Abstract
Environmental pollution is a crucial problem in our society, having nowadays a better understanding of its consequences, which include the increase of contaminant cocktails present in the environment. The contamination of honeybees can occur through their interaction with the nearby environment. Therefore, if honeybees are previously contaminated, there is a possibility of contamination of their products, such as honey as natural, or minimally processed, product, resulting from the honeybees’ activity. Considering that honey is a highly consumed product, it is extremely necessary to control its quality and safety, including evaluating the presence and quantification of contaminants, which should follow monitoring studies and the legislation established by the European Union. This work aims to review the literature of different contaminants reported on honey, including pesticides, persistent organic pollutants, polycyclic aromatic hydrocarbons, and pharmaceuticals, focusing on the reports using the QuEChERS technique for the extraction. Furthermore, reports of microplastics on honey samples were also discussed. Despite the existence of several methods that identify and quantify these contaminants, few methods have been reported to operate with different groups of contaminants simultaneously. The development of methods with this characteristic (while being fast, low cost, and with a lower impact on the environment), monitoring studies to identify the risks, and an update on legislation are priority actions and future perspectives to follow.
1. Introduction
1.1. Honey Contaminants: Overview and Legislation
Over the past decades, the European Union (EU) has been witnessing a decrease in wild pollinator occurrence as well as their diversity, which could be caused by land over exploration, poor management of pesticides application, invasive non-native species, environmental pollution, and, consequently, climate change [1]. Roughly 10,000 honeybees, per beehive, maintain interaction with elements in the surrounding area (over 7 km2) [2]. This activity results in a contact with a vast environment that, if contaminated with different types of pollutants, such as pesticides, persistent organic pollutants (POPs), veterinary drugs, pharmaceuticals, and other emergent pollutants (such as microplastics and plastic-related chemicals), may affect their well-being [3]. Therefore, honeybees, due to their specific body composition, can keep and transport the contaminants to the beehive, potentially leading to the contamination of bee products, such as honey. On the other hand, the inappropriate use of acaricides in the treatment of beehives during honey collection may lead to cross contamination [4].
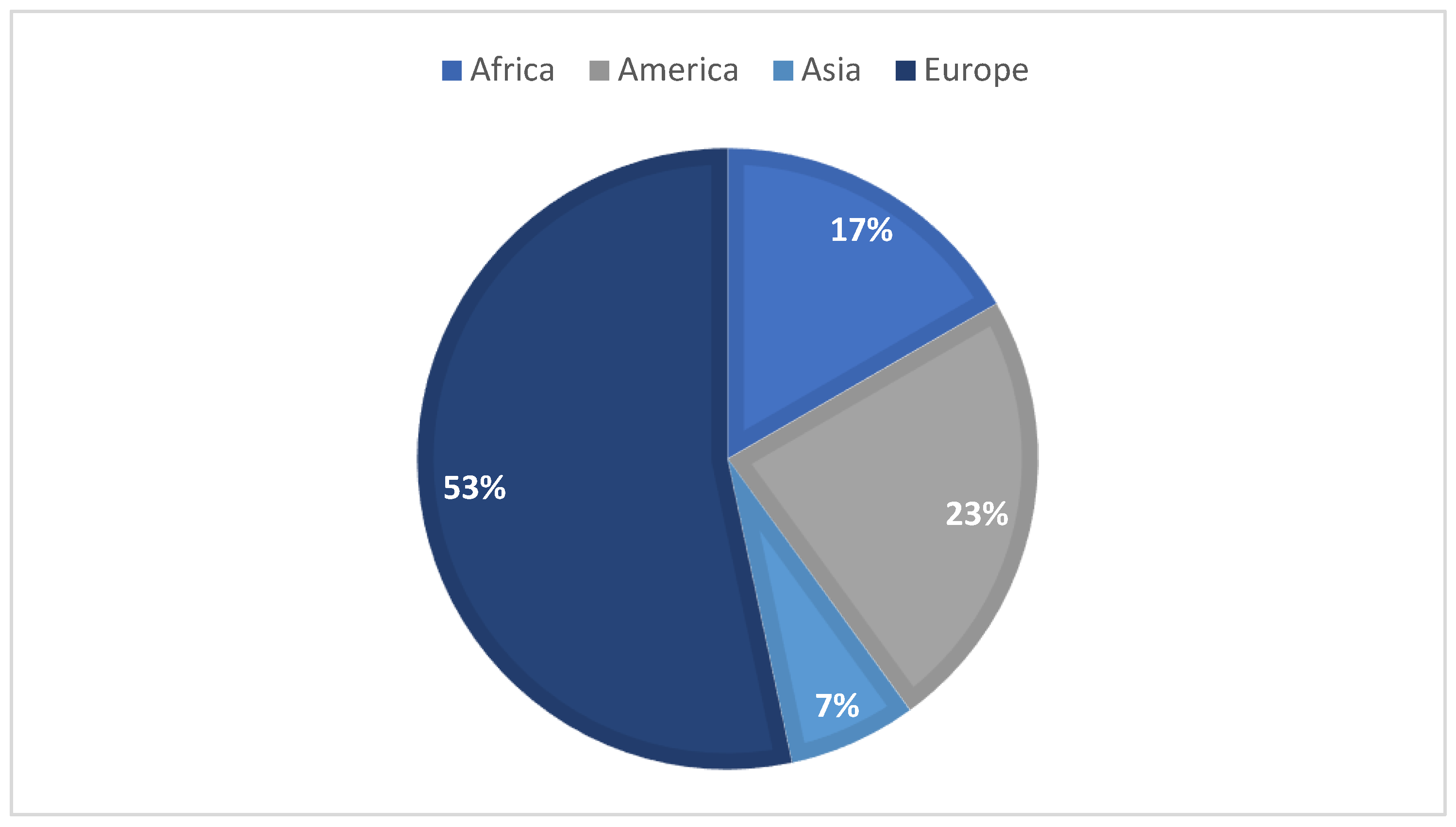
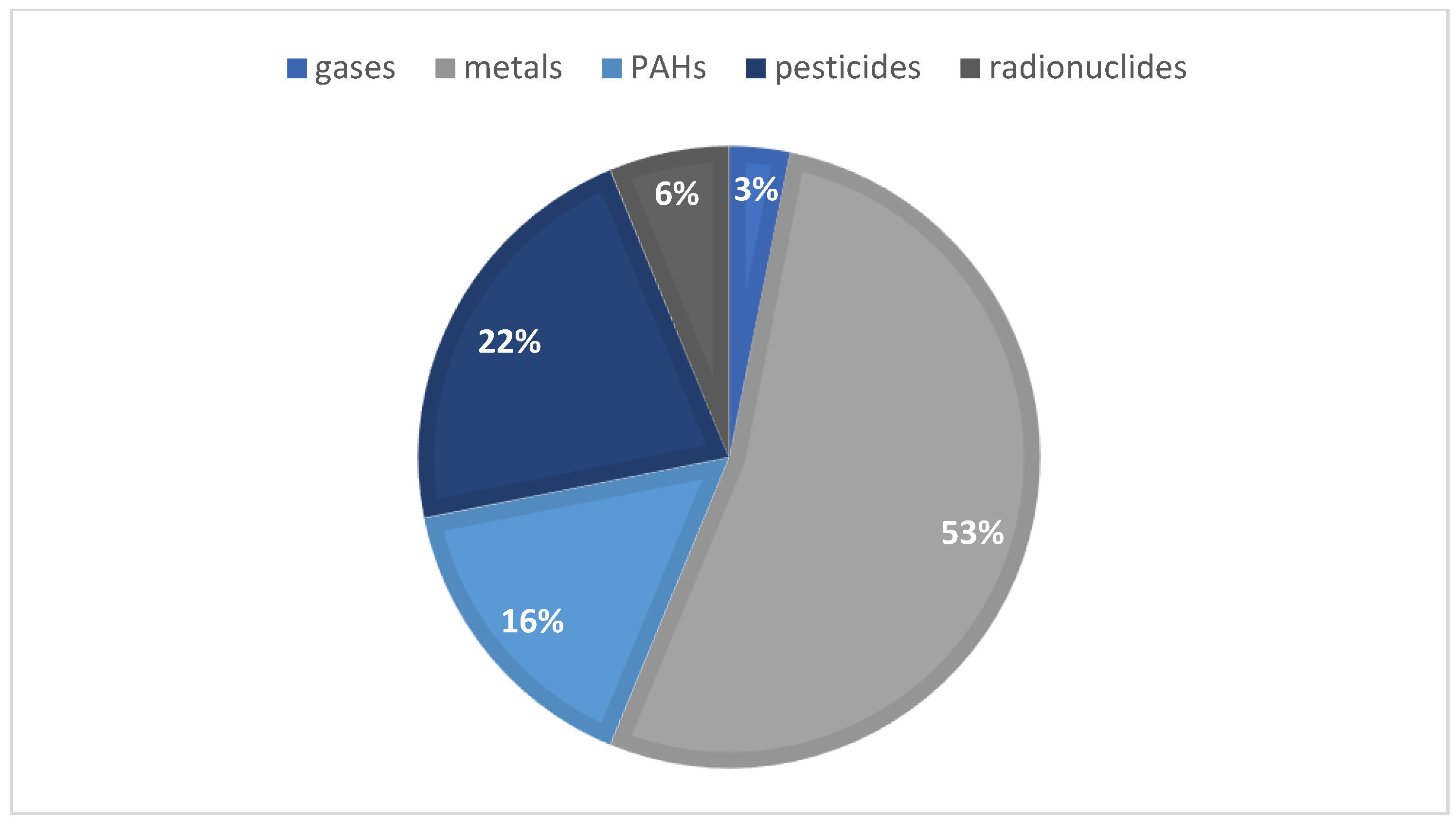
Over the last few years, honey has been noticed as a possible environmental bioindicator. In August 2022, a literature search on the Web of Science featuring the terms “honey” and “bioindicator” in their title, abstract, and/or keywords were made in order to initiate the following study, resulting in 30 reports of interest. Most of these articles target honey as an environmental bioindicator, regarding the presence of pesticides, heavy metals, radionuclides, and polycyclic aromatic hydrocarbons (PAHs). Figure 1 summarizes the articles distribution according to the continent of origin—where, to the best of our knowledge, no portuguese samples were used—while Figure 2 represents the diverse compounds studied in the different articles. As for the extraction techniques used, five articles mentioned the use of QuEChERS when studying pesticides, but also the liquid–liquid extraction (LLE) and the solid–liquid extraction (SPE) techniques; when accessing the presence of PAHs, the most common ones were dispersive liquid–liquid micro-extraction (DLLME) and LLE. For the study of metals, the articles commonly point out acid digestion in order to quantify these.
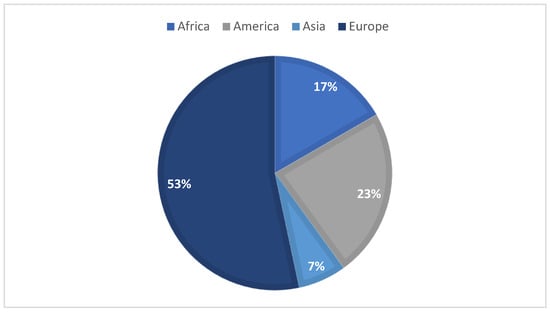
Figure 1.
Graphic distribution of the articles according to the continent of origin.
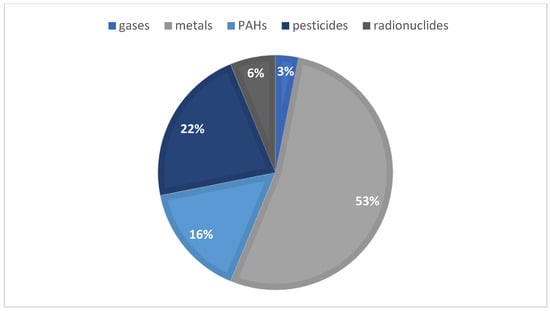
Figure 2.
Graphic distribution of the articles according to the compounds studied in honey samples.
As seen in Figure 2, more than 50% of the articles mentioned the study of metals, followed by pesticides and PAHs. Neonicotinoids, pyrethroids, organochloride, organonitrogen and organophosphorus were the groups of pesticides analyzed.
Within this particular search, few articles mentioned the study of POPs, such as polychlorinated biphenyls (PCBs) or brominated flame retardants (BFRs), and no articles mentioned pharmaceutical drugs or microplastics for instance, which are emerging pollutants of growing concern. However, as mentioned below, studies have been conducted with honey and these contaminants, which could prove, even if it was not the main focus, that honey can also be a bioindicator for these. Nevertheless, more studies should be performed with different pollutants regarding pollinators and their derivatives.
In 2005, two honeybee colonies 100 km apart, in Slovenia, were included in a study [5]. One colony was in Zavodnje, an area known to be polluted by the Šoštanj coal-fired power plant, while the other was in Poljanska dolina, an area without local SO2 pollution. Researchers recovered data from honey originating in the Zavodnje colony and demonstrated that the sulphate quantified in the honey was correlated to the total yearly emissions of SO2 detected by the Environmental Information System (EIS), a system including seven stationary emission-measuring stations. Values of sulphate detected in honey from Poljanska were significantly lower compared to the first colony [5]. Throughout the following years, new studies were developed considering honey as a bioindicator for the pollution of PAHs and heavy metals, among others. In 2008 [6], honey originating from six agricultural areas of Greece (north, center, and south) was evaluated regarding the presence of pesticide residues. The analysis performed by Balayiannis et al. [6] detected residues of phorate, chlorpyrifos, chlorfenvinphos, and coumaphos, an acaricide. A more recent project, published in 2021 [7], analysed the presence of organochlorine pesticides (OCPs), a group of compounds considered POPs. The honey samples were recovered from Masindi district, Uganda, an area that includes a forest reserve. Dichlorodiphenyltrichloroethane (DDT), dieldrin, endosulfan isomers, and lindane were quantified in the honey samples studied, concluding that the monitoring of OCPs should continue [7]. Nowadays, the use of OCPs is banned in several countries, but recently these have been reported in honey samples. Therefore, it is possible to conclude that the use of honey as a sample for the study of pesticides is pertinent, even in banned compounds such as POPs.
Besides these studies, the EU has established regulations regarding the presence of contaminants and their respective maximum residue levels (MRL), but also releases annual reports that assess the pesticide residues. Regulation (EC) No 396/2005 [8] is the legislation submitted by the EU regarding MRL of pesticides in food and feed of plant and animal origin. A total of 315 products, including honey, and the respective MRLs for more than 1000 pesticides currently or formerly used in agriculture, can be found in this regulation [8]. By the EU legislation (Article 32, Regulation (EC) No 396/2005 [8]), the European Food Safety Authority (EFSA) requires an annual report evaluating the pesticide residue levels in foods originating from European markets. The 2019 report includes data provided by the national control activities carried out by the EU Member States, Iceland, and Norway [9]. In this EFSA report, 1302 samples of honey were studied, with 277 samples reporting the presence of contaminants, while 265 honey samples presented residues below or at the MRLs and 12 above. The dominant contaminants in honey samples were neonicotinoids and veterinary medicinal residue products, which include acetamiprid, amitraz, azoxystrobin, benzalkonium chloride (BAC), bromide ion, chlorates, chlorpyrifos, coumaphos, dimoxystrobin, flonicamid, fosetyl, glyphosate, and thiacloprid [9]. Even if in the literature it is possible to find different cases where the presence of contaminants, besides pesticides, are noted, no legislation can be found regardless of their potential harm to the animal and human health. Therefore, it is imperative to stablish new studies approaching these contaminations in order to implement legislation. There are other official documents addressing additional contaminants, such as brominated flame retardants in food. For instance, there is the Commission recommendation (2014/118/EU) of 3 March 2014 on the monitoring of traces of brominated flame retardants in food [10], but it is only a recommendation and does not even mention honey. There is also a Commission regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs [11], namely nitrate, mycotoxins, metals, 3-monochloropropane-1,2-diol, dioxins, PCBs, and PAHs, but it is general and does not specify honey.
1.2. Honey Contaminant Analysis—Extraction Methods and Challenges
Honey composition is dependent on its botanical source, geographical conditions, as well as its processing and storage conditions [12]. Either way, honey has a complex composition [13], being mainly composed of sugars (with a total of about 80%) [14] and water. Smaller amounts of lipids, nitrogen compounds (which includes proteins and free amino acids), organic acids, minerals, vitamins, and phenolic compounds, among others, can also be found [12]. Therefore, this complex composition is problematic when talking about methods of sample preparation and extraction of contaminants in trace amounts. Therefore, the scientific community is working on the urgent need to validate and establish a method with better efficiency regarding recovery and matrix effects for the desired analyte, in this case, contaminants [15].
Efficient separation through chromatographic columns, especially in food analysis, is widely affected by preliminary sample preparation. Nevertheless, some characteristics of the sample must be primarily taken into consideration, such as particle size and homogeneity, as well as the target analyte that will be analyzed, since this information is crucial for the choice of the solvent, extraction, and clean-up technique [16]. The execution of this preliminary preparation enables (i) a clean-up of the sample; (ii) a transfer of the analytes to the medium of injection; and (iii) an enrichment of the target to a concentration that can be measured [17].
Nowadays, a broad spectrum of different sample preparation techniques can be found in the literature, following a common pathway, despite their differences. The extraction process to obtain the analyte from the sample matrix as well as the clean-up procedures [18] could interfere with the detection of the target analyte [17]. These can be used to detect a specific contaminant, a class, or a multiclass, where the last one, when linked to an appropriate detection and analytic method, can provide a technique capable of detecting and quantifying contaminants with the least steps of extraction and purification, increasing the method efficiency [19].
Souza et al. [19] published a review paper that revised the different techniques for sample preparation and pesticides study in honey. The SPE method allows the combination of the extraction and clean-up steps, employing low amounts of solvent and being capable of efficient analysis of samples directly collected from the apiary. A study using the Purge and Trap technique showed that, for specific conditions and coupled with gas chromatography, it is possible to obtain lower limits of detection (LOD) when compared to SPE. LLE, a conventional technique and one of the most used, is associated with some disadvantages, such as extraction of just one chemical class, the use of larger volumes of organic solvents and the extraction of several interferents from the matrix, being, therefore, a very unselective procedure. Even so, adjustments and progresses have been made in the method-development field to increase the efficiency, enabling the study of more than one class of pesticides and other contaminants and allowing its application in different matrices, increasing its versatility. Furthermore, the review also presents a different number of miniaturized techniques used on honey, namely (i) DLLME, a technique that can present different variations in order to achieve higher recoveries; and (ii) Microextraction by packed sorbent (MEPS), which consists in a miniaturized version of the SPE method and, when coupled with GC-MS, allow to detect a multiclass residue, with an extraction time close to 4 min, reusing the sorbent and using lower amounts of sample and organic solvent [19].
A different method was used by Chiesa et al. [20] to analyze the presence of multiresidue pesticides in organic honey from German and Italian beekeepers. In this study, the technique used was the Accelerated Solvent Extraction (ASE), where extraction times and solvent consumption are reduced, being characterized by high temperatures that increase the diffusion rates and the solubility of the analytes into the solvent, as well as high pressure, keeping the viscosity and surface tension of the solvent reduced due to the elevated temperatures employed. Moreover, the method performed was adjusted to combine the extraction and cleanup steps, resulting in an “in-line” method. This allowed to remove the interferences from honey samples, whose recoveries did not depend on the analyte concentration, being overall a cost-effective and minimized-waste method.
Another technique that became popular due to the reduced extraction time and solvent consumption was the Quick, Easy, Cheap, Effective, Rugged and Safe (QuEChERS) method, described in 2003 for the extraction of pesticides from food matrix [21]. Despite its original purpose, nowadays it is also used to recover other analytes from food matrices, such as environmental pollutants (e.g., pesticides, PAHs, etc.) and antidepressants, among others. Similar to other sample preparation methods, QuEChERS can be divided into extraction and clean-up [22]. In the extraction step, a salting-out effect occurs (partitioning of salts to the extract), where the solvent and the inorganic salts (employed to induce the separation between phases and to transfer hydrophobic analytes to the organic layer) are added to the sample. For the clean-up step, a dispersive solid-phase extraction (d-SPE) occurs, adding sorbents (for example, C18 and Z-Sep–removes hydrophobic interferences such as fat; PSA–removes polar interferences such as sugar and organic acids) that remove the matrix interferences to further clean-up and obtain the desired analyte in the extract solution [23]. Since it was first mentioned, various QuEChERS were designed, with different compositions, and the selection of the best one takes into consideration the analyte properties, the matrix, and the analytic technique conditions [16]. Comparing this technique with the traditional ones, QuEChERS are quicker [16], less expensive, employ lower volumes of solvents, and are less toxic [19], but also can provide greater recovery rates and increase the analytical performance [22].
A literature search on Web of Science in August 2022 found 202 articles featuring the terms “honey” and “QuEChERS” in the title, abstract, and/or keywords. Among them, 157 articles featured also “pesticides”, compared to the 21, 18, 3, and 4 articles with titles, abstracts, and/or keywords including “QuEChERS” and either “veterinary drugs”, “antibiotics”, “POPs” or “PAHs”, respectively. In this work, the main goal is to present an overview of the current information about the application of QuEChERS method to different contaminants (such as pesticides, POPs, pharmaceuticals, and veterinary drugs) in honey matrix. In addition, this work also aimed to discuss the presence of microplastics in honey samples.
2. QuEChERS Approach for the Analysis of Several Contaminants in Honey Samples
2.1. Pesticides
To extend the production area, volume of production, shelf time and, simultaneously, improve the appearance of the product, farmers often reach for pesticides–chemicals designed to attack pathogens that could be a threat to their plantations, such as bacteria, weeds, fungi, insects, etc. [16]. Bioaccumulation, high lipophilicity, long half-life, and the potential for long-range transport are characteristics presented by some pesticides on the market, which increase the possibility to contaminate the environment, being possibly a risk to human health [24].
Pesticides are designed to interfere with important mechanisms of several pathogens and, as a side effect, they may also be able to interfere with non-target organisms and plants. Another important point is that these can move freely in the environment, through wind currents and water leaching or runoff, making their transportation over the globe possible [25]. Therefore, and due to their persistence, we can understand that residue levels have been detected in different areas, such as in the air, soil, water, and non-target organisms [26]. Organisms’ contamination can occur through main mechanisms: biomagnification–the higher in the food chain, the higher levels can be found in tissues and organs; and bioconcentration–the accumulation into the organism happens from the neighboring medium [27].
Pesticide contamination in humans can occur directly–such as inhalation, ingestion or dermal absorption–or indirectly–through contaminated food and water–via [26]. Different negative, both acute and chronic health, effects have been reported in the literature, including nausea, nervous system depression, endocrine disruption, and cancer [28]. For example, OCPs are highly associated with the stimulation of the central nervous system; breast, prostate, stomach, and lung cancer, and diabetes type 2, liver malfunctions, endometriosis, among others [24]. Considering these effects on health and their persistence, OCPs have been banned since 1997 [28]. Many pesticides have also the capacity to interfere with the human reproductive process, since they are designed to intervene with the pathogens’ reproductive system [26]. Another interesting effect is the association with psychiatric problems such as depression and depression–anxiety [29].
As for water impact, it has been reported that different insecticides and herbicides can be harmful to different aquatic species, but an alarming point is the report that lower concentrations of malathion can impact plankton populations, an important point of the food chain [26]. Another relevant point is the fact that, besides alteration on aquatic fauna and flora [28], water contamination can not only alter the quality of drinking water, but can also transfer the contaminants to the soil and other living organisms [25]. Soil biodiversity is widely affected by pesticide contamination, impacting different microorganisms presented in the soil biota (with interference on microbial metabolism, molecular interactions, and symbiotic association) [26].
Risk assessments are needed when approving active substances, including pesticides. Their approval is also dependent on criteria relating to honeybees and, for future use, they cannot result in a nefarious exposure for the honeybees and present acute or chronic effects on the colony [30].
Honeybees present an important role in the environment, biodiversity, and food production [31] and, in 2012, neonicotinoids and fipronil were considered high risks for their health [30]. Neonicotinoids, a group of pesticides such as nicotine, are considered more toxic to invertebrates than mammals [32]. These compounds target the central nervous system, resulting in paralysis and death [32]. In 2018, the EU officially banned the use of three neonicotinoids (clothianidin, imidacloprid, and thiamethoxam) on all crops grown outdoors, due to their effect on bees’ health [31]. Honeybees’ exposure to these compounds happens through pollination and, therefore, they can be quantified in honeybee’s products, such as honey and beeswax. Considering this, it is important to explore methods of extraction, quantification, and monitoring pesticides, to better understand the risks associated with these products.
Throughout the years, multiple scientific papers have been approaching the QuEChERS technique for the analysis of pesticides in the honey matrix, combining different detection and quantification analysis methods to achieve a fast and simple screening procedure. Table 1 summarizes the detailed QuEChERS methods used by different research groups, including their method validation. In terms of extraction procedure, most of the articles present the original QuEChERS extraction kit (MgSO4 + NaCl) combined with MgSO4 and PSA sorbent, as the d-SPE, and a mixture of water and acetonitrile as solvent. Few modifications can be seen, for example in Bridi et al. [33], regarding the extraction kit and the d-SPE.
Most works achieved recoveries within the SANTE/11312/2021 [34] regulations (70–120 ± ≤20%), except Calatayud-Vernich et al. [15] and Pang et al. [35] that presented recoveries lower than 70%. Table 2 presents the MRLs values defined by the EU for a selected group of pesticides in honey. Regarding the limits of detection and quantification (LOQs) that are most suitable for the MRLs defined, Calatayud-Vernich et al. [15] developed a method with a LOQ of 0.2–10 ng/g; Almeida et al. [36] presented a LOD of 0.1–4 ng/g and a LOQ of 0.2–8 ng/g; a LOD of 0.34–1.43 ng/g and a LOQ of 0.30–4.76 ng/g was obtained by Bridi et al. [33]; and Pang et al. [35] produced a method with a LOD of 1–4 ng/g.

Table 1.
Application of QuEChERS approach to the analysis of various pesticides in honey samples.
Table 1.
Application of QuEChERS approach to the analysis of various pesticides in honey samples.
| Target Pesticides and/or Chemical Class of Pesticides | Extraction | Clean-Up | Validation Parameters | Analysis | Findings | Sample Origin | Ref. |
|---|---|---|---|---|---|---|---|
| OPPs, PYRs, neonicotinoids, herbicides, insecticides, carbamates, fungicides, and acaricides | Solvent: 7.5 mL water and 10.0 mL acetonitrile Extraction kit: 6.0 g MgSO4 and 1.0 g NaCl | d-SPE: 0.05 g C18, 0.05 g PSA and 0.15 g MgSO4 | Recoveries: 30.0–96.0 ±≤20.0% LOQs: 0.2–10.0 ng/g | LC–MS/MS | - | Spain | [15] |
| OPPs, PYRs, herbicides and phenyl pyrazol | Solvent: 5 mL acetonitrile Extraction kit: 2 g MgSO4 and 1 g of NaCl | d-SPE: 0.15 mg PSA and 1 g MgSO4 | Recoveries: 70.0–120.0 ±<20.0% LOQs: 18.0–410.0 ng/g LODs: 6.0–135.0 ng/g | GC-ECD | HCH, endosulfan, aldrin, heptachlor, malathion, chlorpyrifos, chlorpyrifos methyl, pendimethalin, butachlor, fipronil, bifenthrin, cypermethrin | India | [37] |
| Neonicotinoids, OCPs, PYRs | Solvent: 10 mL water and 10 mL acetonitrile Extraction kit: 4 g MgSO4 and 1.5 g NaCl | d-SPE: 0.750 g MgSO4, 0.250 g PSA and 0.125 g C18 | Recoveries: 74.0–104.0 ±<20.0% LOQs: 10.0–50.0 ng/g | UHPLC-MS/MS | imidacloprid, clothianidin, chlorpyrifos, permethrin, dimethoate, cypermethrin | Brazil | [38] |
| Neonicotinoids | Solvent: 10 mL water and 10 mL acetonitrile Extraction kit: 4 g MgSO4 and 1 g NaCl | d-SPE: 0.150 g MgSO4, 0.050 g PSA and 0.050 g C18 | Recoveries: 86.2–101.7 ±<6.0% LOQs: 60.8–81.0 ng/g LODs: 184.3–245.4 ng/g | UHPLC | acetamiprid, thiacloprid, thiamethoxam, imidacloprid, clothianidin | Poland, Australia, Brazil, Bulgaria, Cameroon, Czech Republic, France, Greece, Italy, Portugal, Romania, Russia, USA, Turkey | [39] |
| PYRs, OCPs, OPPs, neonicotinoids, insecticides, carbamates, growth regulators, herbicides, acaricides, and fungicide | Solvent: 10 mL water and 10 mL acetonitrile and ethyl acetate (70:30, v/v) Extraction kit: 4 g MgSO4, 1 g NaCl, 1 g trisodium citrate dehydrate, and 0.5 g disodium hydrogen citrate sesquihydrate | d-SPE: 0.900 g MgSO4 and 0.150 g PSA | Recoveries: 70.0–120.0 ±≤20.0% LOQs: (LC) 0.2–0.8 ng/g; (GC) 2.0–8.0 ng/g LODs: (LC) 0.1–0.4 ng/g; (GC) 1.0–4.0 ng/g | LC-MS/MS GC-MS/MS | acephate, acetamiprid, azoxystrobin, bifenthrin, boscalid, carbaryl, carbendazim, clomazone, chlorpyrifos, clothianidin, diflubenzuron, dimethoate, diuron, imidacloprid, metoxyphenazide, omethoate, pyraclostrobin, pyrimethanil, pyriproxyfen, tebuconazole, thiabendazole, thiamethoxam, triazophos, trifloxystrobin | Brazil | [36] |
| Neonicotinoids | Solvent: 9 mL H2O:ACN (50:50, v/v) Extraction kit: 2 g MgSO4, 0.5 g NaCl, 0.5 g sodium citrate dihydrate and 0.25 g sodium citrate sesquihydrate | d-SPE: 0.150 g MgSO4, 0.100 g PSA bulk phase and 0.100 g C18 bulk phase | Recoveries: 73.0–95.0 ±≤22.0% LLOQs: 2.0 × 10−3–20.0 × 10−3 pg/g | UHPLC-MS/MS | - | Switzerland | [40] |
| OPPs, OCPs, PYRs | Solvent: 10 mL water and 10 mL acetonitrile acidified with glacial acetic acid (1%) Extraction kit: 6.0 g MgSO4 and 1.5 g CH3COONa | d-SPE: 0.40 g PSA sorbent and 1.20 g MgSO4 | Recoveries: 86.0–107.7 ±≤12.1% LOQs: ≤27.3 ng/g LODs: ≤9.1 ng/g | GC-μECD/FTD GC-MS | dichlorvos, monocrotophos, profenofos, permethrin, ethion, lindane | India | [41] |
| Neonicotinoids | Solvent: 10 mL of water and 10 mL of acetonitrile Extraction kit: MgSO4, NaCl, sodium citrate tribasic dihydrate, and sodium citrate dibasic sesquihydrate | d-SPE: MgSO4, PSA and discovery C18 | Recoveries: 79.0–101.0 ±≤3.3% LOQs: 0.3–4.8 ng/g LODs: 0.3–1.4 ng/g | LC/MS/MS | acetamiprid, thiamethoxam, thiacloprid, imidacloprid | Chile | [33] |
| OCPs, OPPs, PYRs and organonitrogen pesticides | Solvent: 10 mL of water and 10 mL of acetonitrile acidified with acetic acid Extraction kit: 1.0 g sodium acetate, 4.0 g MgSO4 | d-SPE: 0.4 g PSA sorbent and 0.6 g MgSO4 | Recoveries: 84.2–120.3 ±<20.0% LODs: 1.0–168.0 ng/g | GC-NPD GC-ECD | β-HCH, γ-HCH, dicofol, tetradifon, bromopropylate, chlorpyrifos, diazinon, fenitrothion, malathion, pirimicarb, profenofos | Egypt | [42] |
| acylamino acid, anilinopyrimidine, aryloxyphenoxypropionate, benzimidazole, benzofuran, carbamate, carbanilate, carboxamide, chloroacetamide, cyanoimidazole, diacylhydrazine, dicarboximide, dinitroaniline, hydroxyanilide, imidazole, morpholine, neonicotinoid, OPPs, oxadiazine, phenylamide, phenylpyrazole, phenylurea, phosphorothiolate, pyrazole, PYRs, pyridazinone, pyridine, pyrimidine, strobilurin, sulphite ester, tetrazine, tetronic acid, triazine, triazole, urea and other pesticides unclassified | Solvent: 10 mL water and 10 mL acetonitrile:ethyl acetate (70:30) with 1% acetic acid Extraction kit: 1.0 g sodium acetate, 4.0 g MgSO4 | d-SPE: 0.15 g MgSO4, 0.05 g PSA sorbent and 0.05 g Fibrosil | Recoveries: 81.6–108.9 ±≤20.0% LOQs: 10.0–25.0 ng/g LODs: 5.0 ng/g | UHPLC-MS/MS | Trichlorfon | Brazil | [43] |
| OPPs, OCPs, PYRs, strobis, triazoles, chloronitrile, dinitroaniline, and pyrazole | Solvent: 5.0 mL aqueous Na2EDTA (0.1 mol L−1, heated at 45 °C) and 5.0 mL acetonitrile Extraction kit: 1.5 g NaCl, 6.0 g anhydrous MgSO4 | d-SPE: 0.12 g MgSO4 and 0.1 g PSA sorbent | Recoveries: 71.0–119.0 ±≤20.0% LOQs: 10.0–20.0 ng/g LODs: 3.0–6.0 ng/g | GC-ECD | chlorpyrifos ethyl, chlorothalonil, endosulfan sulfate, hexachlorobenzene, malathion | Brazil | [44] |
| Insecticides | Solvent: 6 mL water and 5 mL acetonitrile Extraction kit: 3 g NaCl, 6 g anhydrous MgSO4 | d-SPE: 0.15 g MgSO4, 0.05 g PSA sorbent and 0.001 g graphene | Recoveries: 60.7–116.4 ±<10.0% LODs: 1.0–4.0 ng/g | UPLC-MS/MS | Acetamiprid, chlorpyrifos, imidacloprid | China | [35] |
| organonitrogen pesticides, OPPs, OCPs and PYRs | Solvent: 10 mL water, 10 mL acetonitrile acidified with acetic acid Extraction kit: 1.0 g sodium acetate and 4.0 g MgSO4 | d-SPE: 0.4 g PSA sorbent and 0.6 g MgSO4 | Recoveries: 70.0–120.0 ±≤22% LOQs: 20.0–50.0 ng/g LODs: 1.0–168.0 ng/g | GC-NPD GC-ECD | - | Egypt | [45] |
| Pesticides, PAHs and PCBs OCPs | Solvent: 10 mL ultrapure water and 10 mL acetonitrile Extraction kit: 4 g MgSO4, 1 g NaCl, 1 g trisodium citrate dihydrate, and 0.5 g disodium hydrogencitrate sesquihydrate | d-SPE: 1.20 g MgSO4, 0.400 g PSA, and 0.400 g C18, follows a SPME extraction and concentration | Recoveries: (LC) 55.0–105.0 ±<17.0%; (GC) 51.0–104.0 ±<28.0% LOQs: (LC) 0.2–16.1 ng/g; (GC) 0.2–168.1 ng/g LODs: (LC) 4.8 × 10−2–5.3 ng/g; (GC) 7.0 × 10−2–50.4 ng/g | LC–MS/MS for non-volatile pesticides GC-MS/MS for semivolatile pesticides | diflufenican, pyraclostrobin, diuron, penconazole, fenpropidin, acetochlor, hexachlorobenzene | Lebanon | [46] |
PAHs: polycyclic aromatic hydrocarbons; PYRs: pyrethroids; OCPs: organochlorine pesticides; OPPs: organophosphorus pesticides.
Table 1 also presents the numerous pesticides identified in honey samples tested by different groups, where the most common pesticides reported were chlorpyrifos (maximum levels of 0.34 [36], 27 ng/g [35], and 428 [37]), imidacloprid (maximum levels of 6.18 [36], 7 [33], and 624.91 ng/g [39]), acetamiprid (maximum levels of 78 [33] and 1340.33 ng/g [39]), malathion (maximum levels of 79 ng/g [37]), clothianidin (maximum levels of 0.63 [36] and 598.84 ng/g [39]), and thiamethoxam (maximum levels of 2.09 [36] and 652.42 ng/g [39]). Following the Globally Harmonized System of Classification and Labelling of Chemicals (GHS), most of these pesticides are classified as “Environmental Hazard”, except imidacloprid that is catalogued as “Irritant”, excluding chlorpyrifos. This one is classified as “Acute Toxic”, due to its neurotoxic nature, which could explain the lower MRL (10 ng/g) compared to the 50 ng/g reported for other pesticides, as seen in Table 2. Therefore, the importance of these studies is well-established due to the pesticides’ malicious effect on the environment, animals, and humans.
The presence of these pesticides in the environment is transferred to honey through bee pollination. Comparing the maximum levels registered with the values presented on Table 2, most of the samples exceeded the MRLs values, approved by the EU, which brings up an alarming problem. First, honey is a product highly consumed by humans, which might cause serious problems on human health when in high concentrations. Second, a downgrade of honeybees’ numbers has been observed, these chemicals being part of the cause.
According to the International Agency for Research on Cancer (IARC) Monographs agents are classified as carcinogenic to humans (group 1); probably carcinogenic to humans (group 2A); possibly carcinogenic to humans (group 2B); and not classifiable as to their carcinogenicity to humans (group 3) [47]. Table 2 displays the IARC classification of some reported pesticides.

Table 2.
Published MRLs [48], by EU, for pesticides in honey, and IARC classification [49].
Table 2.
Published MRLs [48], by EU, for pesticides in honey, and IARC classification [49].
| Pesticide | MRL (ng/g) | IARC | Pesticide | MRL (ng/g) | IARC |
|---|---|---|---|---|---|
| Acephate | 20 | - | Fenpropidin | 50 | - |
| Acetochlor | 50 | - | Fipronil | 5 | - |
| Acetamiprid | 50 | - | Fluvalinate | 50 | - |
| Aldrin | 10 | 2A | HCH | 10 | 1 |
| Azoxystrobin | 50 | - | Heptachlor | 10 | 2B |
| Bifenthrin | 50 | - | Hexachlorobenzene | 10 | 2B |
| Boscalid | 150 | - | Imidacloprid | 50 | - |
| Bromopropylate | 10 | - | Lindane | 10 | 1 |
| Carbaryl | 50 | 3 | Malathion | 50 | 2A |
| Carbendazim | 1000 | - | Omethoate | 10 | - |
| Chlorothalonil | 50 | 2B | Penconazole | 50 | - |
| Chlorpyrifos | 10 | - | Pendimethalin | 50 | - |
| Chlorpyrifos-methyl | 10 | - | Pirimicarb | 50 | - |
| Clomazone | 50 | - | Profenofos | 50 | - |
| Clothianidin | 50 | - | Propiconazole | 50 | - |
| Coumaphos | 100 | - | Prothioconazole | 50 | - |
| Cypermethrin | 50 | - | Pyraclostrobin | 50 | - |
| Cyproconazole | 50 | - | Pyrimethanil | 50 | - |
| Diazinon | 10 | 2A | Pyriproxyfen | 50 | - |
| Dicofol | 20 | 3 | Tebuconazole | 50 | - |
| Difenoconazole | 50 | - | Tetraconazole | 20 | - |
| Diflubenzuron | 50 | - | Tetradifon | 50 | - |
| Diflufenican | 50 | - | Thiabendazole | 50 | - |
| Dimethoate | 10 | - | Thiacloprid | 200 | - |
| Dimoxystrobin | 50 | - | Thiamethoxam | 50 | - |
| Diuron | 50 | - | Thiazophos | 50 | - |
| Endosulfan | 10 | - | Trichlorfon | 10 | 3 |
| Ethion | 10 | - | Trifloxystrobin | 50 | - |
| Fenitrothion | 10 | - |
2.2. Persistent Organic Pollutants and Polycyclic Aromatic Hydrocarbons
Persistent organic pollutants are organic chemicals with the ability to remain in the environment for long periods, being widely distributed and toxic to humans and wildlife (with the capability to accumulate in the fatty tissue), due to their specific combination of chemical and physical proprieties [50]. Firstly, the Stockholm Convention targeted nine OCPs, PCBs and polychlorinated dibenzo-p-dioxins, and polychlorinated dibenzo-p-furans (PCDD/Fs) as the “dirty dozen”, but throughout the years, new chemicals have been added, such as BFRs, endosulfan isomers, hexabromocyclododecane, among others [51]. Today, the Stockholm Convention considers 35 POPs that can be divided in three different groups: “pesticides”, “industrial chemicals”, and “unintentional production” [52]—by-products resulting from combustion processes. Polycyclic aromatic hydrocarbons are not considered in the list of POPs by the Stockholm Convention, oppositely to the Aarhus Protocol [53]. PAHs typically result from natural wildfires or incomplete combustion of fossil fuels and are semi-volatile, persistent, organic pollutants widely distributed in the environment [54].
POPs contamination can cause damage in a molecular level, such as neurotoxic effects and metabolic diseases [55]. Due to their nefarious actions, PCBs production has been banned, but their bioaccumulation and persistence in the environment proves to be a problem. For humans, the main route of exposure is inhalation, but others can be considered, such as dermal absorption and oral ingestion [56]. Within effects of PCBs exposure we can find epidemiological studies referring to metabolic and neuro system diseases [57], and these have already been classified, by IARC, as carcinogenic to humans [58]. PCBs can be associated with insulin resistance and diabetes mellitus type 2, due to their capacity to interfere with expression of genes related to these phenomena’s [59]. Another problem is their neurotoxicity, which could be associated with the fact that PCBs metabolism increases the formation of reactive oxygen species (participating in processes within the nervous system), resulting in a oxidative stress environment leading up to inflammation of the cells [60].
When dispersed in the environment, PAHs can affect human health [61]. Naturally, their effects are dependent, for example, on exposure route and duration, as well as their concentration. Among the acute effects that have been associated with these, we can observe skin irritation and inflammation (where naphthalene is consider a direct skin irritant) [62]. Furthermore, naphthalene can induce the disruption of red blood cells, when ingested or inhaled in large quantities. PAHs (such as benzo[a]pyrene) can have a carcinogenic nature when activated, producing epoxides and diols that can bind to DNA. Another chronic effect is the capacity to induce, in humans, reproductive and immune damage [62].
Table 3 represents the QuEChERS approach to determine the presence of POPs and/or PAHs in honey samples. Similar to pesticides, the use of PSA and MgSO4 as d-SPE is common, but better recoveries results were achieved for PAHs and PCBs when using MgSO4 and sodium acetate as the QuEChERS extraction kit. Once again, the most common solvent was a mixture of water and acetonitrile, and the addition of C18 to the d-SPE was observed.
No guidelines are available regarding analytical control and method validation procedures for target analytes, other than pesticides, which is a fault in the literature, so for the following analysis regulations for the pesticides will be considered.
According to the SANTE/11312/2021 [34] regulation, the methods with the most effective recoveries were the ones presented by Petrovic et al. [63], dos Santos et al. [64], and Surma et al. [65]. Regarding the lowest LOD and LOQ registered for PAHs, 0.07 and 0.23 ng/g were reported by Al-Alam et al. [66], respectively, presenting lower recoveries than those that are satisfactory. This method [66] also registered the lowest LOD and LOQ for PCBs. Al-Alam et al. [46] also developed a multiresidue detection method for PAHs and PCBs, where low LOD and LOQ were achieved, but recoveries were not within the SANTE/11312/2021 [34] regulation.

Table 3.
Application of the QuEChERS approach to the analysis of several PCBs, PFAS and PAHs in honey.
Table 3.
Application of the QuEChERS approach to the analysis of several PCBs, PFAS and PAHs in honey.
| Target | Extraction | Clean-Up | Validation Parameters | Analysis | Findings | Sample Origin | Ref. |
|---|---|---|---|---|---|---|---|
| PAHs and PCBs | Solvent: 10 Ml acetonitrile and 10 Ml water Extraction kit: 4 g MgSO4, 1 g NaCl, 1 g trisodium citrate dihydrate and 0.5 g disodium hydrogen citrate sesquihydrate | d-SPE: 1.2 g MgSO4, 0.400 g PSA and 0.400 g C18 | Recoveries: (PAHs) 63.0–104.0 ±<16.0%; (PCBs) 60.0–99.0 ±<16.0% LOQs: (PAHs) 0.2–40.0 ng/g; (PCBs) 3.1–55.9 ng/g LODs: (PAHs) 7 × 10−2–12.0 ng/g; (PCBs) 0.9–16.8 ng/g | GC-MS/MS | naphthalene, acenaphthylene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benzo(a)anthracene, chrysene, benzo(b)fluoranthene, benzo(k)fluoranthene, benzoIpyrene, benzo(a)pyrene, benzo(g,h,i)perylene | Lebanon | [66] |
| PAHs | Solvent: 3 Ml acetonitrile and 3 Ml water Extraction kit: 3 g of MgSO4 and 1 g of anhydrous sodium acetate | d-SPE: 0.150 g MgSO4, 0.100 g of PSA and 0.050 g C18 | Recoveries: 80.0–101.0 ±<15.0% LOQs: 1.0–2.0 ng/g LODs: 0–3–0.5 ng/g | GC-MS | naphthalene, acenaphthalene, acenaphthene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benz[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, indeno [1,2,3cd]pyrene, dibenz[a,h]anthracene, benzo[ghi]perylene | Serbia | [63] |
| PCBs | Solvent: 10 Ml water and 10 Ml acetonitrile (with 1% of acid acetic) Extraction kit: 1 g sodium acetate and 4 g MgSO4 | d-SPE: 0.0500 g PSA and 0.300 g MgSO4 | Recoveries: 81.0–116.0 ±≤20.0% LOQs: 20.0 ng/g LODs: 5.0–10.0 ng/g | GC-µECD | PCBs 28, 77, 81, 101 | Brazil | [64] |
| PFOA and PFOS | Solvent: 5 Ml warm water, 10 Ml acetonitrile with 150 Μl formic acid Extraction kit: 1 g NaCl and 4 g MgSO4 | d-SPE: ENV | Recoveries: (PFOA) 82–0–85.0 ±≤4.9%; (PFOS) 84–0–87.0 ±≤4.8% LOQs: (PFOA) 5.2 × 10−2 ng/g; (PFOS) 0.1 ng/g LODs: (PFOA) 1.6 × 10−2 ng/g; (PFOS) 4.0 × 10−2 ng/g | micro-UHPLC–MS/MS | perfluorooctanoic acid | Scotland, Spain, England, Italy, France | [65] |
| PAHs and PCBs | Solvent: 10 Ml ultrapure water and 10 Ml acetonitrile Extraction kit: 4 g MgSO4, 1 g NaCl, 1 g trisodium citrate dihydrate, and 0.5 g disodium hydrogencitrate sesquihydrate | d-SPE: 1.2 g MgSO4, 0.400 g PSA, and 0.400 g C18, follows a SPME extraction and concentration | Recoveries: 51.0–104.0 ±<28.0% LOQs: (GC) 0.2–168.1 ng/g LODs: (GC) 7.0 × 10−2–50.4 ng/g | GC-MS/MS | naphthalene, acenaphthene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benzo[a]anthracene, chrysene | Lebanon | [46] |
PAHs: polycyclic aromatic hydrocarbons; PCBs: polychlorinated biphenyls; PFAS: perfluoroalkyl and polyfluoroalkyl substances; PFOA: perfluorooctanoic acid; PFOS: perfluorooctane sulfonate.
When looking to the honey samples findings, PAHs were more common than PCBs. The most commons PAHs detected were naphthalene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benz[a]anthracene, and chrysene [46,63,66], with a higher concentration of chrysene (140.6 ng/g) [63]. PCB 28, 77, 81, and 101 were detected by dos Santos et al. [64], with maximum levels of 635, 65, 50, and 194 ng/g, respectively. PFOA residues were also detected at a maximum concentration of 0.223 ng/g by Surma et al. [65]. According to the IARC classification, naphthalene, benz[a]anthracene, and chrysene are possibly carcinogenic to humans (group 2B) [44].
Commission Regulation (EU) No 1259/2011 [67] and No 2020/1255 [68] are the regulations regarding the MRLs of PCBs and PAHs, respectively. Nevertheless, none of these documents were about MRLs allowed on honey. This is a problem that should be taken into consideration since, as seen above, residues of these compounds can be found in this matrix.
2.3. Pharmaceuticals
The negative impact of the presence of pharmaceuticals products on the natural environment is well stablished. However, this remains largely unregulated, despite the extremely toxic impact on both animals and humans.
The presence of pharmaceuticals on honey can occur since honeybees can be susceptible to several different microorganisms and parasites, if the environmental conditions are not the best, and in order to control these plagues, throughout the years, different veterinary drugs have been developed [69]. Within these molecules it is possible to found macrolides, nitroimidazoles, lincosamides, quinolones, sulphonamides, tetracyclines, among others [69,70,71].
Residuals of these pharmaceuticals on honeybees’ products need to be monitored since their presence can result on malicious effects in consumers and bring negative impacts to the bees themselves. For example, macrolides are able to produce allergic reactions [71] but also can induce gastrointestinal disorders as well as residual lincosamides [70]. Sulphonamide contamination can result on allergic reaction, bacteria resistance to antimicrobial resistance, and possible carcinogenicity [71]. Nefarious effects associated with tetracyclines include drug resistance, and allergic and toxic reactions if the individual is hypersensitive [69]. Quinolones have been associated with hepatoxicity, while nitroimidazoles with cell mutation and carcinogenic radionuclides [72].
Table 4 details the QuEChERS method followed by the researchers in the analysis of pharmaceuticals in honey samples. Contrary to the above sections, a more heterogeneous group of solvent, extraction kit, and d-SPE were observed. For the solvent, it is common the use of acidified acetonitrile and buffers solutions, while regarding the d-SPE, ZnO and Z-Sep+ are used as sorbents.
All the studies presented good recoveries, within the 70–120% limits pointed in the SANTE/11312/2021 [34] regulation, except for the method described by Lombardo-Agui et al. [73], which recoveries are between 61.2–99.8%. The lower values described of LOD and LOQ were 0.14 and 0.50 ng/g, respectively, in a multiclass method, for the detection of sulfonamides, macrolides, nitroimidazoles, tetracyclines, etc. [69].

Table 4.
Application of the QuEChERS approach to the analysis of various pharmaceuticals in honey samples.
Table 4.
Application of the QuEChERS approach to the analysis of various pharmaceuticals in honey samples.
| Target | Extraction | Clean-Up | Validation Parameters | Analysis | Findings | Sample Origin | Ref. |
|---|---|---|---|---|---|---|---|
| Quinolones | Solvent: 10 mL water and 15 mL extraction solution (1% acetic acid in can) Extraction kit: 1.5 g NaOAc and 6 g MgSO4 | d-SPE: 1.20 g MgSO4 and 0.400 g PSA | Recoveries: 82.0–117.0 ±<14.0% | UHPLC-ESI-MS/MS | enrofloxacin, danofloxacin, pipemidic acid, lomefloxacin, cinoxacin and, ciprofloxacin | [71] | |
| Sulfonamides, fluoroquinolones, macrolides, nitroimidazoles, tetracyclines, dapsone and trimethoprim | Solvent: 0.200 g Na2EDTA, 0.100 g citric acid, 5.0 mL water, 10 mL acetonitrile containing 1% acetic acid Extraction kit: 4 g anhydrous Na2SO4 and 1 g NaCl | d-SPE: 0.050 g PSA, 0.150 g C18-EC and 0.900 g anhydrous Na2SO4 | Recoveries: 80–4–118.4 ±<20.0% LOQs: 0.5–9.7 ng/g LODs: 0.1 to 2.9 ng/g | HPLC–MS/MS | norfloxacin, ciprofloxacin, ofloxacin, enrofloxacin, metronidazole, sulfamethoxazole and oxytetracycline | China | [69] |
| Quinolones | Solvent: 8 mL NaH2PO4 buffer (30 mM, vpH 7.0), 10 mL formic acid (5%) canACN Extraction kit: 4 g MgSO4, 1 g NaCl, 1 g sodium citrate, 0.5 g disodium citrate sesquihydrate | d-SPE: 0.150 g C18 and 0.900 g MgSO4 | Recoveries: −61.2–99.8 ±<8.0% LOQs: 0.8–5.5 ng/g LODs: 0.2–1.7 ng/g | UHPLC–MS/MS | - | Spain | [73] |
| Nitroimidazoles and quinolones | Solvent: 5 mL Mciluffer bufer (pH = 4.00), 15 mL citric acid- acetonitrile (5:95) Extraction kit: 2.0 g NaCl and 4.0 g MgSO4 | d-SPE: 0.050 g PSA, 0.050 g C18, and 0.100 g Mg2SO4 | Recoveries: −81.0–116.8 ±<6.3% LOQs: 2.1–5.3 ng/g LODs: 0.6–1.6 ng/g | LC-MS/MS | metronidazole, ciprofloxacin | China | [72] |
| Lincosamides and macrolides | Solvent: 10.0 mL acetonitrile Extraction kit: 2.0 g Na2SO4 | d-SPE: 0.1 g ZnO | Recoveries: −81.3–99.0 ±<10.0% LOQs: 0.8–2.3 ng/g LODs: 0.2–0.6 ng/g | HPLC-MS/MS | Lincomycin | China | [70] |
| Neonicotinoids, insecticides, fungicides, herbicides, acaricides, veterinary drugs and growth regulators | Solvent: 10 mL water and 10 mL acetic acid (1%) solution in acetonitrile Extraction kit: 4 g MgSO4 and 1 g sodium acetate | d-SPE: 1.05 g MgSO4, 0.35 g PSA and 0.35 g Z-Sep+ | Recoveries: 70.0–120.0 ±≤20.0% LOQs: 0.1–1.0 ng/g | LC-MS/MS GC-MS/MS | thiacloprid, acetamiprid, carbendazim, amitraz, DMF, DMPF, azoxystrobin, te-buconazole, dimethoate, coumaphos, cyproconazole, boscalid, flutriafol, tau-fluvalinate, tetraconazole, diazinon, dimoxystrobin, p,p′-DDD, difenoconazole, lindane, propiconazole prothioconazole-desthio | Poland | [74] |
The most common drugs detected among the different articles were enrofloxacin (354.5 [71] and 281.4 ng/g [69]) and ciprofloxacin (18.7 [71], 74.2 [69], and 89.43 ng/g [72]). Gawel et al. [74] developed a multiclass method, with good recoveries and low LOQs, which allowed the detection of different classes of pesticides as well as veterinary drugs and growth regulators. With this method, it was possible to quantify multiple contaminants alongside amitraz, an acaricide, at a concentration of 600 ng/g.
According to the Commission Regulation (EU) No 37/2010 [75], the only pharmacologically active substances allowed on honey samples are amitraz and coumaphos–both acaricides–with MRLs of 200 and 100 ng/g, respectively. Both drugs have been identified [74] in concentrations higher than the MRLs presented. Besides these drugs, others were quantified in the samples analysed that were not allowed by the regulation [75]. Once again, these results represent a pollution concern that may affect human health.
3. Microplastics in Honey
Plastic production had a massive increase in the years following the World War II and only in the early 1970s were the first reports about small particles of plastic in the ocean published [76]. The durability, good ductility, light weight, and low price, allowed plastics to become commonplace in daily life. Unfortunately, the limited recovery of plastic waste and, therefore, the natural degradation, has negative impacts on the planet, particularly the accumulation of plastic particles in marine and terrestrial environments [77]. In 1997, Charles More discovered the Great Pacific Garbage Patch, highlighting the problematic side of plastics and their impact on the world [78]. The most recent analysis of the European plastics production, demand, and waste data, dates from 2021, which affirms that the global annual plastics production, in 2021, was more than 390 million tons [79], while in 2020 production numbers were 367 million tons [80], a million tons less than the prior year [81], but 8 million tons more than 2018 [82].
What is commonly known as “microplastics” (MPs) consists of polymeric matrix or synthetic solid plastic particles with sizes that range from 1 to 5 µm (irregular or regular shapes)–the primary types [83]; further degradation can produce smaller particles, with sizes between 1 and 1000 nm (with colloidal behaviors)–secondary types [84]. These secondary MPs can result from the action of sunlight, temperature, humidity, oxygen, or even agricultural films [85]. MPs have the capacity, through contaminated soils and water, to penetrate into different parts of plants during water and nutrient absorption [86], according to their particle size, with the subsequent exposure of animal life to them [87]. Reports of MPs include Asia, Europe, and North American freshwater systems and, naturally, higher volumes were located near densely populated areas, although it is possible to find them in areas with minority populations since plastics can freely move through the atmosphere [86]. Considering MPs capacity for spreading, it is possible to understand their reports on different environments/habitats and foods (such as honey, plants, animals, etc.), but also on other everyday products, such as cosmetics and personal care products [87].
In 2013, Gerd and Elisabeth Liebezeit [88] studied the presence of non-pollen particles in honey collected from producers and local supermarkets in Germany, France, Italy, Spain, and Mexico. Non-pollen particles, confirmed synthetic polymers, were observed in all samples analyzed, with higher concentrations of fibers in samples from Germany, Italy, and Spain. As a possible origin of these, the authors point out that precipitation results in the deposition of particles that can hold on to pollen and, later, be transported to the hive through bee pollination. Later, Gerd and Elisabeth Liebezeit [85] published a study regarding the presence of synthetic particles in honey samples recovered from supermarkets and small-scale beekeepers from Germany. Fibers and fragments were identified in all samples, and no significant differences were reported between them, an indication that harvesting, processing, and packaging are not the main source of these particles. The authors also analyzed plants, and concluded the presence of synthetic fibers and fragments, just as in the honey samples, indicating that the synthetic particles found in honey were transferred from the plants to the hive, through the bees, and consequently to honey. Diaz-Basantes et al. [89] studied liquid foods and source fluids from the Ecuadorian market, including honey packaged industrially and by hand. All honey samples presented MPs, whose numbers were coherent with the ones reported by Gerd and Elisabeth Liebezeit, composed of polyacrylamide, polypropylene, and high- and low-density polyethylene. The authors point out that MP contamination results from the population and industrial activity, associating superior population with higher MP percentage, also depending on the atmospheric conditions, since air currents and precipitation can transport the synthetic particles.
Table 5 summarizes the methods and results presented on the previous studies mentioned. As for the extraction, the different studies opted for a similar method, with differences registered only when filtrating the samples. To study the particles observed, all studies resorted to microscopy. Analyzing the size of the particles identified, fibers were up to few millimeters while fragments reported achieved several micrometers. For the amount, no correlation was found, where Gerd and Elisabeth Liebezeit [85,88] reported higher concentrations of fibers per kg of honey, oppositely to Diaz-Basantes et al. [89] that observed higher amounts of fragments per kg of honey. Only Diaz-Basantes et al. [89] identified the synthetic polymers using FTIR spectroscopy.

Table 5.
Articles reporting MP on honey samples.
Besides honey, honeybees can be considered bioindicators due to their (1) worldwide distribution, (2) wide distance flying capacity, (3) grooming behavior for pollination, and (4) morphologic structures that make them adapted to transport, for instance, pollen. Their body, during flight, become positively charged with static electricity, allowing pollen to adhere, as well as other particles present in the environment [90]. In this way, it is possible to determine if plants that honeybees collect pollen from are contaminated with MPs. Edo et al. [90] conducted a study to test if honeybees can act as bioindicators of MP pollution. For this, honeybees from Danish apiaries (including from urban Copenhagen) were collected, and in all samples were identified MPs shaped as fragments, fibers, filaments, or films, where the most commonly found was polyester. Regarding the concentration, as expected, a higher concentration of MPs was identified in samples from Copenhagen area, and when moving away from the densely populated area was recorded, a decreasing concentration of MPs was observed.
4. Conclusions and Future Perspectives
Nowadays, a cocktail of contaminants can be found in the environment, proving the actual global problem. Among these contaminants are pesticides residues, POPs, PAHs, veterinary drugs, and microplastics. Contamination can occur through different ways, such as poor management, persistence of the contaminants, climate, and human action. Consequently, due to their intimate interaction with the environment, a decline in pollinators has been observed, including honeybees’ numbers and diversity. This represents a serious problem since they play an important role in the environment, living nature, and human beings. Moreover, another major problem is the existence of multiple reports of different honeybee products, such as honey, incorporating these contaminants.
With this in consideration, it is possible to understand the necessity to control the quality of honey samples. This will not only give information about the environment, but also about the product itself, such as safety. For this, it is important to have established MRLs for the different contaminants, despite the gap on legislation, since no values can be found for POPs, PAHs, and microplastics. The QuEChERS technique, the focus technique of this review, has proven to be suitable for the extraction of a cocktail of contaminants and can, therefore, be considered a reference technique to be used for these determinations. The results in terms of analytical validation for the study of pesticides, pharmaceuticals, POPs, and PAHs using this approach of QuEChERS and chromatography were generally within the requirements of the guidelines discussed. However, the major gap is the lack of a standard method for the study of microplastics on honey samples. Finally, it would be interesting to improve and develop methods that can identify and quantify the different groups of contaminants simultaneously, and ideally, these should be eco-friendly, fast and low cost, considering the impact of laboratory procedures in the environment.
Author Contributions
Conceptualization and methodology, M.L. and V.C.F.; resources V.C.F., C.D.-M. and F.R.; writing—original draft preparation, M.L. and V.C.F.; writing—review and editing, M.L., V.C.F., F.R. and M.H.A.; visualization, M.L., V.C.F., F.R., M.H.A. and C.D.-M.; supervision, V.C.F., F.R. and M.H.A.; project administration V.C.F., C.D.-M. and F.R.; and funding acquisition, C.D.-M. and F.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work received financial support from national funds (FCT/MCTES, Fundação para a Ciência e Tecnologia and Ministério da Ciência, Tecnologia e Ensino Superior) through project MTS/SAS/0077/2020—Honey+—New reasons to care honey from the Natural Park of Montesinho: A bioindicator of environmental quality & its therapeutic potential, and by the projects UIDB/50006/2020, UIDP/50006/2020, and LA/P/0008/2020.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are thankful to the Fundação para a Ciência e a Tecnologia (Portugal) through project MTS/SAS/0077/2020—Honey+—New reasons to care honey from the Natural Park of Montesinho: A bioindicator of environmental quality & its therapeutic potential. This work received support from Portuguese national funds (Fundação para a Ciência e a Tecnologia (FCT)/Ministério da Ciência, Tecnologia e Ensino Superior (MCTES)) through projects UIDB/50006/2020, UIDP/50006/2020, and LA/P/0008/2020. Francisca Rodrigues is thankful for her contract (CEECIND/01886/2020) financed by FCT/MCTES—CEEC Individual 2020 Program Contract and Virgínia Cruz Fernandes for the Post Doc fellow (SFRH/BPD/109153/2015).
Conflicts of Interest
The authors declare no conflict of interest.
References
- European Commission. About Pollinators. Available online: https://wikis.ec.europa.eu/display/EUPKH/About+pollinators (accessed on 4 November 2022).
- Sager, M. The Honey as a Bioindicator of the Environment. Ecol. Chem. Eng. S 2017, 24, 583–594. [Google Scholar] [CrossRef]
- Kazazic, M.; Djapo-Lavic, M.; Mehic, E.; Jesenkovic-Habul, L. Monitoring of honey contamination with polycyclic aromatic hydrocarbons in Herzegovina region. Chem. Ecol. 2020, 36, 726–732. [Google Scholar] [CrossRef]
- Herrero-Latorre, C.; Barciela-Garcia, J.; Garcia-Martin, S.; Pena-Crecente, R.M. The use of honeybees and honey as environmental bioindicators for metals and radionuclides: A review. Environ. Rev. 2017, 25, 463–480. [Google Scholar] [CrossRef]
- Ponikvar, M.; Snajder, J.; Sedej, B. Honey as a bioindicator for environmental pollution with SO2. Apidologie 2005, 36, 403–409. [Google Scholar] [CrossRef]
- Balayiannis, G.; Balayiannis, P. Bee honey as an environmental bioindicator of pesticides’ occurrence in six agricultural areas of Greece. Arch. Environ. Contam. Toxicol. 2008, 55, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Ben Mukiibi, S.; Nyanzi, S.A.; Kwetegyeka, J.; Olisah, C.; Taiwo, A.M.; Mubiru, E.; Tebandeke, E.; Matovu, H.; Odongo, S.; Abayi, J.J.M.; et al. Organochlorine pesticide residues in Uganda’s honey as a bioindicator of environmental contamination and reproductive health implications to consumers. Ecotoxicol. Environ. Saf. 2021, 214, 12. [Google Scholar] [CrossRef]
- European Union. Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on Maximum Residue Levels of Pesticides in or on Food and Feed of Plant and Animal Origin and Amending Council Directive 91/414/EEC. 2005. 396/2005. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32005R0396 (accessed on 16 October 2022).
- Cabrera, L.C.; Pastor, P.M. The 2019 European Union report on pesticide residues in food. Efsa J. 2021, 19, e06491. [Google Scholar] [CrossRef]
- European Union. Commission Recommendation of 3 March 2014 on the Monitoring of Traces of Brominated Flame Retardants in Food. 2014. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32014H0118&qid=1676587691936 (accessed on 10 September 2022).
- European Union. Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuff. 2006. 1881/2006. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32006R1881&qid=1676587516415 (accessed on 10 September 2022).
- De-Melo, A.A.M.; de Almeida-Muradian, L.B.; Sancho, M.T.; Pascual-Mate, A. Composition and properties of Apis mellifera honey: A review. J. Apic. Res. 2018, 57, 33. [Google Scholar] [CrossRef]
- Solayman, M.; Islam, M.A.; Paul, S.; Ali, Y.; Khalil, M.I.; Alam, N.; Gan, S.H. Physicochemical Properties, Minerals, Trace Elements, and Heavy Metals in Honey of Different Origins: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 219–233. [Google Scholar] [CrossRef]
- da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Calatayud-Vernich, P.; Calatayud, F.; Simo, E.; Pico, Y. Efficiency of QuEChERS approach for determining 52 pesticide residues in honey and honey bees. MethodsX 2016, 3, 452–458. [Google Scholar] [CrossRef]
- Fernandes, V.C.; Domingues, V.F.; Mateus, N.; Delerue-Matos, C. Determination of Pesticides in Fruit and Fruit Juices by Chromatographic Methods. An Overview. J. Chromatogr. Sci. 2011, 49, 715–730. [Google Scholar] [CrossRef]
- Gjelstad, A.; Pedersen-Bjergaard, S. Challenges and new directions in analytical sample preparation. Anal. Bioanal. Chem. 2014, 406, 375–376. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Narenderan, S.T.; Meyyanathan, S.N.; Babu, B. Review of pesticide residue analysis in fruits and vegetables. Pre-treatment, extraction and detection techniques. Food Res. Int. 2020, 133, 109141. [Google Scholar] [CrossRef]
- Souza Tette, P.A.; Rocha Guidi, L.; de Abreu Gloria, M.B.; Fernandes, C. Pesticides in honey: A review on chromatographic analytical methods. Talanta 2016, 149, 124–141. [Google Scholar] [CrossRef]
- Chiesa, L.M.; Labella, G.F.; Panseri, S.; Britti, D.; Galbiati, F.; Villa, R.; Arioli, F. Accelerated solvent extraction by using an ‘in-line’ clean-up approach for multiresidue analysis of pesticides in organic honey. Food Addit. Contam. Part A-Chem. 2017, 34, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, P.; Porto-Figueira, P.; Pereira, J.A.M.; Silva, C.; Medina, S.; Camara, J.S. QuEChERS—Fundamentals, relevant improvements, applications and future trends. Anal. Chim. Acta 2019, 1070, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Serban Moldoveanu, V.D. Solid-Phase Extraction. In Modern Sample Preparation for Chromatography; Elsevier: Amsterdam, The Netherlands, 2015; pp. 191–286. [Google Scholar]
- Jayaraj, R.; Megha, P.; Sreedev, P. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016, 9, 90–100. [Google Scholar] [CrossRef]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Devi, P.I.; Manjula, M.; Bhavani, R.V. Agrochemicals, Environment, and Human Health. Annu. Rev. Environ. Resour. 2022, 47, 399–421. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El Nemr, A. Pesticides pollution: Classifications, human health impact, extraction and treatment techniques. Egypt J. Aquatic Res. 2020, 46, 207–220. [Google Scholar] [CrossRef]
- Md Meftaul, I.; Venkateswarlu, K.; Dharmarajan, R.; Annamalai, P.; Megharaj, M. Pesticides in the urban environment: A potential threat that knocks at the door. Sci. Total Environ. 2020, 711, 134612. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Medina, A.; Ugalde-Lizarraga, A.; Bojorquez-Cuevas, M.S.; Garnica-Ruiz, J.; Gonzalez-Corral, M.A.; Garcia-Ledezma, A.; Pineda-Garcia, G.; Cornejo-Bravo, J.M. Neuropsychiatric Disorders in Farmers Associated with Organophosphorus Pesticide Exposure in a Rural Village of Northwest Mexico. Int. J. Environ. Res. Public Health 2019, 16, 689. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Pesticides and Bees. Available online: https://food.ec.europa.eu/animals/live-animal-movements/honey-bees/pesticides-and-bees_en (accessed on 29 September 2022).
- Butler, D. Scientists Hail European Ban on Bee-Harming Pesticides. Available online: https://www.nature.com/articles/d41586-018-04987-4 (accessed on 29 September 2022).
- European Commission. Neonicotinoids. Available online: https://food.ec.europa.eu/plants/pesticides/approval-active-substances/renewal-approval/neonicotinoids_en (accessed on 29 September 2022).
- Bridi, R.; Larena, A.; Pizarro, P.N.; Giordano, A.; Montenegro, G. LC-MS/MS analysis of neonicotinoid insecticides: Residue findings in chilean honeys. Cienc. Agrotec. 2018, 42, 51–57. [Google Scholar] [CrossRef]
- European Comission. Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed 2022, 57. Available online: https://www.eurl-pesticides.eu/docs/public/tmplt_article.asp?CntID=727 (accessed on 16 October 2022).
- Pang, X.; Li, C.; Zang, C.; Guan, L.; Zhang, P.; Di, C.; Zou, N.; Li, B.; Mu, W.; Lin, J. Simultaneous detection of ten kinds of insecticide residues in honey and pollen using UPLC-MS/MS with graphene and carbon nanotubes as adsorption and purification materials. Environ. Sci. Pollut. Res. 2022, 29, 21826–21838. [Google Scholar] [CrossRef]
- Almeida, M.O.; Oloris, S.C.S.; Faria, V.H.E.; Ribeiro, M.C.M.; Cantini, D.M.; Soto-Blanco, B. Optimization of Method for Pesticide Detection in Honey by Using Liquid and Gas Chromatography Coupled with Mass Spectrometric Detection. Foods 2020, 9, 1368. [Google Scholar] [CrossRef]
- Nadaf, H.A.; Yadav, G.S.; Kumari, B. Validation and monitoring of pesticide residues in honey using QuEChERS and gas chromatographic analysis. J. Apic. Res. 2015, 54, 260–266. [Google Scholar] [CrossRef]
- Souza, A.P.F.; Petrarca, M.H.; Braga, P.A.D.; Rodrigues, N.R.; Reyes, F.G.R. Analysis of insecticide residues in honey by liquid chromatography tandem mass spectrometry using QuEChERS optimized by the Plackett Burman design. Cyta-J. Food 2021, 19, 326–332. [Google Scholar] [CrossRef]
- Ligor, M.; Bukowska, M.; Ratiu, I.A.; Gadzala-Kopciuch, R.; Buszewski, B. Determination of Neonicotinoids in Honey Samples Originated from Poland and Other World Countries. Molecules 2020, 25, 5817. [Google Scholar] [CrossRef]
- Kammoun, S.; Mulhauser, B.; Aebi, A.; Mitchell, E.A.D.; Glauser, G. Ultra-trace level determination of neonicotinoids in honey as a tool for assessing environmental contamination. Environ. Pollut. 2019, 247, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gill, J.P.S.; Bedi, J.S.; Kumar, A. Pesticide residues in Indian raw honeys, an indicator of environmental pollution. Environ. Sci. Pollut. Res. 2018, 25, 34005–34016. [Google Scholar] [CrossRef]
- Eissa, F.; El-Sawi, S.; Zidan, N.E. Determining Pesticide Residues in Honey and their Potential Risk to Consumers. Pol. J. Environ. Stud. 2014, 23, 1573–1580. [Google Scholar]
- Tette, P.A.; da Silva Oliveira, F.A.; Pereira, E.N.; Silva, G.; de Abreu Gloria, M.B.; Fernandes, C. Multiclass method for pesticides quantification in honey by means of modified QuEChERS and UHPLC-MS/MS. Food Chem. 2016, 211, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Orso, D.; Martins, M.L.; Donato, F.F.; Rizzetti, T.M.; Kemmerich, M.; Adaime, M.B.; Zanella, R. Multiresidue Determination of Pesticide Residues in Honey by Modified QuEChERS Method and Gas Chromatography with Electron Capture Detection. J. Braz. Chem. Soc. 2014, 25, 10. [Google Scholar] [CrossRef]
- Barakat, A.A.; Badawy, H.M.A.; Salama, E.; Attallah, E.; Maatook, G. Simple and rapid method of analysis for determination of pesticide residues in honey using dispersive solid phase extraction and GC determination. J. Food Agric. Environ. 2007, 5, 97–100. [Google Scholar]
- Al-Alam, J.; Fajloun, Z.; Chbani, A.; Millet, M. A multiresidue method for the analysis of 90 pesticides, 16 PAHs, and 22 PCBs in honey using QuEChERS-SPME. Anal. Bioanal. Chem. 2017, 409, 5157–5169. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Agents Classified by the IARC Monographs. Available online: https://monographs.iarc.who.int/agents-classified-by-the-iarc/ (accessed on 12 November 2022).
- European Commission. EU Pesticides Database. Available online: https://food.ec.europa.eu/plants/pesticides/eu-pesticides-database_en (accessed on 12 November 2022).
- International Agency for Research on Cancer. Monographs Available. Available online: https://monographs.iarc.who.int/monographs-available/ (accessed on 12 November 2022).
- UN Environment Programme. What Are POPs? Available online: http://chm.pops.int/TheConvention/ThePOPs/tabid/673/Default.aspx (accessed on 26 December 2022).
- Kim, L.; Lee, D.; Cho, H.K.; Choi, S.D. Review of the QuEChERS method for the analysis of organic pollutants: Persistent organic pollutants, polycyclic aromatic hydrocarbons, and pharmaceuticals. Trends Environ. Anal. Chem. 2019, 22, 16. [Google Scholar] [CrossRef]
- UN Environment Programme. All POPs Listed in the Stockholm Convention. Available online: http://chm.pops.int/TheConvention/ThePOPs/AllPOPs/tabid/2509/Default.aspx (accessed on 26 December 2022).
- Lohmann, R.; Breivik, K.; Dachs, J.; Muir, D. Global fate of POPs: Current and future research directions. Environ. Pollut. 2007, 150, 150–165. [Google Scholar] [CrossRef]
- Alegbeleye, O.O.; Opeolu, B.O.; Jackson, V.A. Polycyclic Aromatic Hydrocarbons: A Critical Review of Environmental Occurrence and Bioremediation. Environ. Manag. 2017, 60, 758–783. [Google Scholar] [CrossRef]
- Kosek, K.; Ruman, M. Arctic Freshwater Environment Altered by the Accumulation of Commonly Determined and Potentially New POPs. Water 2021, 13, 1739. [Google Scholar] [CrossRef]
- Othman, N.; Ismail, Z.; Selamat, M.I.; Sheikh Abdul Kadir, S.H.; Shibraumalisi, N.A. A Review of Polychlorinated Biphenyls (PCBs) Pollution in the Air: Where and How Much Are We Exposed to? Int. J. Environ. Res. Public Health 2022, 19, 13923. [Google Scholar] [CrossRef] [PubMed]
- Hens, B.; Hens, L. Persistent Threats by Persistent Pollutants: Chemical Nature, Concerns and Future Policy Regarding PCBs-What Are We Heading For? Toxics 2017, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. IARC Monographs Volume 107: Polychlorinated Biphenyls and Polybrominated Biphenyls. Available online: https://www.iarc.who.int/news-events/iarc-monographs-volume-107-polychlorinated-biphenyls-and-polybrominated-biphenyls/ (accessed on 10 February 2023).
- Kim, Y.A.; Park, J.B.; Woo, M.S.; Lee, S.Y.; Kim, H.Y.; Yoo, Y.H. Persistent Organic Pollutant-Mediated Insulin Resistance. Int. J. Environ. Res. Public Health 2019, 16, 448. [Google Scholar] [CrossRef]
- Zwierello, W.; Maruszewska, A.; Skorka-Majewicz, M.; Goschorska, M.; Baranowska-Bosiacka, I.; Dec, K.; Styburski, D.; Nowakowska, A.; Gutowska, I. The influence of polyphenols on metabolic disorders caused by compounds released from plastics—Review. Chemosphere 2020, 240, 124901. [Google Scholar] [CrossRef]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 13. [Google Scholar] [CrossRef]
- Ali, M.U.; Siyi, L.Y.; Yousaf, B.; Abbas, Q.; Hameed, R.; Zheng, C.M.; Kuang, X.X.; Wong, M.H. Emission sources and full spectrum of health impacts of black carbon associated polycyclic aromatic hydrocarbons (PAHs) in urban environment: A review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 857–896. [Google Scholar] [CrossRef]
- Petrovic, J.; Kartalovic, B.; Ratajac, R.; Spiric, D.; Djurdjevic, B.; Polacek, V.; Pucarevic, M. PAHs in different honeys from Serbia. Food Addit. Contam. Part B-Surveill. 2019, 12, 116–123. [Google Scholar] [CrossRef]
- dos Santos, M.; Vareli, C.S.; Janisch, B.; Pizzutti, I.R.; Fortes, J.; Sautter, C.K.; Costabeber, I.H. Contamination of polychlorinated biphenyls in honey from the Brazilian state of Rio Grande do Sul. Food Addit. Contam. Part A-Chem. 2021, 38, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Surma, M.; Wiczkowski, W.; Cieslik, E.; Zielinski, H. Method development for the determination of PFOA and PFOS in honey based on the dispersive Solid Phase Extraction (d-SPE) with micro-UHPLC-MS/MS system. Microchem. J. 2015, 121, 150–156. [Google Scholar] [CrossRef]
- Al-Alam, J.; Fajloun, Z.; Chbani, A.; Millet, M. Determination of 16 PAHs and 22 PCBs in honey samples originated from different region of Lebanon and used as environmental biomonitors sentinel. J. Environ. Sci. Health Part A-Toxic/Hazard. Subst. Environ. Eng. 2019, 54, 9–15. [Google Scholar] [CrossRef] [PubMed]
- European Union. Commission Regulation (EU) No 1259/2011 of 2 December 2011 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels for Dioxins, Dioxin-Like PCBs and Non Dioxin-Like PCBs in Foodstuffs. 2011. 1259/2011. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32011R1259&qid=1676587373985 (accessed on 16 October 2022).
- European Union. Commission Regulation (EU) 2020/1255 of 7 September 2020 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Polycyclic Aromatic Hydrocarbons (PAHs) in Traditionally Smoked Meat and Smoked Meat Products and Traditionally Smoked Fish and Smoked Fishery Products and Establishing a Maximum Level of PAHs in Powders of Food of Plant Origin Used for the Preparation of Beverages. 2020. 2020/1255. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32020R1255 (accessed on 16 October 2022).
- Jin, Y.; Zhang, J.Z.; Zhao, W.; Zhang, W.W.; Wang, L.; Zhou, J.H.; Li, Y. Development and validation of a multiclass method for the quantification of veterinary drug residues in honey and royal jelly by liquid chromatography-tandem mass spectrometry. Food Chem. 2017, 221, 1298–1307. [Google Scholar] [CrossRef]
- Xu, J.; Yang, M.; Wang, Y.H.; Yang, Y.; Tu, F.Q.; Yi, J.; Hou, J.; Lu, H.; Jiang, X.M.; Chen, D. Multiresidue analysis of 15 antibiotics in honey using modified QuEChERS and high performance liquid chromatography-tandem mass spectrometry. J. Food Compos. Anal. 2021, 103, 8. [Google Scholar] [CrossRef]
- Emir, A.I.; Ece, Y.K.; Sinem, R.; Sezer, A.; Ozge, E. Validation of two UHPLC-MS/MS methods for fast and reliable determination of quinolone residues in honey. Food Addit. Contam. Part A-Chem. 2021, 38, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.Y.; Guo, J.B.; Lv, Z.; Zhu, X.H.; Xue, X.F.; Wu, L.M.; Cao, W. Simultaneous Determination of Nitroimidazoles and Quinolones in Honey by Modified QuEChERS and LC-MS/MS Analysis. Int. J. Anal. Chem. 2018, 2018, 12. [Google Scholar] [CrossRef]
- Lombardo-Agui, M.; Garcia-Campana, A.M.; Gamiz-Gracia, L.; Cruces-Blanco, C. Determination of quinolones of veterinary use in bee products by ultra-high performance liquid chromatography-tandem mass spectrometry using a QuEChERS extraction procedure. Talanta 2012, 93, 193–199. [Google Scholar] [CrossRef]
- Gawel, M.; Kiljanek, T.; Niewiadowska, A.; Semeniuk, S.; Goliszek, M.; Burek, O.; Posyniak, A. Determination of neonicotinoids and 199 other pesticide residues in honey by liquid and gas chromatography coupled with tandem mass spectrometry. Food Chem. 2019, 282, 36–47. [Google Scholar] [CrossRef]
- European Union. Commission Regulation (EU) No 37/2010 of 22 December 2009 on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin. 2010. 37/2010. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32010R0037&qid=1676587318550 (accessed on 16 October 2022).
- Schymanski, D.; Goldbeck, C.; Humpf, H.U.; Furst, P. Analysis of microplastics in water by micro-Raman spectroscopy: Release of plastic particles from different packaging into mineral water. Water Res. 2018, 129, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, J.; Xing, B. Environmental source, fate, and toxicity of microplastics. J. Hazard. Mater. 2021, 407, 124357. [Google Scholar] [CrossRef]
- Kontrick, A.V. Microplastics and Human Health: Our Great Future to Think About Now. J. Med. Toxicol. 2018, 14, 117–119. [Google Scholar] [CrossRef]
- Plastics Europe. Plastics—the Facts 2022. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/ (accessed on 26 December 2022).
- Plastics Europe. Plastics—the Facts 2021. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2021/ (accessed on 26 December 2022).
- Plastics Europe. Plastics—the Facts 2020. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2020/ (accessed on 26 December 2022).
- Plastics Europe. Plastics—the Facts 2019. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2019/ (accessed on 26 December 2022).
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef]
- Gigault, J.; ter Halle, A.; Baudrimont, M.; Pascal, P.Y.; Gauffre, F.; Phi, T.L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Liebezeit, G.; Liebezeit, E. Origin of Synthetic Particles in Honeys. Pol. J. Food Nutr. Sci. 2015, 65, 5. [Google Scholar] [CrossRef]
- Silva, G.C.; Madrid, F.G.M.; Hernandez, D.; Pincheira, G.; Peralta, A.K.; Gavilan, M.U.; Vergara-Carmona, V.; Fuentes-Penailillo, F. Microplastics and Their Effect in Horticultural Crops: Food Safety and Plant Stress. Agronomy 2021, 11, 1528. [Google Scholar] [CrossRef]
- Conti, G.O.; Ferrante, M.; Banni, M.; Favara, C.; Nicolosi, I.; Cristaldi, A.; Fiore, M.; Zuccarello, P. Micro- and nano-plastics in edible fruit and vegetables. The first diet risks assessment for the general population. Environ. Res. 2020, 187, 7. [Google Scholar] [CrossRef]
- Liebezeit, G.; Liebezeit, E. Non-pollen particulates in honey and sugar. Food Addit. Contam. Part A-Chem. Anal. Control. Expo. Risk Assess. 2013, 30, 2136–2140. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Basantes, M.F.; Conesa, J.A.; Fullana, A. Microplastics in Honey, Beer, Milk and Refreshments in Ecuador as Emerging Contaminants. Sustainability 2020, 12, 5514. [Google Scholar] [CrossRef]
- Edo, C.; Fernandez-Alba, A.R.; Vejsnaes, F.; van der Steen, J.J.M.; Fernandez-Pinas, F.; Rosal, R. Honeybees as active samplers for microplastics. Sci. Total Environ. 2021, 767, 144481. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).