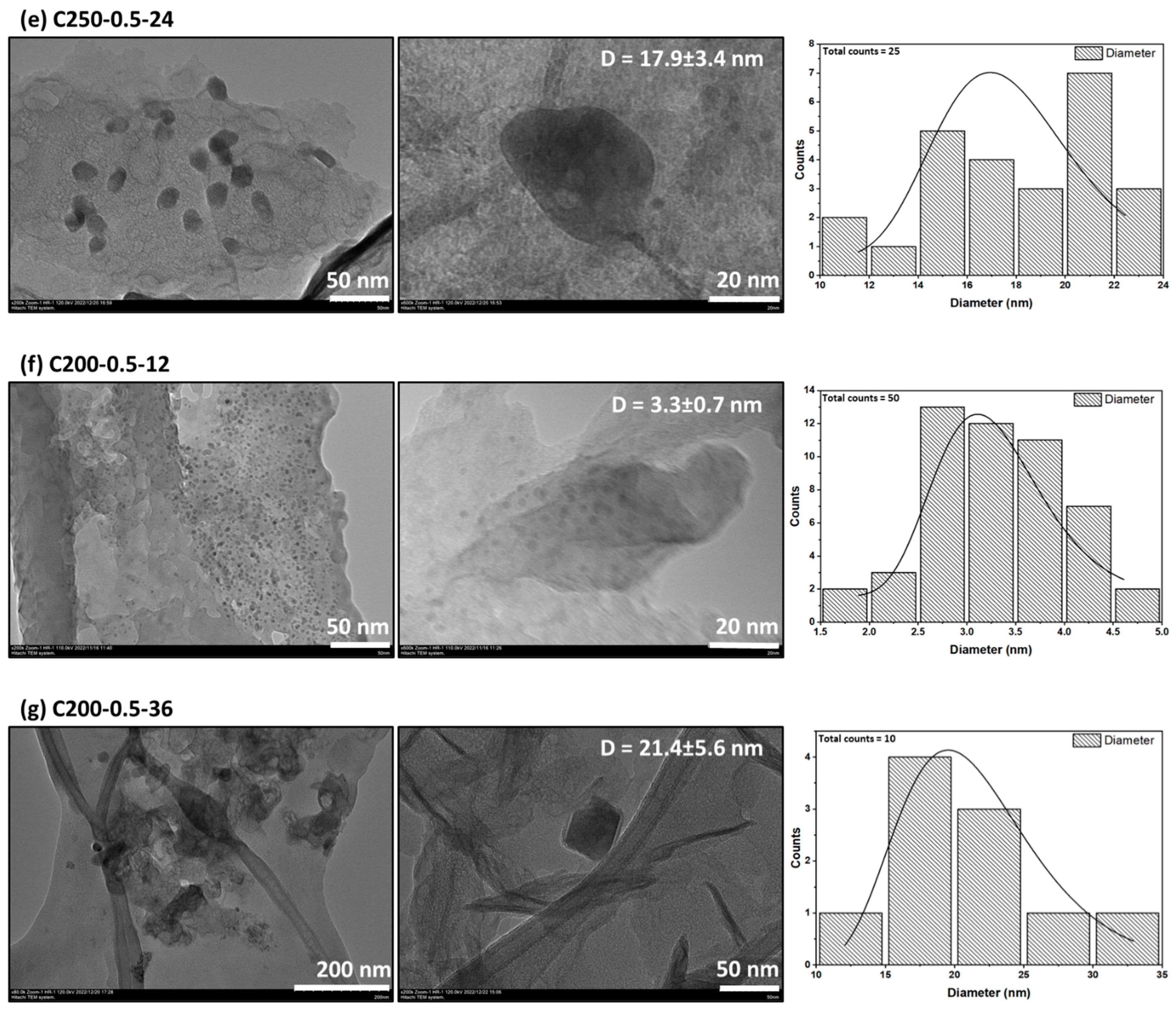
3.2. Morphological Properties
3.3. Optical Properties
3.4. Quantum Yield
3.5. Photocatalytic Degradation Performance of CQDs
3.6. Proposed Photocatalytic Degradation Mechanism
3.7. Reaction Kinetics Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, W.; Sun, M.; Ding, Z.; Gao, B.; Ding, W. Ti3C2 MXene embellished g-C3N4 nanosheets for improving photocatalytic redox capacity. J. Alloys Compd. 2021, 877, 160223. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, J.; Wang, L.; Han, M.; Zhang, M.; Wang, H.; Huang, H.; Liu, Y.; Kang, Z. Carbon dots as solid-state electron mediator for BiVO4/CDs/CdS Z-scheme photocatalyst working under visible light. Appl. Catal. B 2017, 206, 501–509. [Google Scholar] [CrossRef]
- Bajorowicz, B.; Kobyla, M.P.; Go, A.; Nadolna, J.; Zaleska-medynska, A.; Malankowska, A. Quantum dot-decorated semiconductor micro- and nanoparticles: A review of their synthesis, characterization and application in photocatalysis. Adv. Colloid Interface Sci. 2018, 256, 352–372. [Google Scholar] [CrossRef] [PubMed]
- Saafie, N.; Zulfiqar, M.; Samsudin, M.F.R.; Sufian, S. Current Scenario of MXene-Based Nanomaterials for Wastewater Remediation: A Review. Chemistry 2022, 4, 1576–1608. [Google Scholar] [CrossRef]
- Wang, R.; Lu, K.-Q.; Tang, Z.-R.; Xu, Y.-J. Recent progress in carbon quantum dots: Synthesis, properties and applications in photocatalysis. J. Mater. Chem. A Mater. 2017, 5, 3717–3734. [Google Scholar] [CrossRef]
- Li, J.; Ma, Y.; Ye, Z.; Zhou, M.; Wang, H.; Ma, C.; Wang, D.; Huo, P.; Yan, Y. Fast electron transfer and enhanced visible light photocatalytic activity using multi-dimensional components of carbon quantum dots @ 3D daisy-like In2S3/single-wall carbon nanotubes. Appl. Catal. B 2017, 204, 224–238. [Google Scholar] [CrossRef]
- Heng, Z.W.; Chong, W.C.; Pang, Y.L.; Koo, C.H. An overview of the recent advances of carbon quantum dots/metal oxides in the application of heterogeneous photocatalysis in photodegradation of pollutants towards visible-light and solar energy exploitation. J. Environ. Chem. Eng. 2021, 9, 105199. [Google Scholar] [CrossRef]
- Ahuja, V.; Bhatt, A.K.; Varjani, S.; Choi, K.Y.; Kim, S.H.; Yang, Y.H.; Bhatia, S.K. Quantum dot synthesis from waste biomass and its applications in energy and bioremediation. Chemosphere 2022, 293, 133564. [Google Scholar] [CrossRef]
- Chahal, C.; van den Akker, B.; Young, F.; Franco, C.; Blackbeard, J.; Monis, P. Pathogen and Particle Associations in Wastewater: Significance and Implications for Treatment and Disinfection Processes. Adv. Appl. Microbiol. 2016, 97, 63–119. [Google Scholar]
- Boruah, A.; Saikia, M.; Das, T.; Goswamee, R.L.; Saikia, B.K. Blue-emitting fluorescent carbon quantum dots from waste biomass sources and their application in fluoride ion detection in water. J. Photochem. Photobiol. B 2020, 209, 111940. [Google Scholar] [CrossRef]
- Das, R.; Bandyopadhyay, R.; Pramanik, P. Carbon quantum dots from natural resource: A review. Mater. Today Chem. 2018, 8, 96–109. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, P.; Li, X.; Luo, M.; Zhang, W.; Chen, M.; Zhou, X. Green preparation of palm powder-derived carbon dots co-doped with sulfur/chlorine and their application in visible-light photocatalysis. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 227, 117659. [Google Scholar] [CrossRef] [PubMed]
- Wongso, V.; Chung, H.K.; Sambudi, N.S.; Sufian, S.; Abdullah, B.; Wirzal, M.D.H.; Ang, W.L. Silica–carbon quantum dots decorated titanium dioxide as sunlight-driven photocatalyst to diminish acetaminophen from aquatic environment. J. Photochem. Photobiol. A Chem. 2020, 394, 112436. [Google Scholar] [CrossRef]
- Sahoo, N.K.; Jana, G.C.; Aktara, M.N.; Das, S.; Nayim, S.; Patra, A.; Bhattacharjee, P.; Bhadra, K.; Hossain, M. Carbon dots derived from lychee waste: Application for Fe3+ ions sensing in real water and multicolor cell imaging of skin melanoma cells. Mater. Sci. Eng. C 2020, 108, 110429. [Google Scholar] [CrossRef] [PubMed]
- Genc, M.T.; Yanalak, G.; Arslan, G.; Patir, I.H. Green preparation of Carbon Quantum dots using Gingko biloba to sensitize TiO2 for the photohydrogen production. Mater. Sci. Semicond. Process. 2020, 109, 104945. [Google Scholar] [CrossRef]
- Chung Hui, K.; Lun Ang, W.; Soraya Sambudi, N. Nitrogen and bismuth-doped rice husk-derived carbon quantum dots for dye degradation and heavy metal removal. J. Photochem. Photobiol. A Chem. 2021, 418, 113411. [Google Scholar] [CrossRef]
- Chaudhary, N.; Verma, D.; Gopal Sharma, J.; Solanki, P.R. A novel bioinspired carbon quantum dots based optical sensor for ciprofloxacin detection. Mater. Lett. 2022, 308, 131090. [Google Scholar] [CrossRef]
- Wang, T.; Liu, X.; Ma, C.; Zhu, Z.; Liu, Y.; Liu, Z.; Wei, M.; Zhao, X.; Dong, H.; Huo, P.; et al. Bamboo prepared carbon quantum dots (CQDs) for enhancing Bi3Ti4O12 nanosheets photocatalytic activity. J. Alloys Compd. 2018, 752, 106–114. [Google Scholar] [CrossRef]
- Hasija, V.; Sudhaik, A.; Raizada, P.; Hosseini-Bandegharaei, A.; Singh, P. Carbon quantum dots supported AgI/ZnO/phosphorus doped graphitic carbon nitride as Z-scheme photocatalyst for efficient photodegradation of 2,4-dinitrophenol. J. Environ. Chem. Eng. 2019, 7, 103272. [Google Scholar] [CrossRef]
- Khiari, B.; Ghouma, I.; Ibn, A.; Amine, A.; Jellali, S. Kenaf stems: Thermal characterization and conversion for biofuel and biochar production. Fuel 2020, 262, 116654. [Google Scholar] [CrossRef]
- Gan, L.; Fang, X.; Xu, L.; Wang, L.; Wu, Y.; Dai, B.; He, W.; Shi, J. Boosted activity of δ-MnO2 by Kenaf derived carbon fiber for high-efficient oxidative degradation of bisphenol A in water. Mater. Des. 2021, 203, 109596. [Google Scholar] [CrossRef]
- Wongso, V.; Sambudi, N.S.; Sufian, S.; Isnaeni. The effect of hydrothermal conditions on photoluminescence properties of rice husk-derived silica-carbon quantum dots for methylene blue degradation. Biomass Convers. Biorefin. 2021, 11, 2641–2654. [Google Scholar] [CrossRef]
- Liu, N.; Lu, N.; Su, Y.; Wang, P.; Quan, X. Fabrication of g-C3N4/Ti3C2 composite and its visible-light photocatalytic capability for ciprofloxacin degradation. Sep. Purif. Technol. 2019, 211, 782–789. [Google Scholar] [CrossRef]
- Hsiao, P.H.; Timjan, S.; Kuo, K.Y.; Juan, J.C.; Chen, C.Y. Optical management of CQD/AgNP@SiNW arrays with highly efficient capability of dye degradation. Catalysts 2021, 11, 399. [Google Scholar] [CrossRef]
- Jafarov, M.A.; Nasirov, E.F.; Kazımzade, A.H.; Jahangirova, S.A. Synthesis and characterization of nanoscale material ZnS in porous silicon by chemical method. Chalcogenide Lett. 2021, 18, 791–795. [Google Scholar]
- Ţucureanu, V.; Matei, A.; Avram, A.M. FTIR Spectroscopy for Carbon Family Study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.A.; Peng, W.; Zare, Y.; Rhee, K.Y. Effects of Size and Aggregation/Agglomeration of Nanoparticles on the Interfacial/Interphase Properties and Tensile Strength of Polymer Nanocomposites. Nanoscale Res. Lett. 2018, 13, 214. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, J.; Wang, Y.; Yang, Y.; Liu, X.; Xu, B. Facile and Rapid Synthesis of Yellow-Emission Carbon Dots for White Light-Emitting Diodes. J. Electron. Mater. 2018, 47, 7497–7504. [Google Scholar] [CrossRef]
- Pellegrino, F.; Pellutiè, L.; Sordello, F.; Minero, C.; Ortel, E.; Hodoroaba, V.D.; Maurino, V. Influence of agglomeration and aggregation on the photocatalytic activity of TiO2 nanoparticles. Appl. Catal. B 2017, 216, 80–87. [Google Scholar] [CrossRef]
- Yue, X.; Zhu, C.; Gu, R.; Hu, J.; Xu, Y.; Ye, S.; Zhu, J. Copper-Modified Double-Emission Carbon Dots for Rapid Detection of Thiophanate Methyl in Food. Foods 2022, 11, 3336. [Google Scholar] [CrossRef]
- Kainth, S.; Maity, B.; Shetti, N.P.; Basu, S.; Kakarla, R.R. Dual emissive carbon dots: Synthesis strategies, properties and its ratiometric sensing applications. Nano-Struct. Nano-Objects 2023, 33, 100931. [Google Scholar] [CrossRef]
- Jing, S.; Zhao, Y.; Sun, R.C.; Zhong, L.; Peng, X. Facile and High-Yield Synthesis of Carbon Quantum Dots from Biomass-Derived Carbons at Mild Condition. ACS Sustain. Chem. Eng. 2019, 7, 7833–7843. [Google Scholar] [CrossRef]
- Mai, X.D.; Phan, Y.T.H.; Nguyen, V.Q. Excitation-Independent Emission of Carbon Quantum Dot Solids. Adv. Mater. Sci. Eng. 2020, 2020, 9643168. [Google Scholar] [CrossRef]
- Jiang, K.; Feng, X.; Gao, X.; Wang, Y.; Cai, C.; Li, Z.; Lin, H. Preparation of multicolor photoluminescent carbon dots by tuning surface states. Nanomaterials 2019, 9, 529. [Google Scholar] [CrossRef]
- Hoan, B.T.; Tam, P.D.; Pham, V.H. Green Synthesis of Highly Luminescent Carbon Quantum Dots from Lemon Juice. J. Nanotechnol. 2019, 2019, 2852816. [Google Scholar] [CrossRef]
- Devi, S.; Gupta, R.K.; Paul, A.K.; Tyagi, S. Waste carbon paper derivatized Carbon Quantum Dots/(3-Aminopropyl)triethoxysilane based fluorescent probe for trinitrotoluene detection. Mater. Res. Express 2019, 6, 025605. [Google Scholar] [CrossRef]
- Ghezzi, F.; Donnini, R.; Sansonetti, A.; Giovanella, U.; la Ferla, B.; Vercelli, B. Nitrogen-Doped Carbon Quantum Dots for Biosensing Applications: The Effect of the Thermal Treatments on Electrochemical and Optical Properties. Molecules 2022, 28, 72. [Google Scholar] [CrossRef]
- Surendran, P.; Lakshmanan, A.; Vinitha, G.; Ramalingam, G.; Rameshkumar, P. Facile preparation of high fluorescent carbon quantum dots from orange waste peels for nonlinear optical applications. Luminescence 2020, 35, 196–202. [Google Scholar] [CrossRef]
- Lai, C.; Lin, S.; Xiong, L.; Wu, Y.; Liu, C.; Jin, Y. High quantum yield nitrogen-doped carbon quantum dots: Green synthesis and application as “on-off” fluorescent sensors for specific Fe3+ ions detection and cell imaging. Diam. Relat. Mater. 2023, 133, 109702. [Google Scholar] [CrossRef]
- Amjadi, M.; Manzoori, J.L.; Hallaj, T.; Azizi, N. Sulfur and nitrogen co-doped carbon quantum dots as the chemiluminescence probe for detection of Cu2+ ions. J. Lumin. 2017, 182, 246–251. [Google Scholar] [CrossRef]
- Chaudhary, N.; Gupta, P.K.; Eremin, S.; Solanki, P.R. One-step green approach to synthesize highly fluorescent carbon quantum dots from banana juice for selective detection of copper ions. J. Environ. Chem. Eng. 2020, 8, 103720. [Google Scholar] [CrossRef]
- Gengan, S.; Ananda Murthy, H.C.; Sillanpää, M.; Nhat, T. Carbon dots and their application as photocatalyst in dye degradation studies- Mini review. Results Chem. 2022, 4, 100674. [Google Scholar] [CrossRef]
- Baaloudj, O.; Kenfoud, H.; Badawi, A.K.; Assadi, A.A.; el Jery, A.; Assadi, A.A.; Amrane, A. Bismuth Sillenite Crystals as Recent Photocatalysts for Water Treatment and Energy Generation: A Critical Review. Catalysts 2022, 12, 500. [Google Scholar] [CrossRef]
- Jamaludin, N.; Rashid, S.A.; Tan, T. Natural Biomass as Carbon Sources for the Synthesis of Photoluminescent Carbon Dots. In Synthesis, Technology and Applications of Carbon Nanomaterials; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Shahzad, W.; Badawi, A.K.; Rehan, Z.A.; Muhammad, A.; Ali, R.; Shah, F.; Ali, S.; Ismail, B. Enhanced visible light photocatalytic performance of Sr0.3(Ba,Mn)0.7ZrO3 perovskites anchored on graphene oxide. Ceram. Int. 2022, 48, 24979–24988. [Google Scholar] [CrossRef]
- Das, G.S.; Shim, J.P.; Bhatnagar, A.; Tripathi, K.M. Biomass-derived Carbon Quantum Dots for Visible-Light-Induced Photocatalysis and Label-Free Detection of Fe(III) and Ascorbic acid. Sci. Rep. 2019, 9, 15084. [Google Scholar] [CrossRef]
- Liu, J.; Wong, L.M.; Gurudayal; Wong, L.H.; Chiam, S.Y.; Yau Li, S.F.; Ren, Y. Immobilization of dye pollutants on iron hydroxide coated substrates: Kinetics, efficiency and the adsorption mechanism. J. Mater. Chem. A Mater. 2016, 4, 13280–13288. [Google Scholar] [CrossRef]









| CQD Precursor | Method | Precursor Mass (g) | Solvent (Volume) | Temp (°C) | Time (h) | Performances | Ref. |
|---|---|---|---|---|---|---|---|
| Taro peel | Microprocessor-based sonicator | 2.0 | H2O2 (100 mL) | RT | 4 | F−detection in water: (10–160 ppm) | [10] |
| Raw palm veneers | Hydrothermal | 0.1 | H2O (10 mL) + thionyl chloride (200 µL) | 200 | 7 | Degradation of RhB: 71.7% in 45 min | [12] |
| Rice husk | Hydrothermal | 0.1 | H2O (30 mL) | 200 | 10 | Degradation of acetaminophen: 100% in 240 min | [13] |
| Lychee waste | Hydrothermal | 1.0 | H2O + ethanol (10 mL solution) | 180 | 5 | Detection of Fe3+ ions 23.6 nM | [14] |
| Ginkgo biloba leaves | Microwave-assisted irradiation | 1.0 | H2O (50 mL) | RT | 10 min | H2 generation: HER: 665 μmol/g.h | [15] |
| Rice husk | Hydrothermal | 0.5 | H2O (50 mL) | 190 | 4 | Methylene blue degradation: N-QDs 72.16%, Bi-QDs 68.91% within 2 h Copper II ion removal: N-QDs 56.23%, Bi-QDs 33.13% within 1 h | [16] |
| Cinnamomum tamala leaves extract | Hydrothermal | Chopped leaves boiled in 100 mL H2O | Leaves extract (40 mL) | 200 | 12 | Detection of CIP: 1 to 100 μM, the detection limit of 6.06 μM | [17] |
| Sample | Temperature (°C) | Mass (g) | Time (h) |
|---|---|---|---|
| C200-0.1-24 | 200 | 0.1 | 24 |
| C200-0.5-24 | 200 | 0.5 | 24 |
| C200-1.0-24 | 200 | 1.0 | 24 |
| C150-0.5-24 | 150 | 0.5 | 24 |
| C250-0.5-24 | 250 | 0.5 | 24 |
| C200-0.5-12 | 200 | 0.5 | 12 |
| C200-0.5-36 | 200 | 0.5 | 36 |
| Carbon Precursors | Quantum Yield (%) | References |
|---|---|---|
| Raw palm veneers | 0.90 | [12] |
| Rice husk | 20.80 | [22] |
| Lemon juice | 21.37 | [35] |
| Waste carbon paper | 5.10 | [36] |
| Citric acid (CA) and urea | 0.37 | [37] |
| Orange peels | 11.37 | [38] |
| CA and o-phenylenediamine | 33.55 | [39] |
| CA and l-cysteine | 82.00 | [40] |
| Banana juice | 32.00 | [41] |
| Kenaf fiber | 5.00–76.12 | This work |
| Sample | PFO | PSO | ||
|---|---|---|---|---|
| k1 (min−1) | R2 | k2 (min−1) | R2 | |
| C200-0.1-24 | 0.006 | 0.890 | 0.0002 | 0.947 |
| C200-0.5-24 | 0.023 | 0.960 | 0.0049 | 0.740 |
| C200-1.0-24 | 0.009 | 0.946 | 0.0005 | 0.890 |
| C150-0.5-24 | 0.004 | 0.880 | 0.0001 | 0.961 |
| C250-0.5-24 | 0.003 | 0.802 | 0.0001 | 0.873 |
| C200-0.5-12 | 0.007 | 0.826 | 0.0002 | 0.945 |
| C200-0.5-36 | 0.005 | 0.689 | 0.0001 | 0.808 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saafie, N.; Sambudi, N.S.; Wirzal, M.D.H.; Sufian, S. Effect of Hydrothermal Conditions on Kenaf-Based Carbon Quantum Dots Properties and Photocatalytic Degradation. Separations 2023, 10, 137. https://doi.org/10.3390/separations10020137
Saafie N, Sambudi NS, Wirzal MDH, Sufian S. Effect of Hydrothermal Conditions on Kenaf-Based Carbon Quantum Dots Properties and Photocatalytic Degradation. Separations. 2023; 10(2):137. https://doi.org/10.3390/separations10020137
Chicago/Turabian StyleSaafie, Nabilah, Nonni Soraya Sambudi, Mohd Dzul Hakim Wirzal, and Suriati Sufian. 2023. "Effect of Hydrothermal Conditions on Kenaf-Based Carbon Quantum Dots Properties and Photocatalytic Degradation" Separations 10, no. 2: 137. https://doi.org/10.3390/separations10020137
APA StyleSaafie, N., Sambudi, N. S., Wirzal, M. D. H., & Sufian, S. (2023). Effect of Hydrothermal Conditions on Kenaf-Based Carbon Quantum Dots Properties and Photocatalytic Degradation. Separations, 10(2), 137. https://doi.org/10.3390/separations10020137