Abstract
Vanadium titanomagnetite (VTM) is an important mineral for developing titanium resources, but the comprehensive recovery of ilmenite separation is extremely poor, resulting in the low-efficiency utilization of titanium resources. Here, the separation of ilmenite from VTM ore is studied by combining magnetic separation and flotation technologies. In particular, the floatability of mixed MOH/PG-1 collectors is thoroughly investigated. The results show that a concentrate with a TiO2 grade of 9.90% can be separated via weak magnetic separation and coarse particle tailing dumping. The concentrate grade is then increased to 14.32% via strong magnetic separation and floating separation of sulfur minerals. Finally, a TiO2 grade of 46.34% is obtained through closed-circuit flotation using mixed MOH/PG-1 collectors. The mixed collectors are very efficient and can enhance the chemical adsorption of the Ti4+, Fe3+, and Fe2+ ions in the ilmenite concentrate compared with the MOH collector, thereby increasing the TiO2 grade and recovery by 3.31% and 1.20%, respectively. This is beneficial for improving the comprehensive utilization of titanium resources in VTM ores.
1. Introduction
Titanium, which is mainly extracted from rutile ores and ilmenite, is the basic raw material for the production of titanium dioxide and titanium metal [1,2]. According to the statistics related to the distribution of titanium resources in the world (TiO2 m) published by the United States Geological Survey (USGS 2021), the global titanium reserves are about 742 million tons [3]. However, high-quality rutile reserves only account for 6.2%. With the gradual depletion of these rutile reserves, ilmenite, which accounts for 93.8% of the global titanium reserves, has become the main titanium-bearing mineral considered. Vanadium titanomagnetite (VTM) is rich in Fe, V, Ti, and trace elements, such as Co, Ni, and Ga; this mineral has a high utilization value, and its development is extremely important [4,5]. It is estimated that the global VTM reserves exceed 40 billion tons; they are concentrated in Russia, China, South Africa, the United States, Canada, Norway, Finland, India, and Sweden [6,7,8]. Ilmenite and titanomagnetite are the two most useful minerals contained in VTM. Therefore, VTM has become the main target mineral for extracting titanium, especially in China [9].
VTM resources in the Panzhihua–Xichang region of China are very abundant, accounting for 27.9% of the world’s titanium reserves [10]. According to the difference in the magnetic properties between titanomagnetite and other minerals, VTM can provide a beneficiation process consisting of stage grinding and stage separation to obtain the VTM concentrate and Fe-separation tailing. Ilmenite can then be separated from the Fe-separation tailings through a strong magnetic enrichment process (or gravity separation), followed by a flotation separation process (or electric separation) [11,12]. With the gradual reduction of rich ore resources, the ore grade decreases, the particles are refined, and the mineral composition becomes more complex; thus, a smaller grinding particle size is required to obtain a high-grade concentrate. However, ultra-fine grinding particles can no longer be separated efficiently through the electric separation process. Therefore, the flotation process has become the mainstream process for ilmenite separation at present, and numerous research works have been carried out regarding ilmenite surface activation, gangue mineral inhibition, and flotation agent optimization and modification (see Table 1).

Table 1.
Summary of previous results on ilmenite flotation.
Since the surface-active point of ilmenite has a significant influence on its floatability, the flotation performance can be enhanced by adding metal ions (such as Al3+, Pt2+, and Fe3+) or chemical reagents [13,14,15,16,17,18]. Furthermore, the surface-active point of ilmenite can also be increased through ultrasonic excitation, magnetic fields, microwave excitation, etc. Carboxymethyl cells (CMCs), acidified water glass (AWG), sodium polystyrene sulfide (PSSNa), and other reagents can effectively depress titanopyroxene from entering ilmenite, thus improving the grade and recovery of ilmenite [19,20,21,22]. Currently, studies on collectors have gradually shifted their focus from single fat acids, phosic acid, arsonic acid, and hydrogen acid to a series of combined collectors, such as sodium oleate/styrene phosic acid (NaOL/SPA), Pt-BHA-NaOL (PBA), MOH (composed of A, B, and C organic compounds), sodium oleate/benzohydroxamic acid (NaOL/BHA), benzhydroxamic acid/decyclamine (BHA/DDA), and XT (prepared using the A, B, and C collectors in a certain ratio) [23,24,25,26,27,28,29,30,31,32]. The flotation effect of combined collectors on ilmenite is significantly improved compared with that of a single collector.
It should be noted in Table 1 that the grade and recovery of ilmenite concentrate after flotation are affected not only by flotation factors such as activator, depressant, and collector but also by factors such as feed grade and its mineral characteristics. As some mineral characteristics of VTM ores in Panzhihua have changed with mining, combination collectors, such as MOH and XT, which are widely used at present, can no longer provide efficient flotation of ilmenite. Thus, it is necessary to further study the mineral characteristics and flotation process parameters of VTM ores. Therefore, in this work, the separation process of ilmenite from the Panzhihua VTM is studied using the mixed MOH/PG-1 collectors. The results show that the grade and yield of the ilmenite concentrate obtained using the mixed collectors are higher than those obtained using the MOH collector.
2. Experimental Methods
2.1. Materials and Reagents
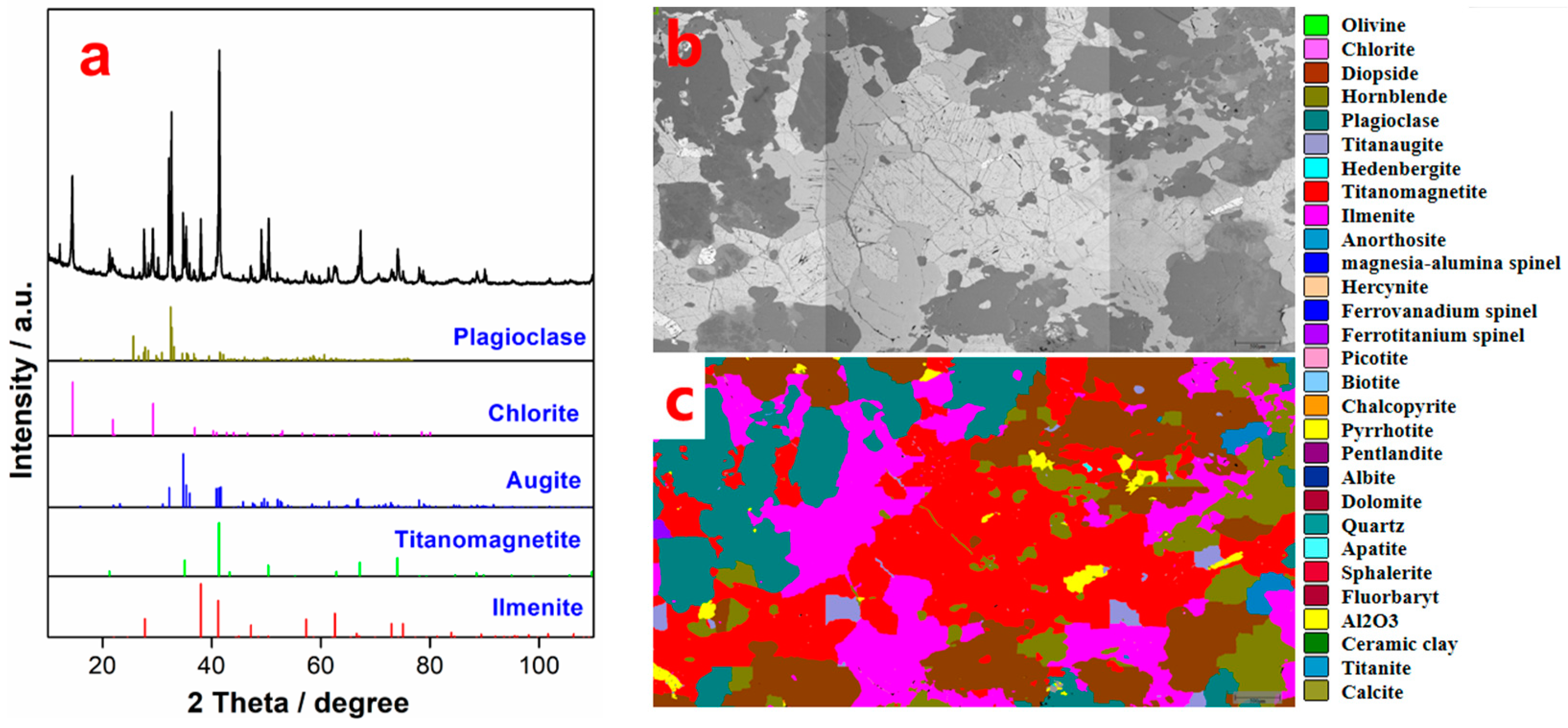
The MOH, which is a commercial collector, was provided by Hubei Jingjiang Mineral Processing Reagent Co., Ltd., China. Butyl xanthate was purchased from Condice Chemical Co., Ltd. (Wuhan, Hubei province, China). 2# oil and diesel oil were supplied by Wuhan Jiyesheng Chemical Co., Ltd., China, and China National Petroleum Corporation, respectively. The hydroxamate PG-1 collector was developed by Pangang Group Co., Ltd., China. The VTM ore with ~11.3% TiO2 and ~27.7% total iron (TFe) was also provided by Pangang Group Co., Ltd., China (see Table 2). The mineral compositions listed in Table 3 show that the contents of ilmenite and titanomagnetite are 10.48% and 28.97%, respectively. In addition, there are a large number of pyroxenes (augite, chlorite, olivine, hornblende, etc.), feldspar gangue minerals (plagioclase, anorthosite, etc.), and sulphides (pyrrhotite, pyrite, etc.) (Figure 1).

Table 2.
Chemical composition of the VTM ores (%).

Table 3.
Mineral contents of the VTM ores (%).
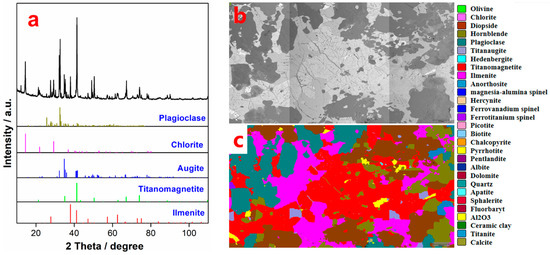
Figure 1.
XRD patterns (a), SEM images (b), and MLA diagrams (c) of VTM ores.
2.2. Magnetic Separation Enrichment
Since ilmenite and titanomagnetite have the form of close aggregates with a large particle size and are filled with plagioclase, pyroxene, and other minerals (Figure 1b,c). The VTM sample has to be ground to obtain the monomer dissociation of each mineral. Therefore, a sealed laboratory pulverizer (GJ100-1, Jiangxi Hongxing Mineral Processing Equipment Manufacturing Co., Ltd., Ganzhou, Jiangxi province, China) was used to pre-grind the ores for 15 s (particle size <0.38 mm). The ores were then crushed using a planetary ball mill (QM3SP01L, Nanjing University Instrument Factory, Nanjiang, Jiangsu province, China). To analyze the effect of the grinding particle size on the separation of titanomagnetite and other strong magnetic minerals, about 20 g of the coarsely ground ores was placed inside a wet magnetic separation tube (XCGS50, Jiangxi Hengcheng Mineral Processing Equipment Co., Ltd., Ganzhou, Jiangxi province, China) at 0.12 T.
As the particle size has a considerable impact on the grade and yield of the ilmenite concentrate in the flotation process, the particles of the low-intensity magnetic separation (LIMS) concentrate (weak magnetic and non-magnetic minerals) was sieved using a Tyler standard sieve. The TiO2 content of the LIMS concentrate with different particle sizes was then measured using inductively coupled plasma atomic emission spectrometry (ICP-AES, NexION 300D, PerkinElmer, Waltham, MA, USA) [33], and the part of the screened material with a low grade was directly discarded as tailing.
To improve the grade of ilmenite concentrate and reduce the consumption of collectors in the flotation process, the non-magnetic minerals in the LIMS concentrate can be removed through high-intensity magnetic separation (HIMS). Therefore, the LIMS concentrate was used as a raw material in a high magnetic separator (XCSQ-50 × 70, Wuhan Prospecting Machinery Factory, Wuhan, Hubei province, China) to obtain a HIMS concentrate. The influence of different magnetic intensities (0.6, 0.8, 1.0, and 1.2 T) on the yield, TiO2 content, and recovery was investigated.
The yield (η) and recovery (R) during the magnetic separation can be calculated using Equation (1) and Equation (2), respectively.
where Q1 and Q2 refer to the weight of concentrate and tailing, respectively (g); β1 and β2 represent the TiO2 content in concentrate and tailing, respectively (%).
2.3. Flotation Separation
Since sulfide minerals have an impact on the grade of the ilmenite concentrate, it is necessary to perform the separation before the ilmenite flotation. Gou et al. used 1000 g t−1 sulfuric acid, 100 g t−1 butyl xanthate, and 70 g t−1 2# oil as the surfactant, collector, and foaming agent, respectively, for the flotation separation of the metal sulfides [34]. Therefore, 25 g of the HIMS concentrate was first added to the flotation cell (XFDIV, Jilin Prospecting Machinery Factory, Changchun, Jilin province, China), which contained about 0.5 L deionized water. This process was performed while rotating the cell at a speed of 1600 rpm. 0.250 g sulfuric acid with a concentration of 10% was added to the pulp to be activated for 3 min, and 0.075 g of butyl xanthate with a concentration of 10% and 0.018 g 2# oil were then added to the activated pulp; the mixture was then stirred for 3 min. Finally, air was introduced for bubbling, and the scraper was opened for 3 min to separate metal sulfides containing foam (tailing).
The pH of the above residual pulp was adjusted by adding H2SO4 (or NaOH) with a concentration of 10% and stirring the obtained mixture for 2 min. Subsequently, a certain amount of the mixed MOH/PG-1 collectors was added to the pulp under continuous stirring for 3 min. After that, compressed air was blown into the pulp, and the scraper started to separate the foam containing ilmenite concentrate, and the flotation duration was controlled at 3 min. The foam flotation concentrate was finally filtered, dried, and weighed. All flotation experiments were repeated three times under the same conditions. Flotation experiments were also carried out using the MOH collector under the same conditions to analyze the collection ability of the mixed collector. In these experiments, 150 g t−1 of diesel oil was added to the pulp as the foaming agent [27].
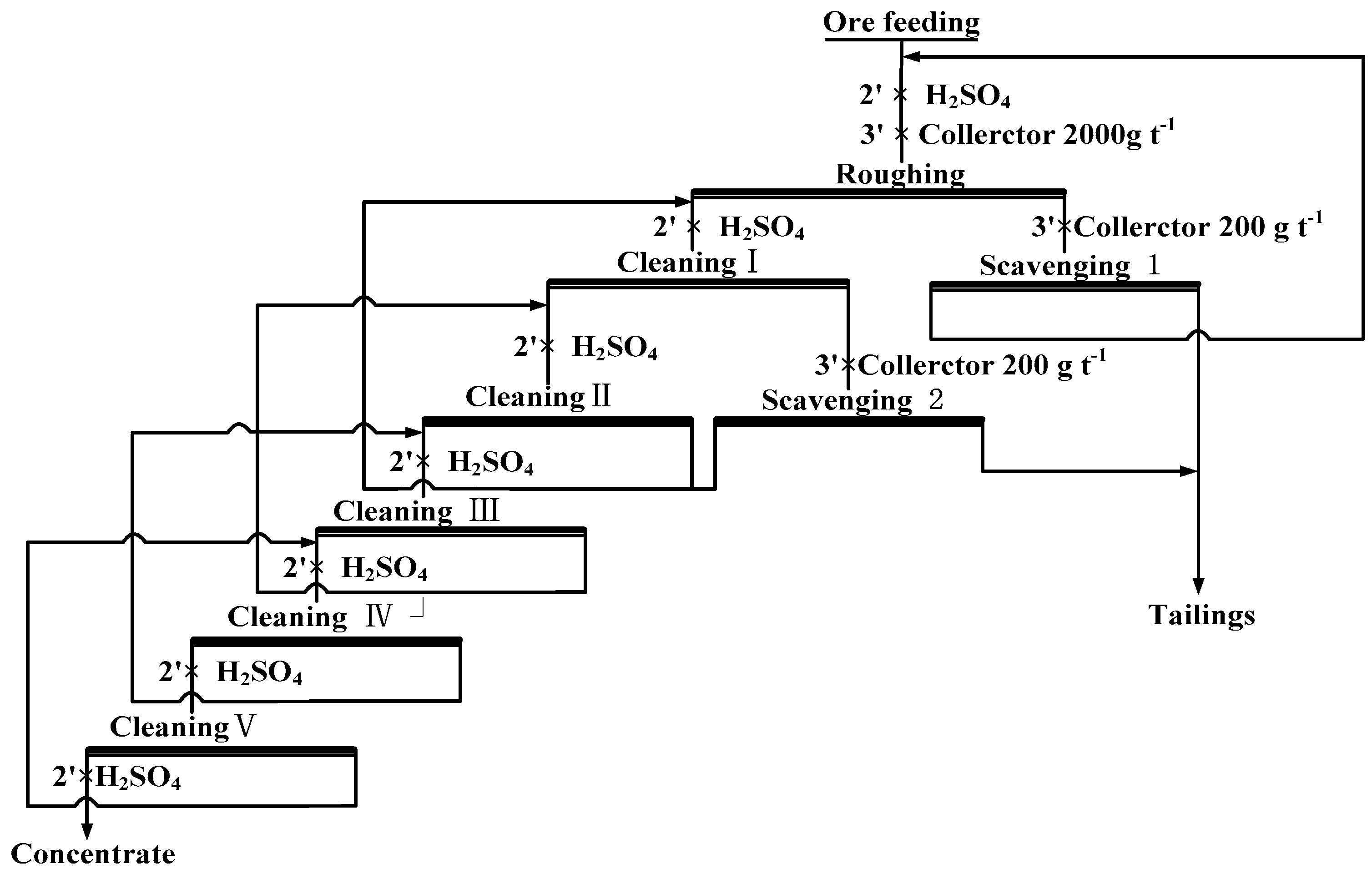
Since it is difficult to obtain high-grade ilmenite concentrate by conducting mirco-flotation tests, a closed-circuit flotation system with one roughing stage, two scavenging stages, and five cleaning stages was designed, as shown in Figure 2. The operation of this closed-circuit flotation system has been described in detail by Liu et al. [21].
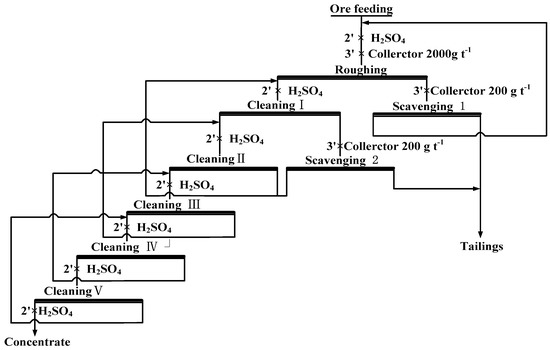
Figure 2.
Flow chart of the closed-circuit flotation process.
2.4. Characterization Methods
The phases of the SLMS concentrate and SLMS tailing were detected using an X-ray diffractometer (XRD, X’pert PRO, PANalytical, Almelo, Netherlands), equipped with an X-ray gun (Co Kα radiations), operating at 35 kV and 50 mA. To study the flotation mechanism of the MOH and PG-1 collectors, zeta potentials at different pH values were measured using a potentiometer (Nano-ZS90, Malvern Instruments, Malvern, WOR, UK). In addition, Fourier transform infrared spectroscopy (FTIR, Nicolet iS50, Thermo Fisher Scientific Co., Waltham, MA, USA) and X-ray photoelectron spectroscopy (XPS, ESCALAB Xi+Thermo Fisher Scientific Co., Waltham, MA, USA) measurements were conducted to analyze the surface chemical structure characteristics of the ilmenite concentrate.
3. Results and Discussion
3.1. Magnetic Separation
3.1.1. Low-Intensity Magnetic Separation (LIMS)
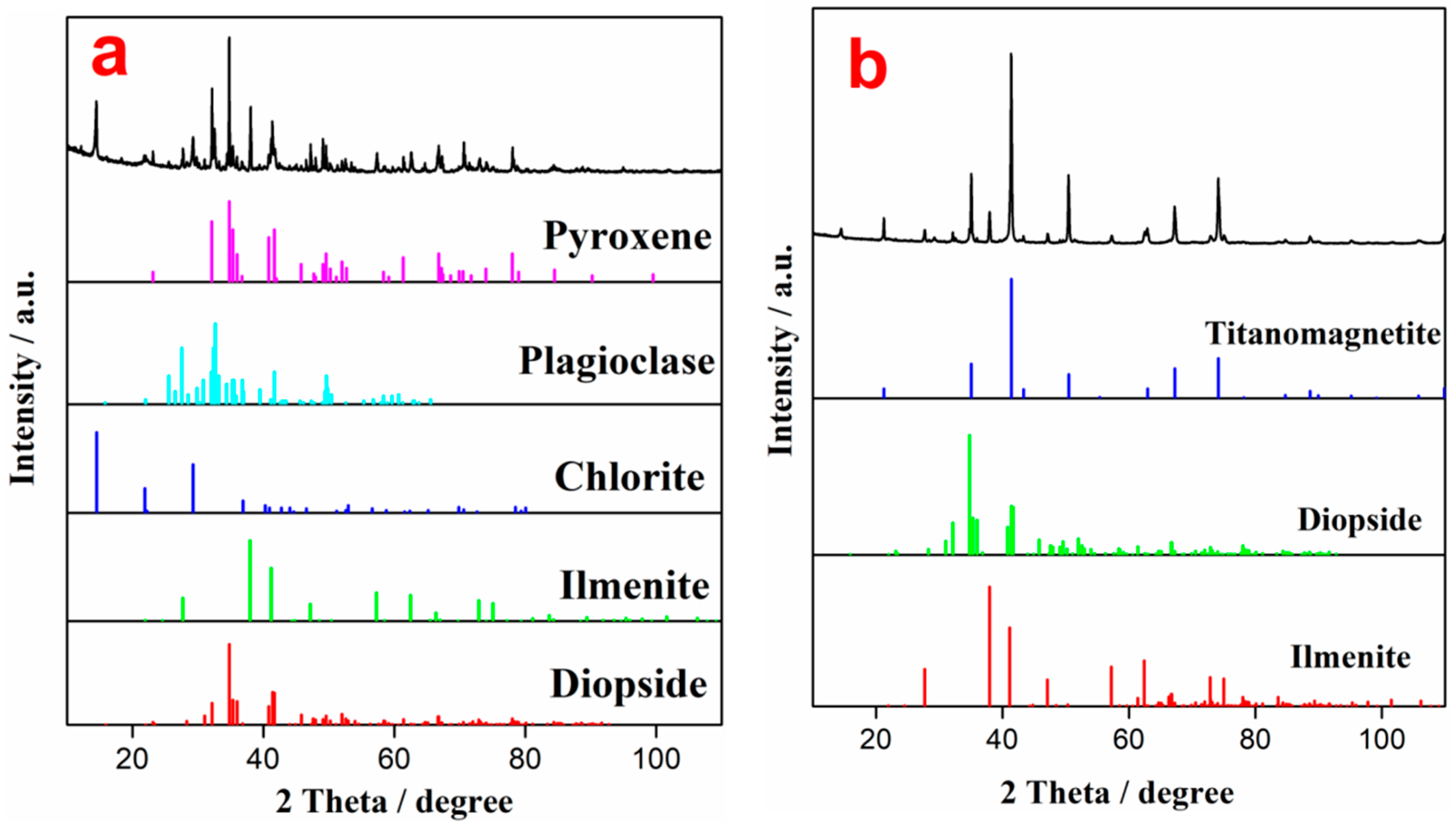
The effect of the grinding particle size on LIMS concentrate is shown in Table 4. It can be seen that both TiO2 content and recovery of the LIMS concentrate are the lowest, corresponding to the highest TiO2 content of the LIMS tailing, which indicates that the degree of monomer dissociation of ilmenite and titanomagnetite without ball milling is not sufficient [35]. With increasing ball milling time, the degree of monomer dissociation of each mineral increases, resulting in an increase in the yield, TiO2 content, and recovery of the LIMS concentrate after the weak magnetic separation process. The yield, TiO2 content, and recovery of the LIMS concentrate are 57.07%, 9.87%, and 50.27% after grinding for 60 min, respectively. As the grinding time increases further, although the TiO2 content and recovery of the LIMS concentrate continue to increase, the rate at which they increase becomes significantly lower. These results suggest that the influence of prolonged grinding on the weak magnetic separation process is reduced. Thus, the best grinding time is found to be 60 min. To further analyze the ore separation for a grinding time of 60 min, the LIMS concentrate and tailing were characterized via XRD, and the results are shown in Figure 3. It can be seen that the main phases of the LIMS concentrate are ilmenite, diopside, chlorite, plagioclase, and pyroxene. However, magnetite (titanomagnetite), diopside, and ilmenite appear in the LIMS tailing, indicating that most of the titanomagnetite enters the tailing for separation.

Table 4.
Effect of the grinding time on the LIMS process.
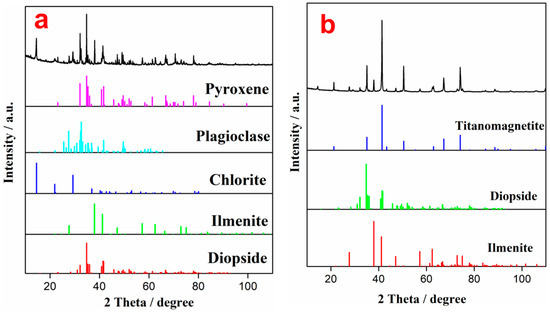
Figure 3.
XRD patterns of the SLMS concentrate (a) and SLMS tailing (b).
The yield, TiO2 content, and recovery for different particle sizes of the LIMS concentrate are shown in Table 5. It can be seen from the table that the particle size of the LIMS concentrate is mainly in the range of 0.15–0.1 mm (53.34%), while the proportion of fine particles (with size less than 0.04 mm) is only 5.79%, which is beneficial for the separation of ilmenite. The TiO2 content for different particle sizes of the LIMS concentrate is clearly different, with the TiO2 content for particle sizes exceeding 0.25 mm being the lowest (only 3.35%). These large particles can be directly discarded as the tailing (sieving separation, or SP), and the TiO2 content of the LIMS concentrate is up to 9.90%. The largest proportion of TiO2 can be found in particles with a size in the range of 0.15–0.074 mm (around 87%), which can improve the grade and recovery of ilmenite during flotation [36].

Table 5.
Mineral contents and element distributions of the LIMS concentrate.
3.1.2. High-Intensity Magnetic Separation (HIMS)
The HIMS separation results under different magnetic field strengths are shown in Table 6. It can be seen from the table that with increasing magnetic field strength, the yield and recovery of the HIMS concentrate increase, while the TiO2 content gradually decreases, which corresponds to a decrease in the TiO2 content of the HIMS tailing. The recovery of the HIMS concentrate can reach 87.19%, and the corresponding TiO2 content is 14.24% at 1.0 T. Although the TiO2 content at 1.0 T is 0.74% lower than that at 0.6 T, the recovery is increased by around 11.6%. As the magnetic field intensity is increased further (1.2 T), the TiO2 content of the HIMS concentrate decreases significantly, but the yield increases slightly. Therefore, the magnetic field intensity of 1.0 T was selected for strong magnetic separation, and the TiO2 content of the HIMS tailing was found to be 3.22%, which was also directly discarded.

Table 6.
Effect of the magnetic field strength on the HIMS process.
3.2. Flotation Separation
3.2.1. Desulfide Flotation
The concentrate grade and yield indices obtained by desulfurization flotation (DSF) are shown in Table 7. It can be seen that the DFS concentrate obtained through the flotation separation process is relatively high (99.21%). Although the TiO2 grade has been slightly improved, the removal rate of the S impurity reaches 58.71%; therefore, this process can effectively reduce the impact of the S impurity on the quality of ilmenite. In addition, TFe in the DFS concentrate decreases slightly due to the removal of a large amount of the sulfide minerals.

Table 7.
Experimental results of the desulfide flotation process.
3.2.2. Micro-Flotation
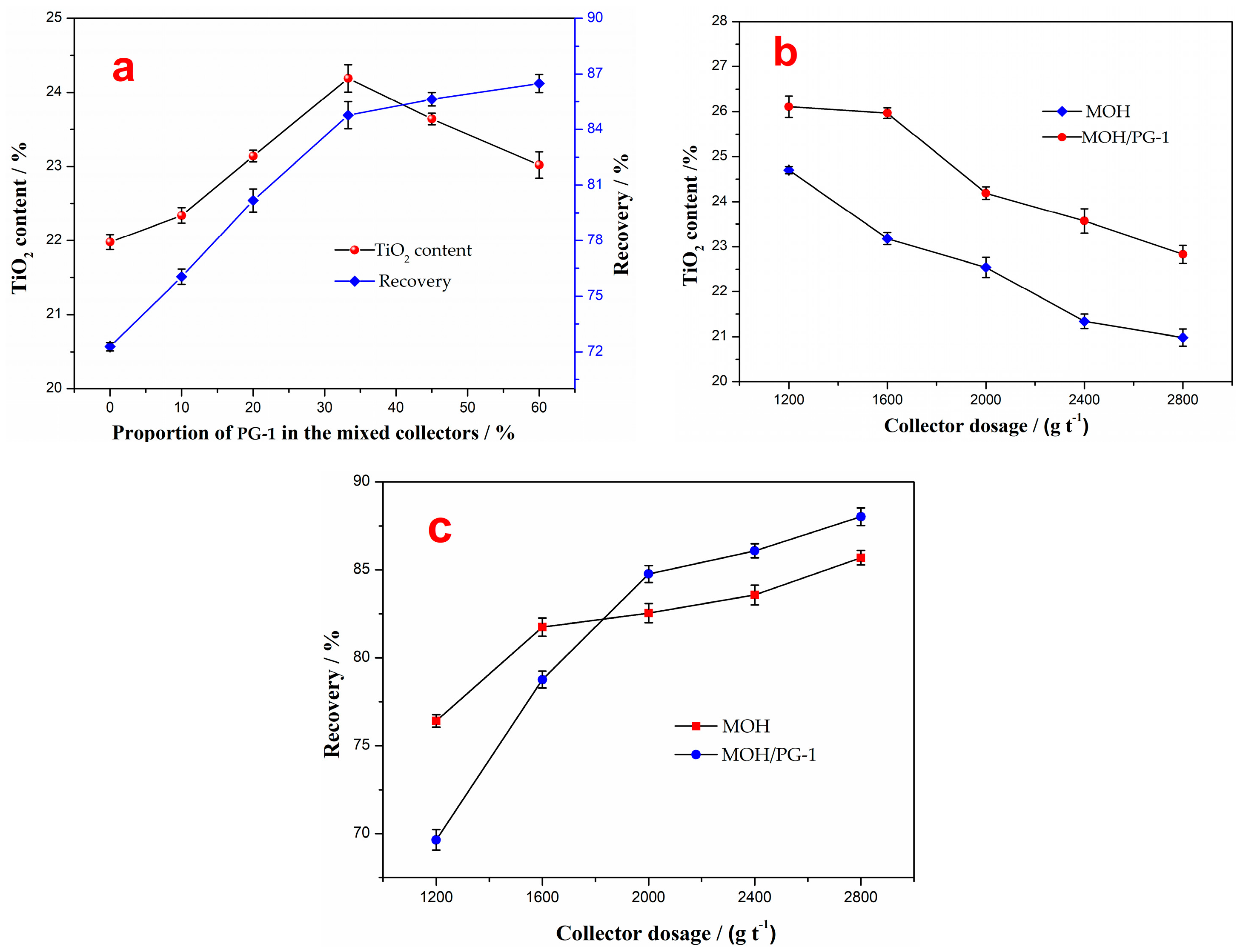
The effect of the proportion of the PG-1 collector in mixed collectors on the flotation concentrate was investigated at a pulp pH of 5 and for a collector amount of 2000 g t−1, and the results are shown in Figure 4a. It can be seen from the figure that as the proportion of PG-1 in the mixed MOH/PG collectors increases, the TiO2 content of the flotation concentrate first increases and then decreases, while the recovery increases, indicating that the collection effect of the mixed MOH/PG-1 collectors is stronger than that of MOH. However, an excessive amount of PG-1 leads to a significant decline in the TiO2 grade, resulting in a poor separation of ilmenite and the gangue minerals. Therefore, the best proportion of PG-1 in the mixed MOH/PG-1 collectors is 33.3%, at which the TiO2 content and recovery of the flotation concentrate are 24.19% and 84.77%, respectively.
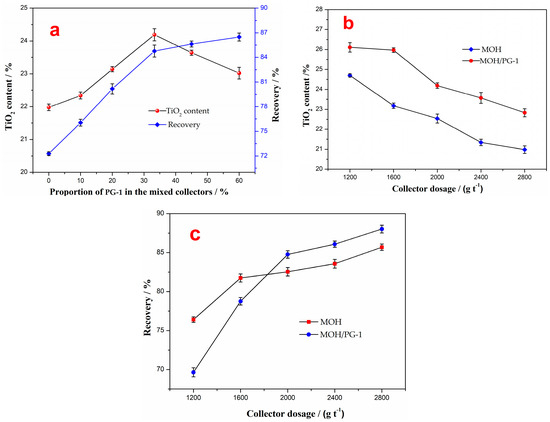
Figure 4.
Effect of the proportion of PG-1 collector in the mixed collectors on the flotation concentrate (a). TiO2 content (b) and recovery (c) of the flotation concentrate as a function of the collector dosage.
The influence of the collector dosage on the flotation of ilmenite was studied for a proportion of PG-1 in the mixed collectors of 33.3% and a pH of 5, and the results were compared with the case in which the MOH collector was used. The results show that with the increase in the amount of the mixed collector, the TiO2 content of the flotation concentrate decreases while the recovery gradually increases (Figure 4b,c). For the same amount of collector, the TiO2 content of the concentrate obtained using the mixed MOH/PG-1 collectors is around 1.5% higher than that obtained using the MOH collector. When the amount of the collector is less than 1600 g t−1, the recovery of the flotation concentrate obtained using the MOH collector is higher than that obtained using the mixed MOH/PG-1 collectors. However, the recovery of the former becomes lower than that of the latter once the amount in the collector is increased to 2000 g t−1. It should be noted that reducing the amount of collector is beneficial to improving the grade of the flotation concentrate. However, the recovery is relatively low, and there is still a large amount of ilmenite remaining in the flotation tailing. When the amount of the collector is 2000 g t−1, the recovery of the flotation concentrates obtained using the MOH and MOH/PG-1 collectors is 82.54% and 84.77%, respectively. The increase in the recovery is considerably lower than the decrease in the TiO2 content upon continually increasing the amount of the collector. Therefore, the amount of the mixed collector was selected to be 2000 g t−1.
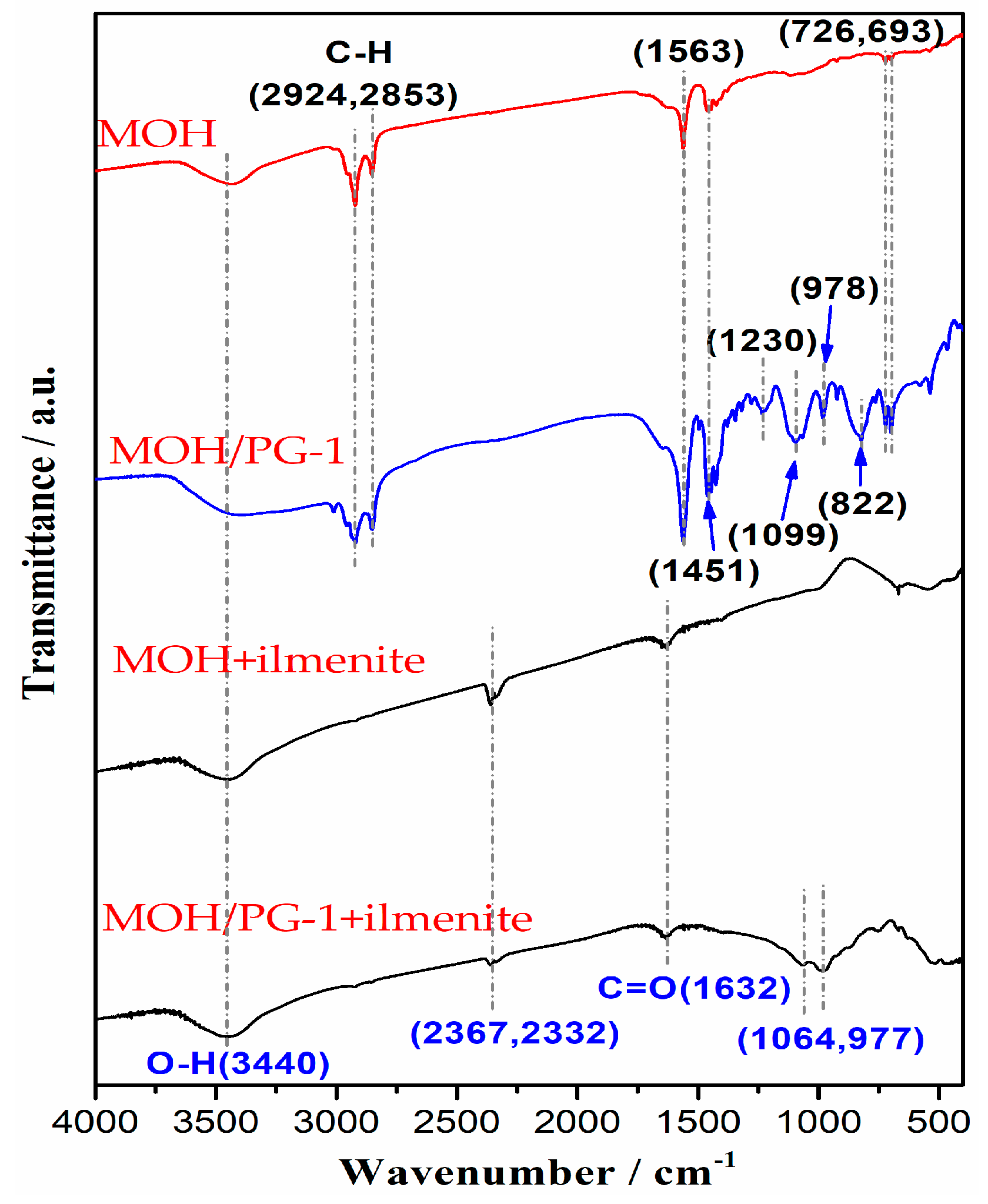
To further analyze the influence of the mixed MOH/PG-1 collectors on the ilmenite separation, the flotation concentrate obtained using a collector amount of 2000 g t−1 was investigated via FTIR. It can be seen from Figure 5 that there are seven typical absorption peaks of the MOH collector in the wavenumber range of 400–4000 cm−1; in particular, the peak at 3440 cm−1 is the stretching vibration of the O–H bond, and the peaks at 2924 and 2853 cm−1 are the vibration absorption peaks of the C–H bond [37,38]. The asymmetric stretching and symmetric vibration absorption peaks of the C=O group are observed at 1563 and 1451 cm−1, respectively, while the rocking vibration absorption peaks of the long carbon chain are observed at 726 and 693 cm−1 [39,40]. In the infrared spectrum of the mixed MOH/PG-1 collectors, in addition to the absorption peak of the MOH collector, four characteristic peaks can be observed at 1230, 1099, 978, and 822 cm−1; the two former peaks are the stretching vibration absorption peaks of the C–N and N–O bonds, respectively, while the two latter peaks are the long carbon chain vibration absorption peaks of PG-1 [41,42]. With the addition of the PG-1 collector, the characteristic absorption peaks of MOH located at 1563, 1451, 726, and 693 cm−1 become more intense, which indicates that these groups are more easily adsorbed on the surface of ilmenite. When the MOH collector interacts with ilmenite, the typical absorption peaks of the ilmenite concentrate appear at 3440, 2367, 2332, and 1632 cm−1. Compared with the MOH collector, it can be seen that the vibration absorption peak of the C=O group of the mixed MOH/PG-1 collectors is shifted by 69 cm−1 toward a higher wavenumber. This indicates that the MOH collector is chemisorbed on the surface of ilmenite, but the shift of the O–H characteristic peak is not clear; it may be caused by the physical adsorption of the collector and ilmenite [14]. In addition, the vibration absorption peaks at 2367 and 2332 cm−1 may be caused by the CO2 adsorption on the sample surface [43]. When the mixed MOH/PG-1 collectors are used for flotation, in addition to the characteristic peaks of the MOH collector, the spectrum also shows the presence of characteristic peaks at 1064 and 977 cm−1. Compared with the MOH collector, it can be seen that the N–O characteristic peak of the mixed MOH/PG-1 collectors is shifted by 35 cm−1 toward a lower wavenumber, indicating that the mixed collector is chemisorbed on the surface of ilmenite. These results suggest that the flotation effect of the MOH+PG-1 collector on ilmenite is better than that of the MOH collector.
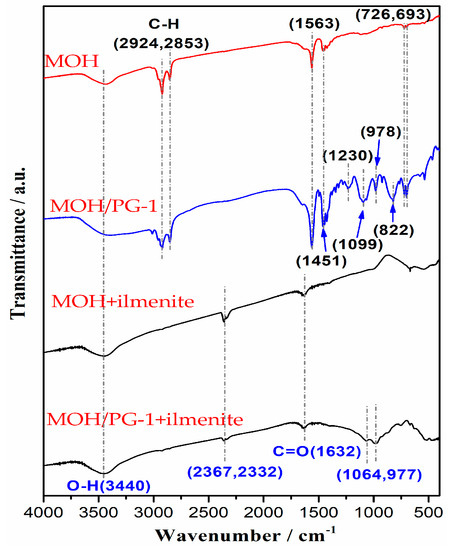
Figure 5.
Infrared spectrum of the flotation concentrates for different collectors.
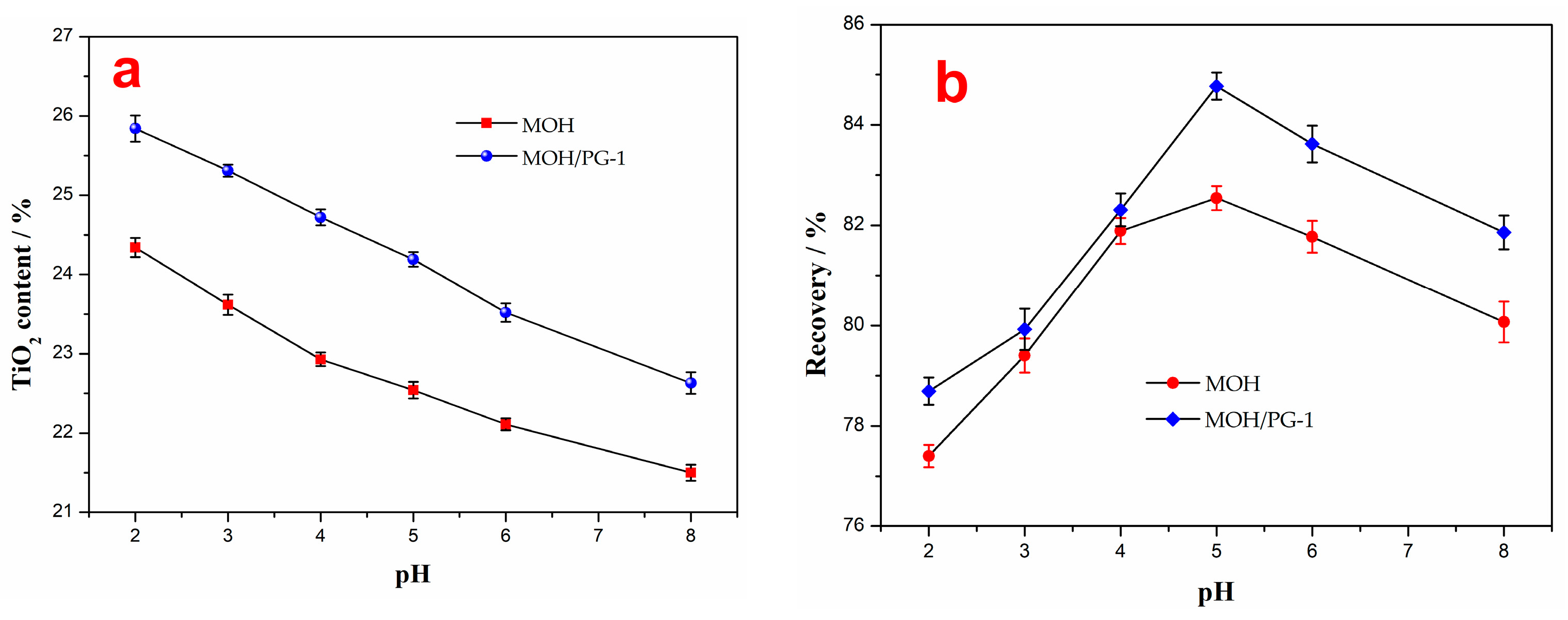
Since sulfuric acid can oxidize Fe2+ on the surface of ilmenite to Fe3+ with active sites and increase the floatability of ilmenite, it is often used to adjust the pH value of pulp [44]. Therefore, the effect of different pH values on the grade and recovery of the flotation concentrate with a collector amount of 2000 g t−1 and a proportion of PG-1 of 33.3% in the mixed collectors was investigated, and the results are shown in Figure 6. The results show that with an increasing pH value, the TiO2 content of the flotation concentrate decreases gradually, while the recovery first increases and then decreases. However, the mixed MOH/PG-1 collectors are superior to the MOH collector in separating ilmenite. It should be noted that a reduction in pH improves the grade of the flotation concentrate; however, this leads to an increase in the sulfuric acid reagent consumption and a reduction in the recovery. The recovery obtained using the MOH and MOH/PG-1 collectors is the highest at a pH value of 5 (82.54% and 84.77%, respectively), as shown in Figure 6, corresponding to TiO2 contents of 22.54% and 24.15%, respectively. Therefore, the best pH value for flotation is 5.
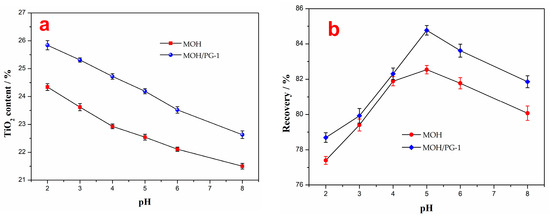
Figure 6.
TiO2 content (a) and recovery (b) of the flotation concentrate as a function of the pH value.
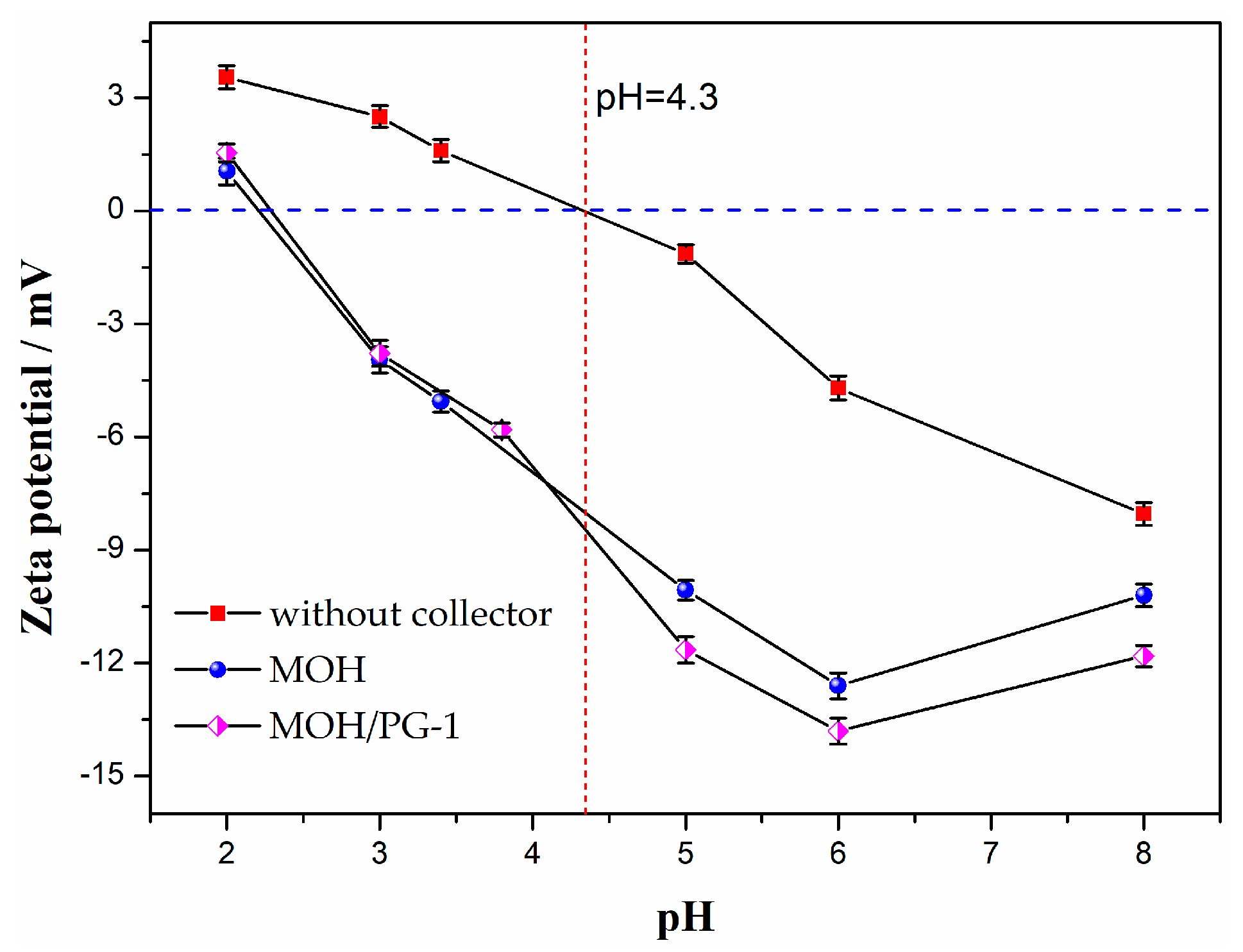
To further analyze the influence of the pH on the mechanism of the two collectors, the zeta potentials of the ilmenite concentrate obtained using the two collectors are shown in Figure 7. It can be seen that the equipotential point of ilmenite without a collector is pH = 4.3, which is consistent with the results of Xu et al. (pH = 4.6) [45]. However, the equipotential point of ilmenite is shifted considerably toward a negative value after the addition of the collector, indicating that the collector is chemisorbed on the surface of ilmenite. In particular, the shift is more pronounced for the mixed MOH/PG-1 collectors than for the MOH collector, indicating that the adsorption energy of the mixed collector on the surface of ilmenite is higher than that of the MOH collector. The Ti–O and Fe–O bonds in ilmenite are destroyed during grinding to form Ti4+ and Fe2+ unsaturated bonds, which can form Ti(OH)n4−n, Fe(OH)m2−m, and other hydroxyl complexes with OH− or H2O in aqueous solution [46]. As the solubility of Fe2+ in solution is significantly higher than that of Ti4+, positive charge ions, such as Ti(OH)3+, Ti(OH)22+, and Ti(OH)3+, can be formed on the surface of ilmenite; these cations can directly combine with the anionic collectors, resulting in the observed negative zeta potentials [47]. In addition, since the PG-1 collector containing the hydroxamate group can form five-membered ring chelate complexes with Ti or Fe in ilmenite, the mixed MOH/PG-1 collectors are superior for ilmenite collection [48].
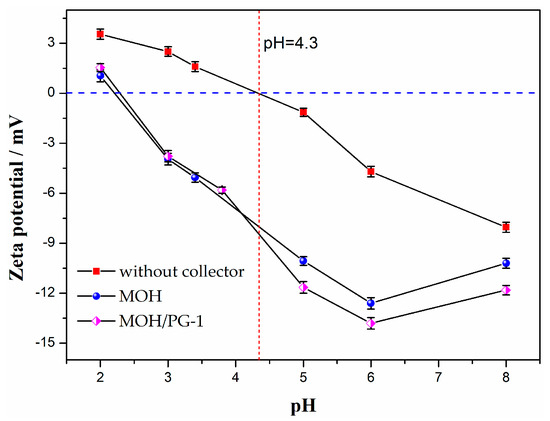
Figure 7.
Zeta potentials of ilmenite as a function of the pH value for different collectors.
3.2.3. Closed-Circuit Flotation
The results of the closed-circuit flotation experiments are shown in Table 8. It can be seen from the table that a TiO2 grade of 43.03% can be obtained via the closed-circuit flotation process using MOH as the collector; the corresponding yield and recovery are 23.32% and 70.7%, respectively. However, an ilmenite concentrate with a 46.34% TiO2 content can be obtained using MOH/PG-1 as the collector; this value is 3.31% higher than that obtained using MOH, and the corresponding recovery is 1.20% higher. By contrast, the TiO2 content of the tailing obtained using MOH+PG-1 is 0.31% lower than that obtained using MOH and can thus be neglected.

Table 8.
Results of the closed-circuit flotation process.
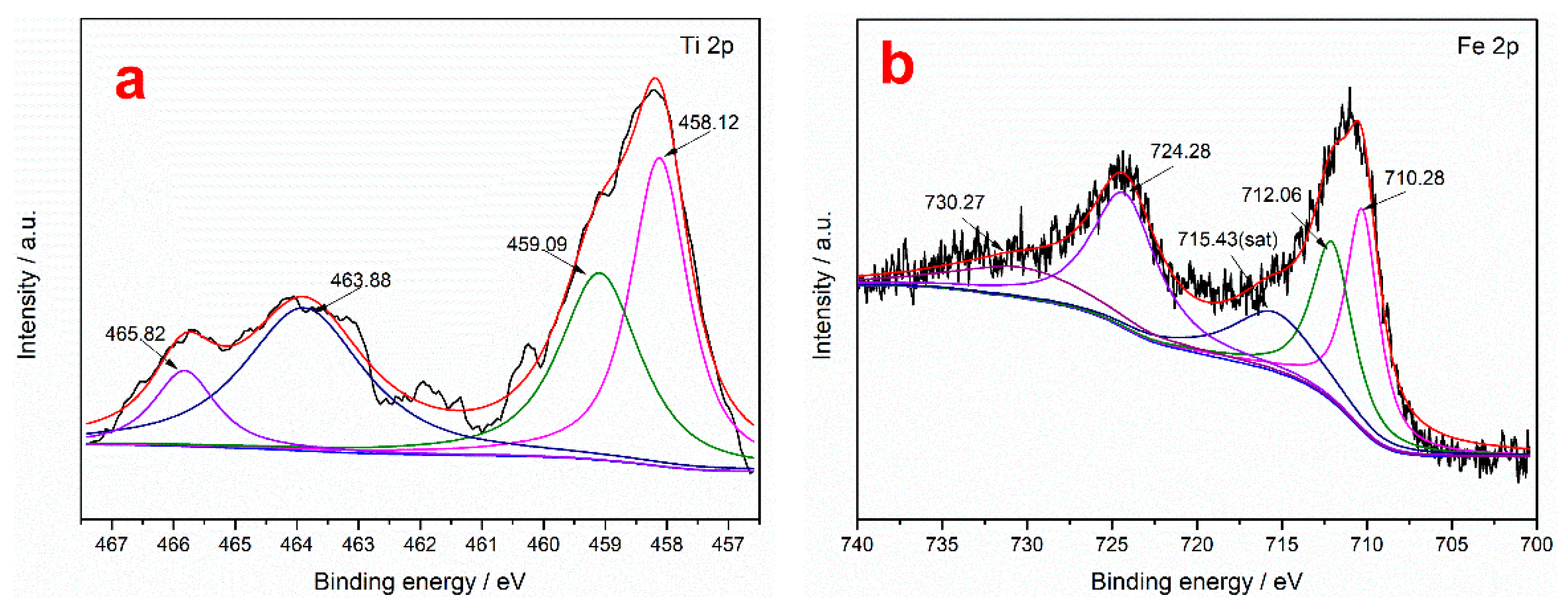
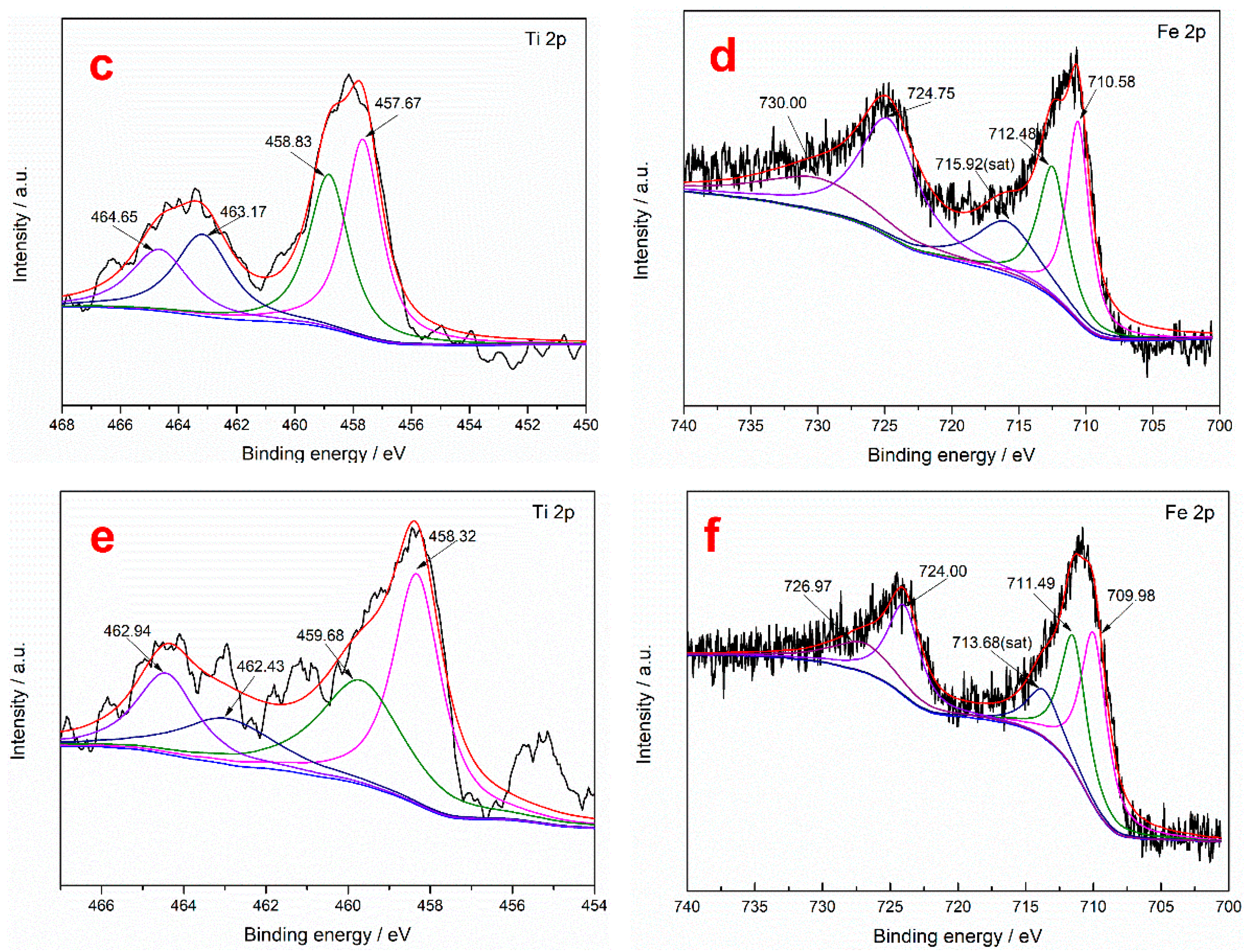
To further study the action mechanism of the collector on the surface of ilmenite, XPS measurements were performed to analyze the content of the main elements in the ilmenite concentrate obtained using the two collectors. It can be seen from Table 9 that the C and N contents of the ilmenite concentrate obtained using the mixed MOH/PG-1 collectors are higher than those obtained using the MOH collector, indicating that both the MOH and PG-1 collectors are adsorbed on the surface of the ilmenite concentrate [49]. The separation of ilmenite is enhanced due to the synergism between the MOH and PG-1 collectors. The chemical binding energies of the Fe 2p and Ti 2p peaks in the ilmenite concentrate obtained using the two collectors were determined through peak deconvolution to determine the type of flotation adsorption. It can be seen from Figure 8 that the Ti 2p photoelectron spectrum of ilmenite exhibits four characteristic peaks: The characteristic peaks at 463.88 and 458.12 eV correspond to the spin–orbit–split photoelectrons of the Ti–O–Fe 2p1/2 and Ti–O–Fe 2p3/2 orbitals in ilmenite, while the characteristic peaks at 465.82 and 459.09 eV correspond to the Ti4+ ions on the TiO2(110) surface, which are labeled TiO2 2p1/2 and TiO2 2p3/2, respectively [21]. To detect the Ti4+ signal originating from the Ti–O–Fe bond, the Ti4+ ions on the TiO2 surface were labeled Ti4+*. The Fe 2p photoelectron spectra of ilmenite shows five characteristic peaks: The characteristic peaks at 724.28 and 710.28 eV are attributed to Fe2+ 2p1/2 and Fe2+ 2p3/2, respectively; those at 730.27 and 712.06 eV represent Fe3+ 2p1/2 and Fe3+ 2p3/2, respectively; and that at 715.43 eV is a satellite peak of Fe 2p [50]. The Ti4+ and Ti4+* binding energies of the ilmenite concentrate obtained using the MOH collector shift by 0.23 and 0.63 eV in the Ti 2p3/2 spectrum, respectively, while they shift by 0.53 and 0.96 eV in the Ti 2p1/2 spectrum, respectively (Table 10). The change in the binding energies of both ions is greater than 0.2 eV, indicating that the MOH collector is chemisorbed on the Ti atoms of ilmenite [51]. Similarly, the shifts in the binding energies of Ti4+ and Ti4+* in the Ti 2p3/2 spectrum of ilmenite obtained using the mixed MOH/PG-1 collectors are 0.20 and 0.59 eV, respectively, and the corresponding shifts in the Ti 2p1/2 spectrum are 0.94 and 1.39 eV, respectively, indicating that both the MOH and PG-1 collectors are absorbed on the Ti atoms of ilmenite. For the Fe atoms in the ilmenite concentrate, the Fe2+ and Fe3+ binding energies for the MOH collector shift by 0.30 and 0.42 eV in the 2p3/2 spectrum, respectively, and the corresponding shifts in the Fe 2p1/2 spectrum are 0.47 and 0.27 eV, respectively, indicating that MOH is adsorbed on the Fe2+ and Fe3+ ions of the ilmenite concentrate. When using the mixed MOH/PG-1 collectors, the Fe2+ and Fe3+ binding energies shift by 0.30 and 0.57 eV in the 2p3/2 spectrum, respectively, and the corresponding shifts in the Fe 2p1/2 spectrum are 0.28 and 3.30 eV, respectively. The chemisorption of the mixed collectors on Fe3+ is enhanced compared with that of the MOH collector; the chemisorption of the mixed collectors on the Fe3+ ions is stronger than that on the Fe2+ ions. Therefore, the flotation capacity of ilmenite is enhanced. The above results indicate that the mixed MOH/PG-1 collectors are absorbed on the Ti4+, Fe2+, and especially the Fe3+ ions of ilmenite during flotation.

Table 9.
Relative atomic concentrations on the ilmenite surface for different flotation agents (at. %).
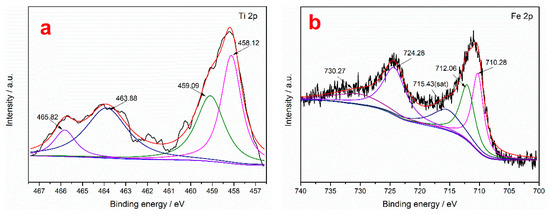
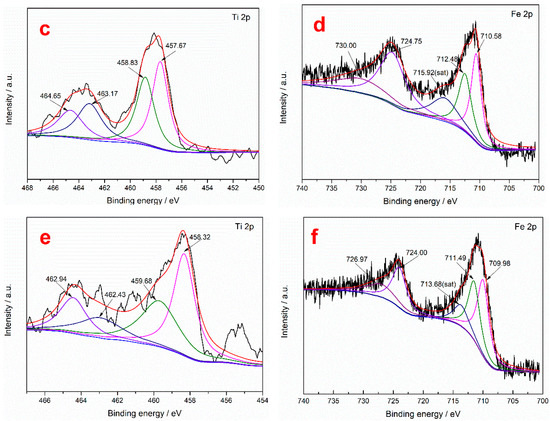
Figure 8.
Fe 2p and Ti 2p XPS spectra of ilmenite for different flotation agents: without collector (a,b), with the MOH collector (c,d), and with the mixed MOH/PG-1 collectors (e,f).

Table 10.
Binding energies of the Fe 2p and Ti 2p peaks on the ilmenite surface for different flotation agents.
4. Conclusions
In this work, ilmenite from Panzhihua VTM ore was separated by combining magnetic separation and flotation processes. The effect of the mixed MOH/PG-1 collectors on the flotation separation of ilmenite was studied via XRD, FTIR, XPS, and other techniques. The results show that an ilmenite concentrate with 46.34% TiO2 can be separated from VTM ores through the LIMS process, sieving separation process, HIMS process, DSF process, and flotation separation process using MOH/PG-1 as the collector. Compared with the MOH collector, the TiO2 grade and recovery of the ilmenite concentrate are increased by 3.31% and 1.20%, respectively, because the mixed MOH/PG-1 collectors are more efficient in strengthening the chemical adsorption of the Ti4+, Fe3+, and Fe2+ ions in the ilmenite concentrate. These findings will be useful in improving the comprehensive utilization of titanium resources in VTM ores.
Author Contributions
Conceptualization, F.Z. and Z.M.; methodology, F.Z.; software, W.P.; validation, F.Z., Z.M. and K.Q.; formal analysis, F.Z.; investigation, F.Z.; resources, K.Q.; data curation, Z.M.; writing—original draft preparation, F.Z.; writing—review and editing, K.Q.; visualization, F.Z.; supervision, K.Q.; project administration, Z.M.; funding acquisition, F.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The research presented in this work was funded by the National Basic Research and Development Program of China (2013CB632600).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data is availabile to contact the author (pzhzfx@sina.com).
Acknowledgments
This work was supported by the National Basic Research and Development Program of China.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fray, D.J. Novel methods for the production of titanium. Int. Mater. Rev. 2013, 53, 317–325. [Google Scholar] [CrossRef]
- Hu, X.; Luo, F.; Lin, J.; Wang, M.; Li, X. Dynamic material flow analysis of titanium sponge in China: 2000–2019. J. Clean. Prod. 2022, 371, 1333704. [Google Scholar] [CrossRef]
- Mineral Commodity Summaries 2021; U.S. Geological Survey: Reston, VA, USA, 2021; pp. 174–176. [CrossRef]
- Jena, B.C.; Dresler, W.; Reilly, I.G. Extraction of titanium, vanadium and iron from titanomagnetite deposits at pipestone lake, Manitoba, Canada. Miner. Eng. 1995, 8, 159–168. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Wang, L.N.; Chen, D.S.; Wang, W.J.; Liu, Y.H.; Zhao, H.X.; Qi, T. A method for recovery of iron, titanium, and vanadium from vanadium-bearing titanomagnetite. Int. J. Min. Met. Mater. 2018, 25, 131–144. [Google Scholar] [CrossRef]
- Chen, S.; Fu, X.; Chu, M.; Liu, Z.; Tang, J. Life cycle assessment of the comprehensive utilisation of vanadium titano-magnetite. J. Clean. Prod. 2015, 101, 122–128. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, L.; Liu, Y.; Qi, T.; Wang, J.; Wang, L. A novel process for recovery of iron, titanium, and vanadium from titanomagnetite concentrates: NaOH molten salt roasting and water leaching processes. J. Hazard. Mater. 2013, 244–245, 588–595. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, X.; Xu, C.; Li, W.; Kwauk, M. A new process for comprehensive utilization of complex titania ore. Miner. Eng. 2006, 19, 975. [Google Scholar] [CrossRef]
- Liu, X.J.; Chen, D.S.; Chu, J.L.; Wang, W.J.; Li, Y.L.; Qi, T. Recovery of titanium and vanadium from titanium-vanadium slag obtained by direct reduction of titanomagnetite concentrates. Rare Metals 2022, 41, 1688–1696. [Google Scholar] [CrossRef]
- Pang, K.; Zhou, M.; Qi, L.; Shellnutt, G.; Wang, C.Y.; Zhao, D. Flood basalt-related Fe–Ti oxide deposits in the Emeishan large igneous province, SW china. Lithos 2010, 119, 123–136. [Google Scholar] [CrossRef]
- Guo, X.; Dai, S.; Wang, Q. Influence of different comminution flowsheets on the separation of vanadium titano-magnetite. Miner. Eng. 2020, 149, 106268. [Google Scholar] [CrossRef]
- Hao, Z.; Fei, H.; Liu, L.; Turner, S. Comprehensive Utilization ofVanadium-Titanium Magnetite Deposits in China Has Come to a New Level. Acta Geol. Sin-Engl. 2012, 87, 286–287. [Google Scholar]
- Wei, X.; Shao, Y.; Yu, J.; Zhang, B.; Shu, H.; Zhang, Y. Activation of ilmenite flotation by Al3+ in the benzohydroxamic acid (BHA) system. Sep. Purif. Technol. 2022, 299, 121770. [Google Scholar] [CrossRef]
- Xu, L.; Tian, J.; Wu, H.; Lu, Z.; Yang, Y.; Sun, W.; Hu, Y. Effect of Pb2+ ions on ilmenite flotation and adsorption of benzohydroxamic acid as a collector. Appl. Surf. Sci. 2017, 425, 795–802. [Google Scholar] [CrossRef]
- Liao, R.; Wen, S.; Liu, J.; Zuo, Q.; Zheng, Y.; Luo, D. Flotation behavior and mechanism of ilmenite using ferrate as activator. Miner. Eng. 2020, 178, 107400. [Google Scholar] [CrossRef]
- Wu, H.; Fang, S.; Shu, K.; Xu, Y.; Wang, Z.; Luo, L.; Yang, J.; Xu, L. Selective flotation and adsorption of ilmenite from titanaugite by a novel method: Ultrasonic treatment. Powder Technol. 2020, 363, 38–47. [Google Scholar] [CrossRef]
- Xu, Y.; Yuan, Z.; Meng, Q.; Zhao, X.; Du, Y. Enhancing the flotation performance of ilmenite with the magnetic treatment of water. Sep. Purif. Technol. 2022, 57, 83–93. [Google Scholar] [CrossRef]
- Zhai, J.; Chen, P.; Wang, H.; Hu, Y.; Sun, W. Flotability Improvement of Ilmenite Using Attrition-Scrubbing as a Pretreatment Method. Minerals 2017, 7, 13. [Google Scholar] [CrossRef]
- Yuan, Z.; Du, Y.; Meng, Q.; Zhang, C.; Xu, Y.; Zhao, X. Adsorption differences of carboxymethyl cellulose depressant on ilmenite and titanaugite. Miner. Eng. 2021, 166, 106887. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, L.; Tian, J.; Liu, Y.; Han, Y. Selective flotation of ilmenite from olivine using the acidified water glass as depressant. Int. J. Miner. Process 2016, 157, 73–79. [Google Scholar] [CrossRef]
- Liu, N.; Wang, Z.; Xiao, J.; Wang, H.; Deng, B.; Zhang, Y.; Chen, C. Novel Selective Depressant of Titanaugite and Implication for Ilmenite Flotation. Minerals 2019, 9, 703. [Google Scholar] [CrossRef]
- Du, Y.; Meng, Q.; Yuan, Z.; Ma, L.; Zhao, X.; Xu, Y. Study on the flotation behavior and mechanism of ilmenite and titanaugite with sodium oleate. Miner. Eng. 2020, 152, 106366. [Google Scholar] [CrossRef]
- Deng, L.; Zhu, L.; Fei, L.; Ma, X.; Deng, F.; Zuo, R.; Huang, Z.; Li, L.; Xie, Y.; Xiao, Z.; et al. Froth flotation of ilmenite by using the dendritic surfactant 2-decanoylamino-pentanedioic acid. Miner. Eng. 2021, 165, 106861. [Google Scholar] [CrossRef]
- Wang, S.; Xiao, W.; Ma, X.; Li, J.; Chen, L.; Yao, H. Analysis of the Application Potential of Coffee Oil as an Ilmenite Flotation Collector. Minerals 2019, 9, 505. [Google Scholar] [CrossRef]
- Wu, H.; Luo, L.; Zhang, Y.; Meng, J.; Huo, X.; Zhou, H.; Xu, L. New insight into adsorption of novel ternary mixed collector in ilmenite–titanaugite flotation system. Miner. Eng. 2022, 176, 107319. [Google Scholar] [CrossRef]
- Yang, S.; Xu, Y.; Liu, C.; Sorya, D.A.D.; Li, C.; Li, H. Investigations on the synergistic effect of combined NaOl/SPA collector in ilmenite flotation. Colloid. Surface. A 2021, 628, 127267. [Google Scholar] [CrossRef]
- Fan, G.; Liu, J.; Cao, Y. Optimization of flotation process on fine ilmenite using flotation column. Physicochem. Probl. Miner. Process. 2014, 50, 823–834. [Google Scholar] [CrossRef]
- Zhang, H.; Zeng, J.; Xie, H.; Guan, C.; Chen, L. Enhanced separation for ilmenite tailing with a novel HGMS-flotation process. Sep. Purif. Technol. 2020, 55, 752–760. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, L.; Liu, Y.; Han, Y. Flotation separation of ilmenite from titanaugite using mixed collectors. Sep. Purif. Technol. 2016, 51, 1840–1846. [Google Scholar] [CrossRef]
- Luo, L.; Wu, H.; Xu, L.; Meng, J.; Lu, J.; Zhou, H.; Huo, X.; Huang, L. An in situ ATR-FTIR study of mixed collectors BHA/DDA adsorption in ilmenite-titanaugite flotation system. Int. J. Sci. Technol. 2021, 31, 689–697. [Google Scholar] [CrossRef]
- Yu, Q.; Tian, F.; Cao, Y.; Fan, G.; Hao, H.; Peng, W.; Zhou, G.; Li, P. Application of Waste Engine Oil for Improving Ilmenite Flotation Combined with Sodium Oleate Collector. Minerals 2021, 11, 1242. [Google Scholar] [CrossRef]
- Cai, J.; Deng, J.; Wang, L.; Hu, M.; Xu, H.; Hou, X.; Wu, B.; Li, S. Reagent types and action mechanisms in ilmenite flotation: A review. Int. J. Miner. Metall. Mater. 2022, 29, 1656–1669. [Google Scholar] [CrossRef]
- Hirata, S.; Umezaki, Y.; Ikeda, M. Determination of chromium(III), titanium, vanadium, iron(III), and aluminum by inductively coupled plasma atomic emission spectrometry with an on-line preconcentrating ion-exchange column. Anal. Chem. 1986, 58, 2602–2606. [Google Scholar] [CrossRef]
- Gou, M.; Yang, L.; Xue, G.; Wu, J. Application of Mixed Collector in Flotation of Low-grade Ilmenite. Multipurp. Util. Miner. Resour. 2015, 3, 40–44. (In Chinese) [Google Scholar] [CrossRef]
- Ursula, S.; Dominique, L.; Michael, B.; Ralf, E. The titanomagnetite-ilmenite equilibrium: New experimental data and thermo-oxybarometric application to the crystallization of basic to intermediate rocks. J. Petrol. 2008, 49, 1161–1185. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, L.; Deng, J.; Liu, S. Influence of particle size on flotation separation of ilmenite, olivine, and pyroxene. Physicochem. Probl. Miner. Process. 2021, 57, 106–117. [Google Scholar] [CrossRef]
- Meng, Q.; Yuan, Z.; Yu, L.; Xu, Y.; Du, Y.; Zhang, C. Selective depression of titanaugite in the ilmenite flotation with carboxymethyl starch. Appl. Surf. Sci. 2018, 440, 955–962. [Google Scholar] [CrossRef]
- Song, Q.; Tsai, S.C. Flotation of ilmenite using benzyl arsonic acid and acidified sodium silicate. Int. J. Miner. 1989, 26, 111–121. [Google Scholar] [CrossRef]
- Fang, S.; Xu, L.; Wu, H.; Jia, T.; Hu, Y. Adsorption of Pb(Ⅱ)/benzohydroxamic acid collector complexes for ilmenite flotation. Miner. Eng. 2018, 126, 16–23. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhao, X.; Meng, Q.; Zhang, Y.; Xu, Y.; Li, L. Adsorption mode of sodium citrate for achieving effective flotation separation of ilmenite from titanaugite. Miner. Eng. 2021, 171, 107086. [Google Scholar] [CrossRef]
- Zhang, C.; Li, P.; Cao, Y.; Hao, H.; Peng, W.; Teng, D.; Fan, G. Synthesis of sodium oleate hydroxamate and its application as a novel flotation collector on the ilmenite-forsterite separation. Sep. Purif. Technol. 2022, 284, 120283. [Google Scholar] [CrossRef]
- Bai, S.; Yu, P.; Ding, Z.; Bi, Y.; Wen, S. New insights into lead ions activation for microfine particle ilmenite flotation in sulfuric acid system: Visual MINTEQ models, XPS, and ToF-SIMS studies. Miner. Eng. 2020, 155, 106473. [Google Scholar] [CrossRef]
- Liu, X.; Xie, J.; Huang, G.; Li, C. Low-temperature performance of cationic collector undecyl propyl ether amine for ilmenite flotation. Miner. Eng. 2017, 114, 50–56. [Google Scholar] [CrossRef]
- Rezai, I.B. Effect of chemical composition and crystal chemistry on the zeta potential of ilmenite. Colloid. Surface. A 2013, 428, 111–119. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, H.; Tang, Q.; Wang, S.; Zhao, G.; Liu, G. A novel collector 2-ethyl-2-hexenoic hydroxamic acid: Flotation performance and adsorption mechanism to ilmenite. Appl. Surf. Sci. 2015, 353, 882–889. [Google Scholar] [CrossRef]
- Taran, M.N. Electronic intervalence Fe2+ + Ti4+ → Fe3+ + Ti3+ charge-transfer transition in ilmenite. Phys. Chem. Miner. 2019, 46, 839–843. [Google Scholar] [CrossRef]
- Parapari, P.S.; Irannajad, M.; Mehdilo, A. Modification of ilmenite surface properties by superficial dissolution method. Miner. Eng. 2016, 92, 160–167. [Google Scholar] [CrossRef]
- Wei, Z.; Hu, Y.; Han, H.; Sun, W. Configurations of lead(Ⅱ)-benzohydroxamic acid complexes in colloid and interface: A new perspective. J. Colloid Interf. Sci. 2019, 562, 342–351. [Google Scholar] [CrossRef]
- Li, M.; Liu, Z.; Wang, B.; Teng, X.; Jiang, H. Selective flotation separation of fluorite from calcite using mixed anionic/cationic collectors. Miner. Eng. 2022, 178, 107423. [Google Scholar] [CrossRef]
- Chen, P.; Zhai, J.; Sun, W.; Hu, Y.; Yin, Z. The activation mechanism of lead ions in the flotation of ilmenite using sodium oleate as a collector. Miner. Eng. 2017, 111, 100–107. [Google Scholar] [CrossRef]
- Zhai, J.; Chen, P.; Sun, W.; Chen, W.; Wan, S. A review of mineral processing of ilmenite by flotation. Miner. Eng. 2020, 157, 106558. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).