Effect of Surfactants on Reverse Osmosis Membrane Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feed Solution:
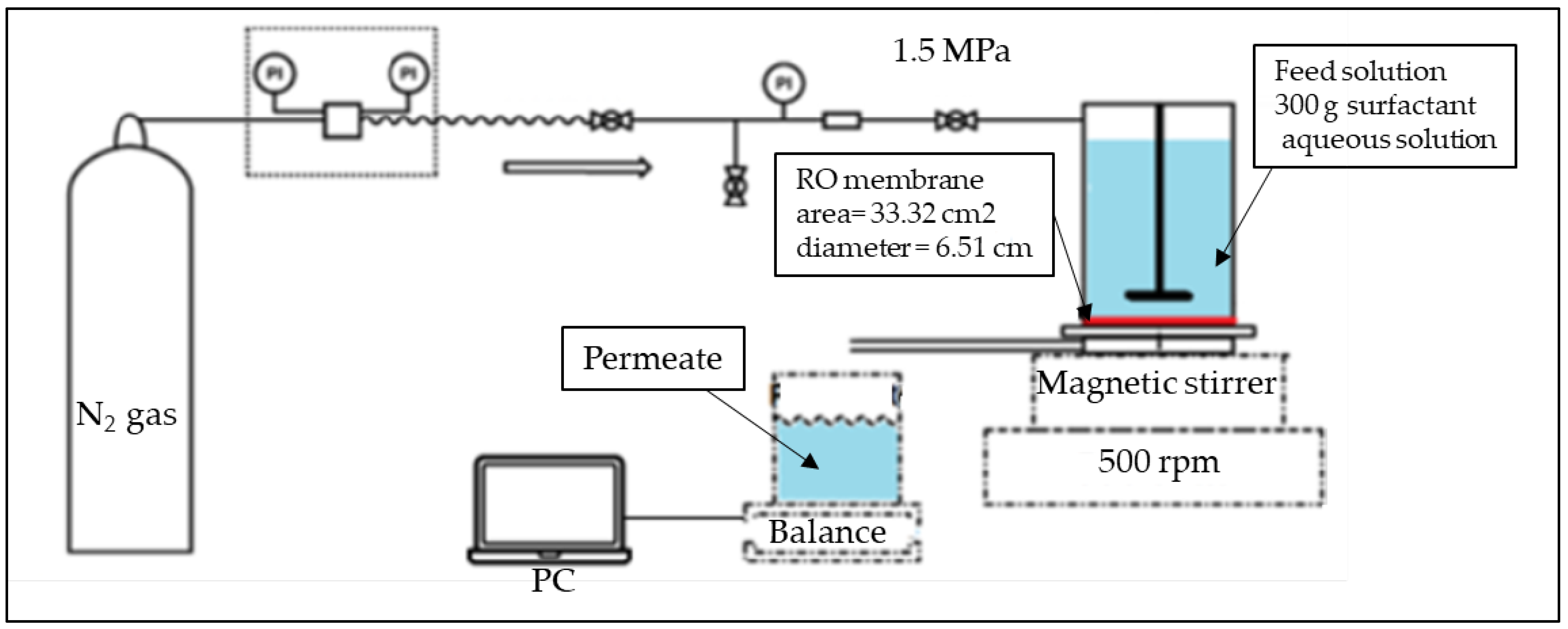
2.2. Separation Using RO Membrane:
- -
- “UTC-73” as PA thin film composite (TFC) membrane.
- -
- “SC-3000” as CA membrane.
3. Results and Discussion
3.1. Surfactant Aqueous Solution Separation
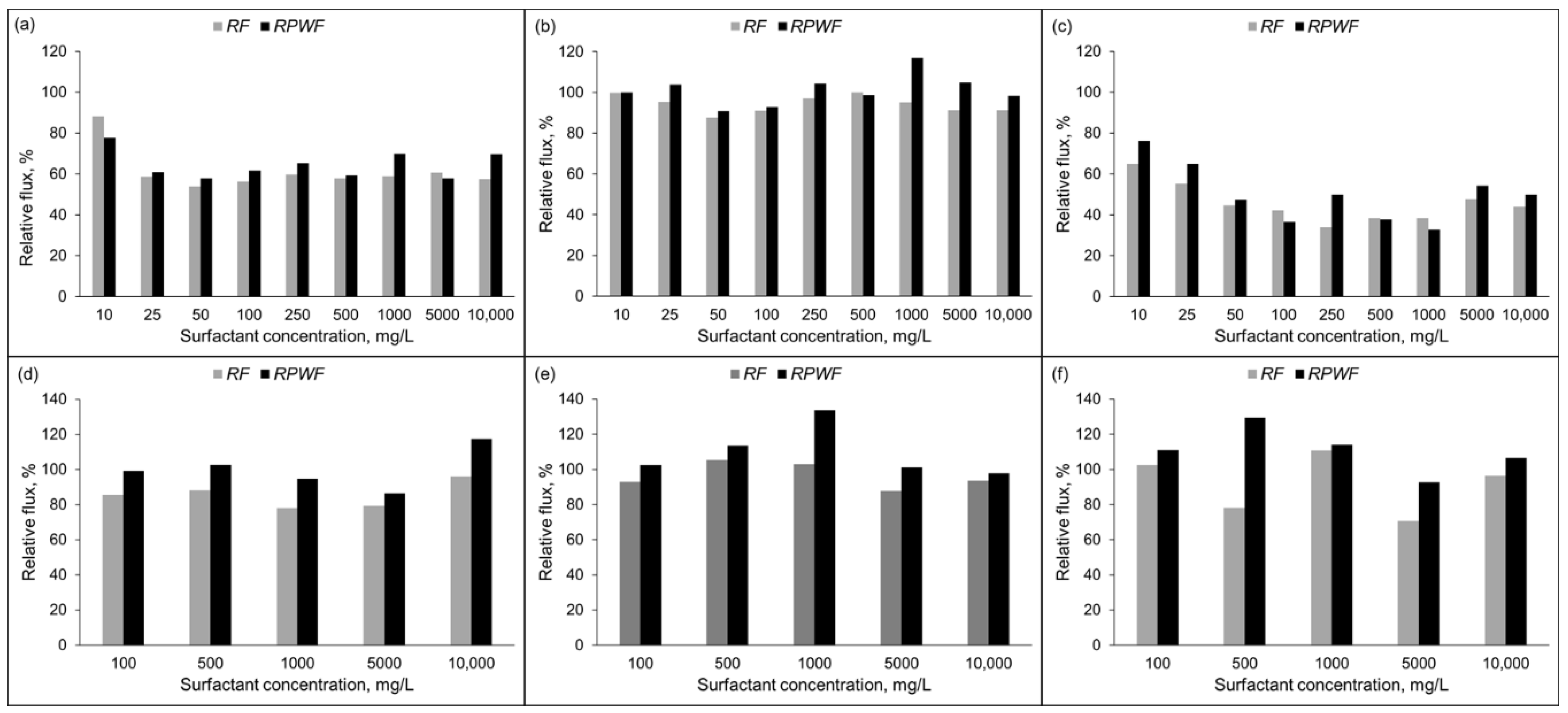
3.1.1. Membranes Fluxes as Function of Test Time
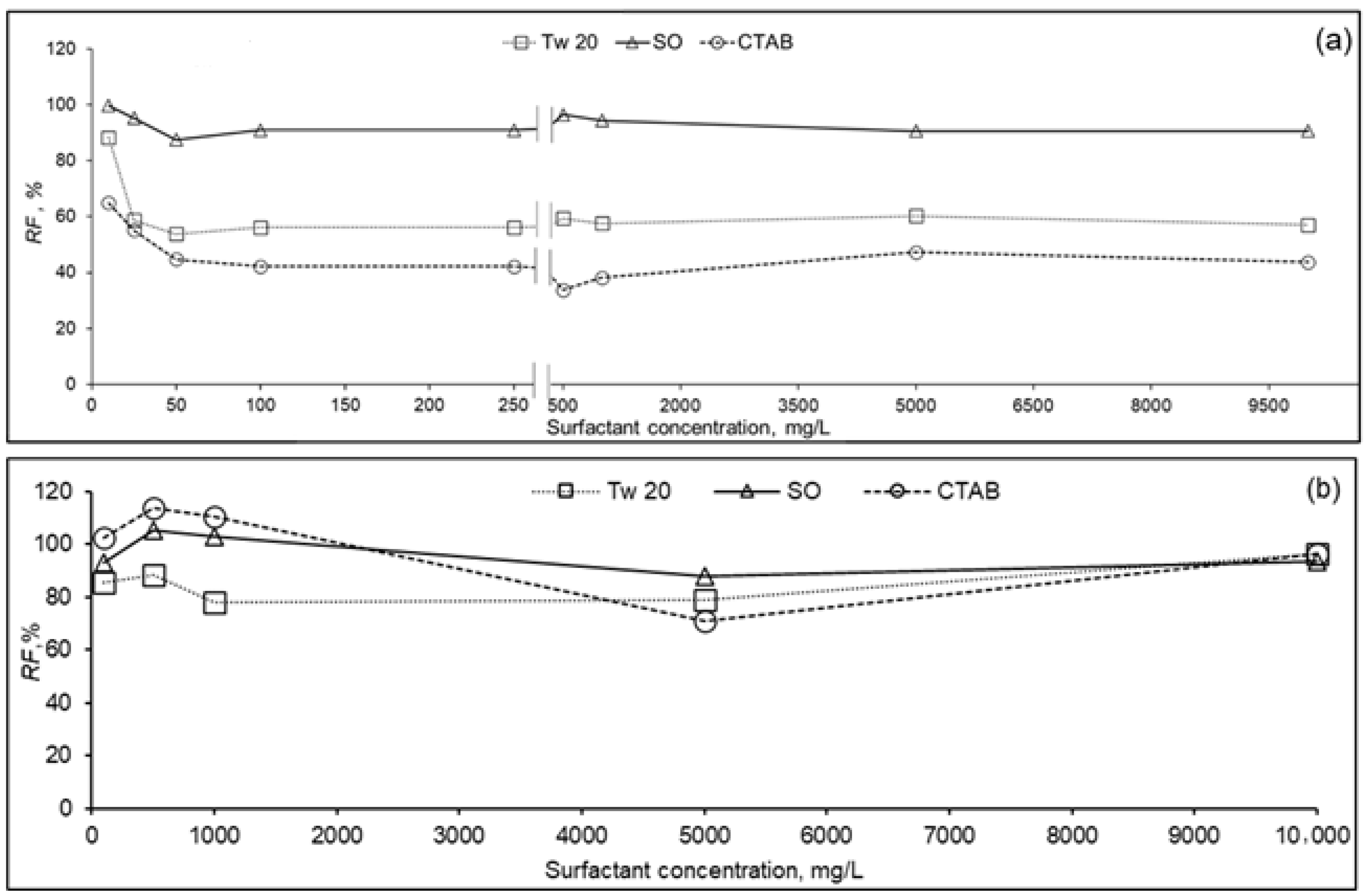
3.1.2. Membranes Fluxes as Function of Surfactant Concentration
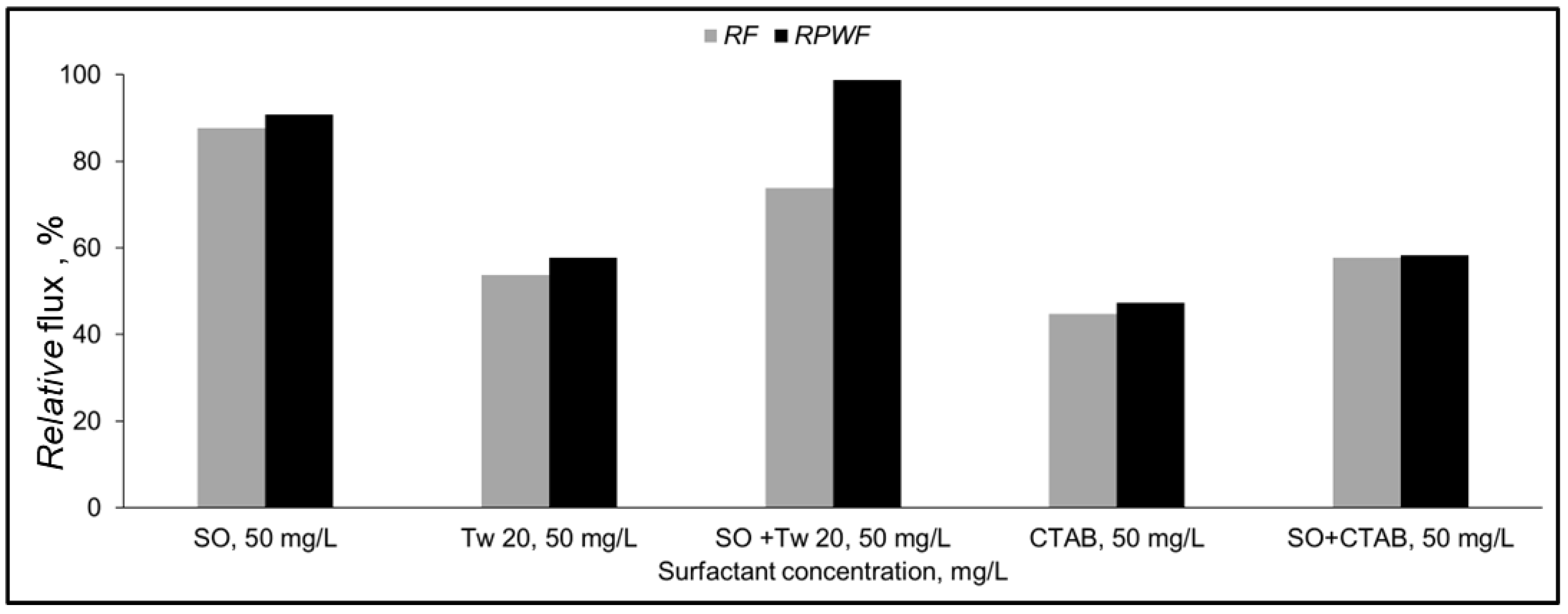
3.1.3. Separation of Surfactant Mixture
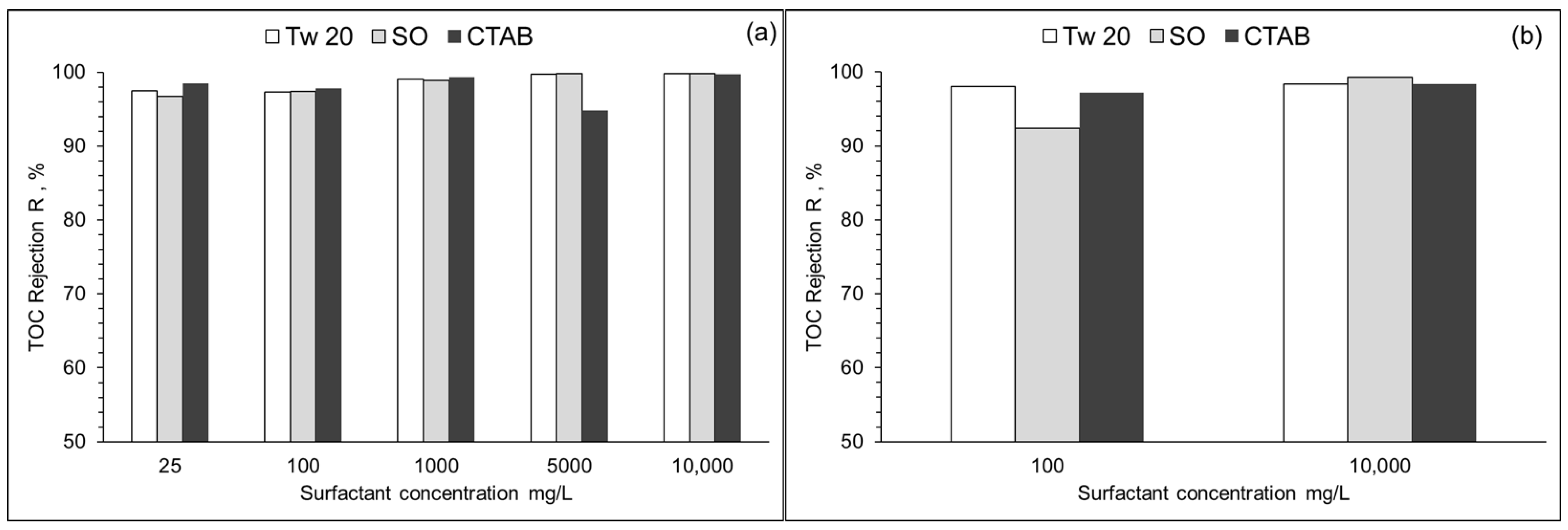
3.2. Membrane Rejection
3.3. Process Implementation and Scalability
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jönsson, A.S.; Jönsson, B. The Influence of Nonionic and Ionic Surfactants on Hydrophobic and Hydrophilic Ultrafiltration Membranes. J. Membr. Sci. 1991, 56, 49–76. [Google Scholar] [CrossRef]
- Kowalska, I.; Kabsch-Korbutowicz, M.; Majewska-Nowak, K.; Winnicki, T. Separation of Anionic Surfactants on Ultrafiltration Membranes. Desalination 2004, 162, 33–40. [Google Scholar] [CrossRef]
- Myers, D. Surfactant Science and Technology, 3rd ed.; J. Wiley: Hoboken, NJ, USA, 2006; ISBN 978-0-471-68024-6. [Google Scholar]
- Aboulhassan, M.A.; Souabi, S.; Yaacoubi, A.; Baudu, M. Removal of Surfactant from Industrial Wastewaters by Coagulation Flocculation Process. Int. J. Environ. Sci. Technol. 2006, 3, 327–332. [Google Scholar] [CrossRef] [Green Version]
- Palmer, M.; Hatley, H. The Role of Surfactants in Wastewater Treatment: Impact, Removal and Future Techniques: A Critical Review. Water Res. 2018, 147, 60–72. [Google Scholar] [CrossRef]
- Aloui, F.; Kchaou, S.; Sayadi, S. Physicochemical Treatments of Anionic Surfactants Wastewater: Effect on Aerobic Biodegradability. J. Hazard. Mater. 2009, 164, 353–359. [Google Scholar] [CrossRef]
- Orlandi, M.; Filosa, N.; Bettonte, M.; Fendrich, M.; Girardini, M.; Battistini, T.; Miotello, A. Treatment of Surfactant-Rich Industrial Wastewaters with Concentrated Sunlight: Toward Solar Wastewater Remediation. Int. J. Environ. Sci. Technol. 2019, 16, 2109–2114. [Google Scholar] [CrossRef]
- González, S.; Petrović, M.; Radetic, M.; Jovancic, P.; Ilic, V.; Barceló, D. Characterization and Quantitative Analysis of Surfactants in Textile Wastewater by Liquid Chromatography/Quadrupole-time-of-flight Mass Spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Lara-Martín, P.A.; González-Mazo, E.; Petrovic, M.; Barceló, D.; Brownawell, B.J. Occurrence, Distribution and Partitioning of Nonionic Surfactants and Pharmaceuticals in the Urbanized Long Island Sound Estuary (NY). Mar. Pollut. Bull. 2014, 85, 710–719. [Google Scholar] [CrossRef]
- Kowalska, I.; Kabsch-Korbutowicz, M.; Majewska-Nowak, K.; Pietraszek, M. Removal of Detergents from Industrial Wastewater in Ultrafiltration Process. Environ. Prot. Eng. 2005, 31, 207–219. [Google Scholar]
- Mondal, B.; Adak, A.; Datta, P. Degradation of Anionic Surfactant in Municipal Wastewater by UV-H2O2: Process Optimization Using Response Surface Methodology. J. Photochem. Photobiol. A Chem. 2019, 375, 237–243. [Google Scholar] [CrossRef]
- Mondal, B.; Adak, A.; Datta, P. Integrated UV–H2O2 and Biological Treatment Processes for the Removal of Cationic Surfactant. J. Environ. Eng. Sci. 2021, 16, 85–93. [Google Scholar] [CrossRef]
- Klimonda, A.; Kowalska, I. Membrane Technology for the Treatment of Industrial Wastewater Containing Cationic Surfactants. Water Resour. Ind. 2021, 26, 100157. [Google Scholar] [CrossRef]
- Ivanković, T.; Hrenović, J. Surfactants in the Environment. Arch. Ind. Hyg. Toxicol. 2010, 61, 95–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, F.-J.; Ma, W.-L.; Xu, T.-F.; Ding, Y.; Zhao, X.; Li, W.-L.; Liu, L.-Y.; Song, W.-W.; Li, Y.-F.; Zhang, Z.-F. Removal Characteristic of Surfactants in Typical Industrial and Domestic Wastewater Treatment Plants in Northeast China. Ecotoxicol. Environ. Saf. 2018, 153, 84–90. [Google Scholar] [CrossRef]
- Venhuis, S.H.; Mehrvar, M. Health Effects, Environmental Impacts, and Photochemical Degradation of Selected Surfactants in Water. Int. J. Photoenergy 1900, 6, 631840. [Google Scholar] [CrossRef] [Green Version]
- Tandukar, M.; Oh, S.; Tezel, U.; Konstantinidis, K.T.; Pavlostathis, S.G. Long-Term Exposure to Benzalkonium Chloride Disinfectants Results in Change of Microbial Community Structure and Increased Antimicrobial Resistance. Environ. Sci. Technol. 2013, 47, 9730–9738. [Google Scholar] [CrossRef] [PubMed]
- Nunes, R.F.; Teixeira, A.C.S.C. An Overview on Surfactants as Pollutants of Concern: Occurrence, Impacts and Persulfate-Based Remediation Technologies. Chemosphere 2022, 300, 134507. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Safer Choice Criteria for Surfactants. Available online: https://www.epa.gov/saferchoice/safer-choice-criteria-surfactants (accessed on 6 January 2023).
- Regulation (EC) No 648/2004 of the European Parliament and of the Council of 31 March 2004 on Detergents (Text with EEA Relevance). 2004. Available online: https://www.ecolex.org/details/legislation/regulation-ec-no-6482004-of-the-european-parliament-and-of-the-council-on-detergents-lex-faoc042319/ (accessed on 2 February 2023).
- Dhouib, A.; Hamad, N.; Hassaїri, I.; Sayadi, S. Degradation of Anionic Surfactants by Citrobacter Braakii. Process Biochem. 2003, 38, 1245–1250. [Google Scholar] [CrossRef]
- Mungray, A.K.; Kumar, P. Anionic Surfactants in Treated Sewage and Sludges: Risk Assessment to Aquatic and Terrestrial Environments. Bioresour. Technol. 2008, 99, 2919–2929. [Google Scholar] [CrossRef]
- Kaleta, J.; Elektorowicz, M. The Removal of Anionic Surfactants from Water in Coagulation Process. Environ. Technol. 2013, 34, 999–1005. [Google Scholar] [CrossRef]
- Amat, A.M.; Arques, A.; Miranda, M.A.; Seguí, S. Photo-Fenton Reaction for the Abatement of Commercial Surfactants in a Solar Pilot Plant. Sol. Energy 2004, 77, 559–566. [Google Scholar] [CrossRef]
- Suárez, L.; Díez, M.A.; García, R.; Riera, F.A. Membrane Technology for the Recovery of Detergent Compounds: A Review. J. Ind. Eng. Chem. 2012, 18, 1859–1873. [Google Scholar] [CrossRef]
- Kowalska, I.; Majewska-Nowak, K.; Kabsch-Korbutowicz, M. Influence of Temperature on Anionic Surface Active Agent Removal from a Water Solution by Ultrafiltration. Desalination 2006, 198, 124–131. [Google Scholar] [CrossRef]
- Archer, A.C.; Mendes, A.M.; Boaventura, R.A.R. Separation of an Anionic Surfactant by Nanofiltration. Environ. Sci. Technol. 1999, 33, 2758–2764. [Google Scholar] [CrossRef]
- Morris, B.G.; Aziz, M.; Kasongo, G. Remediation of Laundry Wastewater with a Low-Pressure Aromatic Polyamide Thin-Film Composite Reverse Osmosis Membrane for Membrane Fouling Minimisation and Reuse Application. Environ. Protect. Eng. 2022, 48, 43–57. [Google Scholar] [CrossRef]
- Kurihara, M. Current Status and Future Trend of Dominant Commercial Reverse Osmosis Membranes. Membranes 2021, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Sekikawa, T.; Toshiki, K. A New Method for Efficient Detection of Cryptosporidium RNA by Real-Time Reverse Transcription-PCR with Surfactants. Water Sci. Technol. Water Supply 2015, 15, 1061–1068. [Google Scholar] [CrossRef] [Green Version]
- Akhter, M.S. Effect of N-Methyl Acetamide on the Critical Micelle Concentration of Aqueous Solutions of Some Surfactants. Colloids Surf. 1997, 121, 103–109. [Google Scholar] [CrossRef]
- Virga, E.; Bos, B.; Biesheuvel, P.M.; Nijmeijer, A.; de Vos, W.M. Surfactant-Dependent Critical Interfacial Tension in Silicon Carbide Membranes for Produced Water Treatment. J. Colloid Interface Sci. 2020, 571, 222–231. [Google Scholar] [CrossRef]
- Halleb, A.; Yokoyama, F.; das Neves, M.A.; Nakajima, M. Effects of Surfactants and Oil-in-Water Emulsions on Reverse Osmosis Membrane Performance. Euro-Mediterr. J. Environ. Integr. 2021, 6, 44. [Google Scholar] [CrossRef]
- Kurihara, M.; Sasaki, T. The Pursuits of Ultimate Membrane Technology Including Low Pressure Seawater Reverse Osmosis Membrane Developed by Mega-Ton Water System Project. J. Membr. Sci. Res. 2017, 3, 157–173. [Google Scholar]
- Dammak, I.; Neves, M.A.; Nabetani, H.; Isoda, H. Food and Bioproducts Processing Transport Properties of Oleuropein through Nanofiltration Membranes. Food Bioprod. Process. 2015, 94, 342–353. [Google Scholar] [CrossRef] [Green Version]
- Dickhout, J.M.; Moreno, J.; Biesheuvel, P.M.; Boels, L.; Lammertink, R.G.H.; de Vos, W.M. Produced Water Treatment by Membranes: A Review from a Colloidal Perspective. J. Colloid Interface Sci. 2017, 487, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Srisukphun, T.; Chiemchaisri, C.; Urase, T.; Yamamoto, K. Experimentation and Modeling of Foulant Interaction and Reverse Osmosis Membrane Fouling during Textile Wastewater Reclamation. Sep. Purif. Technol. 2009, 68, 37–49. [Google Scholar] [CrossRef]
- Elimelech, M.; Zhu, X.; Childress, A.E.; Hong, S.; Shi, L.; Huang, J.; Zeng, G.; Zhu, L.; Gu, Y.; Shi, Y.; et al. Role of Membrane Surface Morphology in Colloidal Fouling of Cellulose Acetate and Composite Aromatic Polyamide Reverse Osmosis Membranes. J. Membr. Sci. 1997, 127, 101–109. [Google Scholar] [CrossRef]
- Childress, A.E.; Elimelech, M. Effect of Solution Chemistry on the Surface Charge of Polymeric Reverse Osmosis and Nanofiltration Membranes. J. Membr. Sci. 1996, 119, 253–268. [Google Scholar] [CrossRef]
- Asaka, K. Dielectric Properties of Cellulose Acetate Reverse Osmosis Membranes in Aqueous Salt Solutions. J. Membr. Sci. 1990, 50, 71–84. [Google Scholar] [CrossRef]
- Kishimoto, N.; Kimura, H. Fouling Behaviour of a Reverse Osmosis Membrane by Three Types of Surfactants. J. Water Reuse Desalination 2012, 2, 40–46. [Google Scholar] [CrossRef]
- Childress, A.E.; Elimelech, M. Relating Nanofiltration Membrane Performance to Membrane Charge (Electrokinetic) Characteristics. Environ. Sci. Technol. 2000, 34, 3710–3716. [Google Scholar] [CrossRef]
- Vrijenhoek, E.M.; Hong, S.; Elimelech, M. Influence of Membrane Surface Properties on Initial Rate of Colloidal Fouling of Reverse Osmosis and Nanofiltration Membranes. J. Membr. Sci. 2001, 188, 115–128. [Google Scholar] [CrossRef]
- Chen, B.; Yu, S.; Zhao, X. The Influence of RO Membrane Surface Properties on Surfactant Fouling in Radioactive Wastewater Treatment. Process Saf. Environ. Prot. 2021, 149, 858–865. [Google Scholar] [CrossRef]
- Bai, Y.; Shan, F.; Zhu, Y.; Xu, J.; Wu, Y.; Luo, X.; Wu, Y.; Hu, H.-Y.; Zhang, B. Long-Term Performance and Economic Evaluation of Full-Scale MF and RO Process–A Case Study of the Changi NEWater Project Phase 2 in Singapore. Water Cycle 2020, 1, 128–135. [Google Scholar] [CrossRef]
- Kurihara, M. Sustainable Seawater Reverse Osmosis Desalination as Green Desalination in the 21st Century. J. Membr. Sci. Res. 2020, 6, 20–29. [Google Scholar]
- Aleisa, E.; Alshayji, K. Analysis on Reclamation and Reuse of Wastewater in Kuwait. J. Eng. Res. 2019, 7, 1–13. [Google Scholar]




| Surfactant | Concentration (mg/L) | Matrix | Reference |
|---|---|---|---|
| Nonionic | 3370 | Industrial wastewater | [7] |
| 0.93–5.68 | Textile effluent | [8] | |
| 0.265–2.72 | Sewage | [9] | |
| Anionic | 1552–1650 | Detergent production | [10] |
| 14,800 | Industrial wastewater | [7] | |
| 1.8 | Municipal wastewater | [11] | |
| Cationic | 362 | Textile effluent | [12] |
| 2890–3390 | Surfactant plant effluent | [13] | |
| 780 | Industrial wastewater | [7] |
| Surfactant | Molecular Weight (g/mol) | CMC (mg/L) |
|---|---|---|
| Nonionic Tw 20 | 1226 | 60 [30] |
| Anionic SO | 304 | 912 [31] |
| Cationic CTAB | 365 | 346 [32] |
| Feed | Zeta Potential (mV) at Surfactant Concentrations of: | ||
|---|---|---|---|
| 50 mg/L | 100 mg/L | 1000 mg/L | |
| Tw 20 | −22.3 ± 1.5 | −13.3 ± 2.3 | −11.4 ± 3 |
| SO | − 62.7 ± 1.5 | −59.6 ± 1.5 | −79.1 ± 4.3 |
| CTAB | 46.5 ± 2.3 | 38.5 ± 9.8 | 79.1 ± 14.4 |
| SO + Tw 20 | −58.6 ± 2.0 | - | - |
| SO + CTAB | −16.7 ± 1.8 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halleb, A.; Nakajima, M.; Yokoyama, F.; Neves, M.A. Effect of Surfactants on Reverse Osmosis Membrane Performance. Separations 2023, 10, 168. https://doi.org/10.3390/separations10030168
Halleb A, Nakajima M, Yokoyama F, Neves MA. Effect of Surfactants on Reverse Osmosis Membrane Performance. Separations. 2023; 10(3):168. https://doi.org/10.3390/separations10030168
Chicago/Turabian StyleHalleb, Aymen, Mitsutoshi Nakajima, Fumio Yokoyama, and Marcos Antonio Neves. 2023. "Effect of Surfactants on Reverse Osmosis Membrane Performance" Separations 10, no. 3: 168. https://doi.org/10.3390/separations10030168
APA StyleHalleb, A., Nakajima, M., Yokoyama, F., & Neves, M. A. (2023). Effect of Surfactants on Reverse Osmosis Membrane Performance. Separations, 10(3), 168. https://doi.org/10.3390/separations10030168







