Characterization of the Aroma Profile of Food Smoke at Controllable Pyrolysis Temperatures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Production and Sampling of Food Smoke
2.3. Headspace Solid-Phase Microextraction of Food Smoke
2.4. Gas Chromatography-Mass Spectrometry
2.5. Compound Identification
2.6. Data Evaluation and Determination of Relative Peak Areas
2.7. Statistical Analysis
3. Results and Discussion
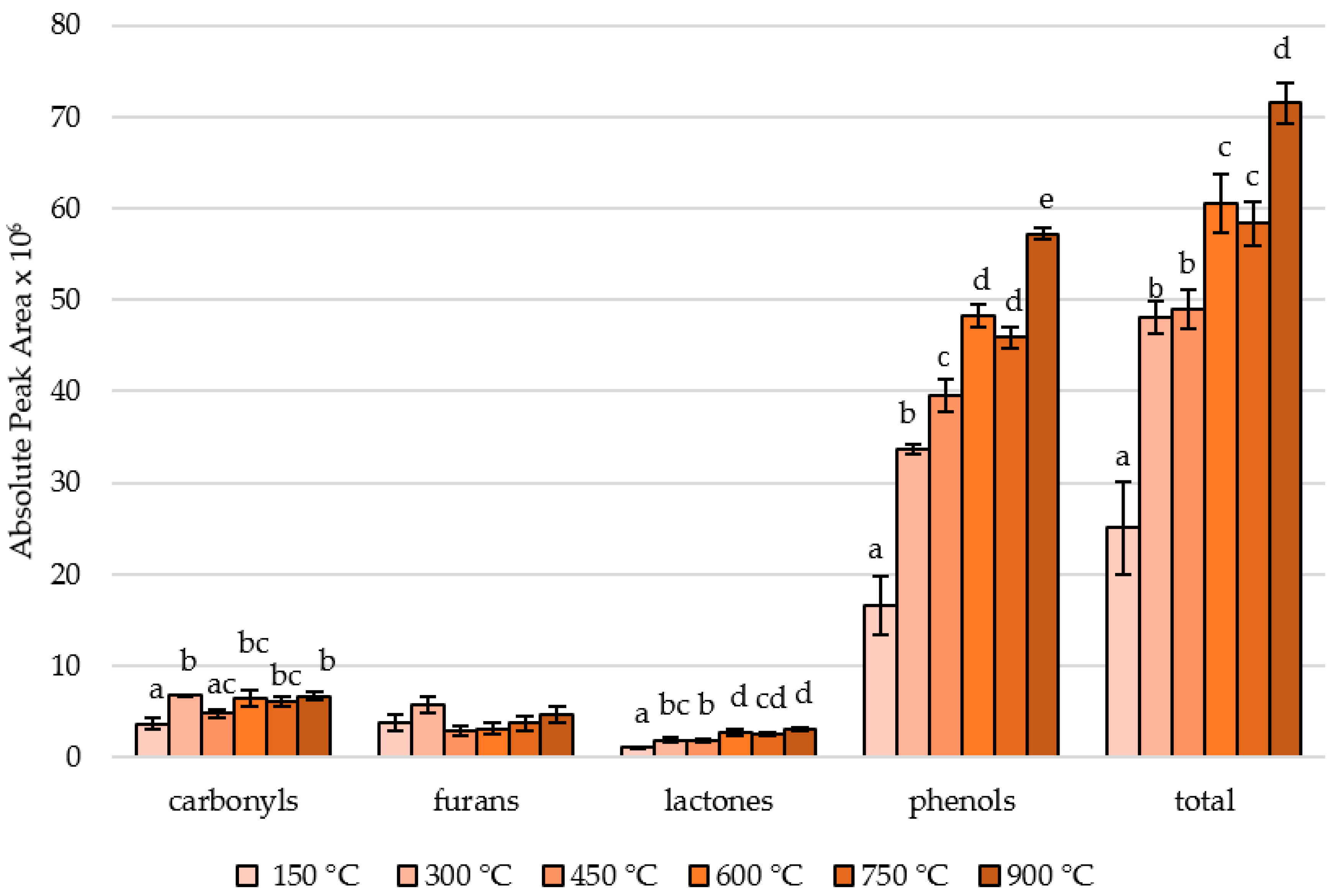
3.1. Effect of Controlled Pyrolysis Temperature on the Areas and Ratios of Different Substance Classes in Smoke
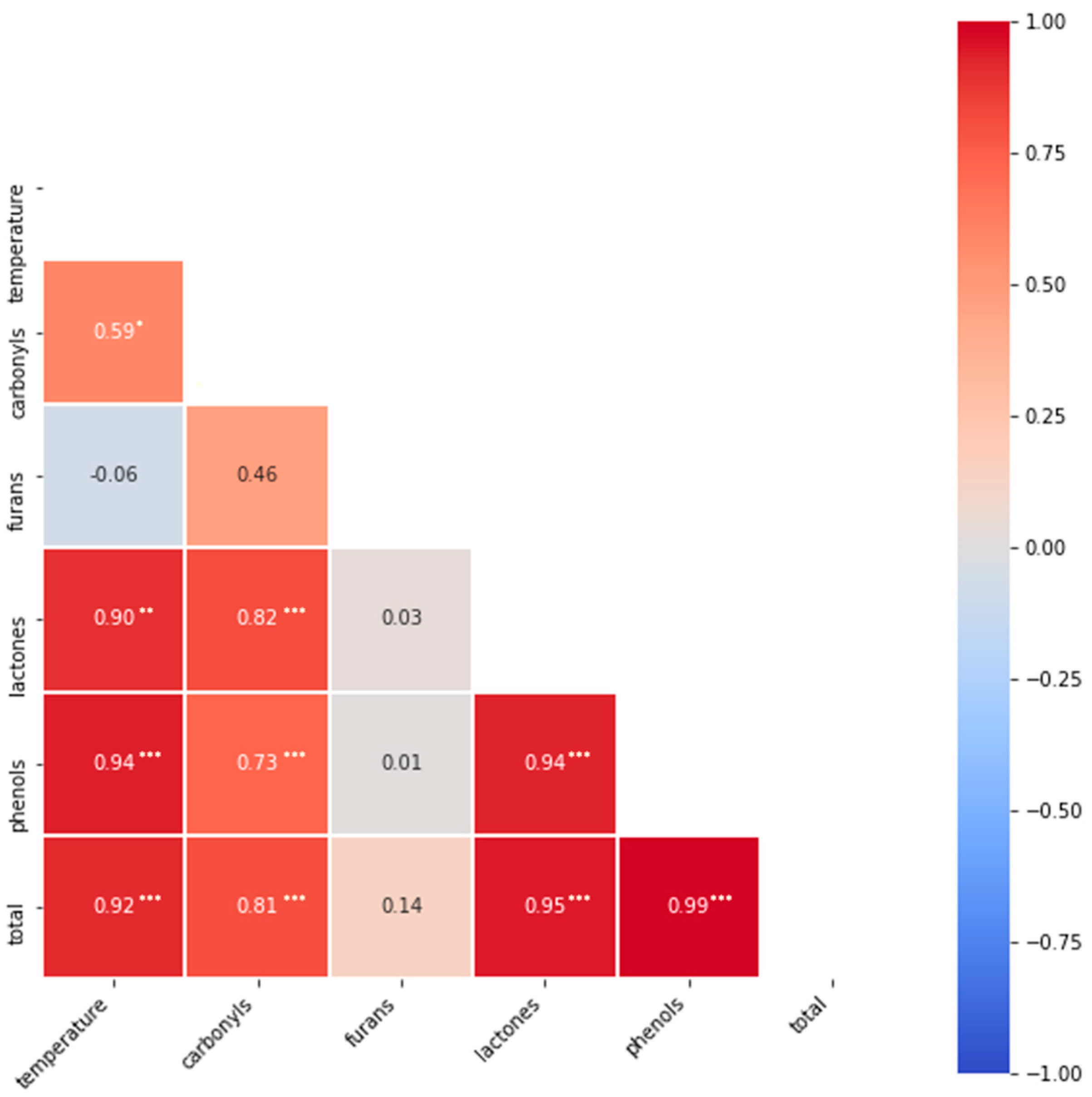
3.2. Correlations of Different Substance Classes with the Pyrolysis Temperature
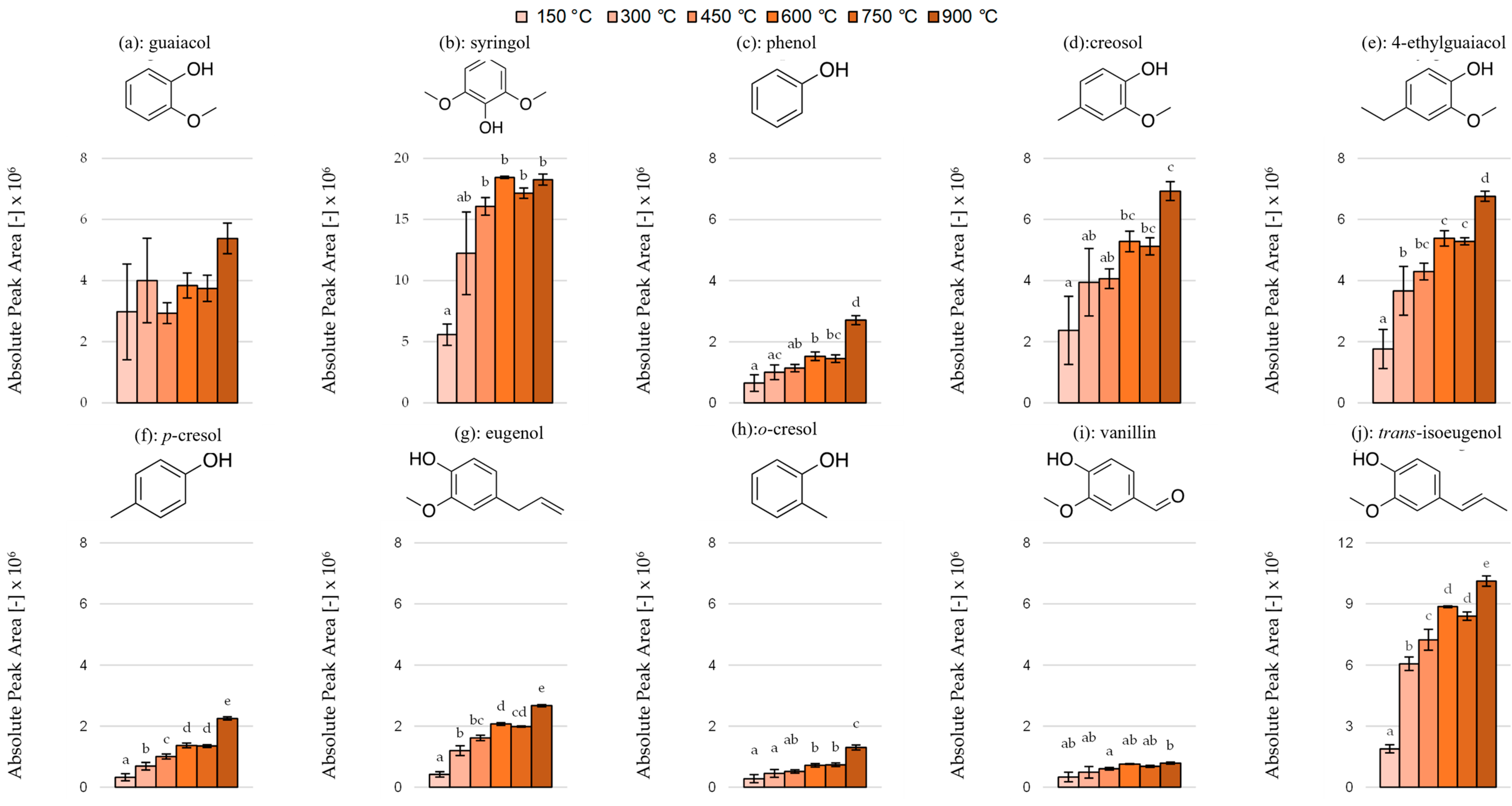
3.3. Effect of Pyrolysis Temperature on the Area and Ratios of Individual Phenols and Lactones
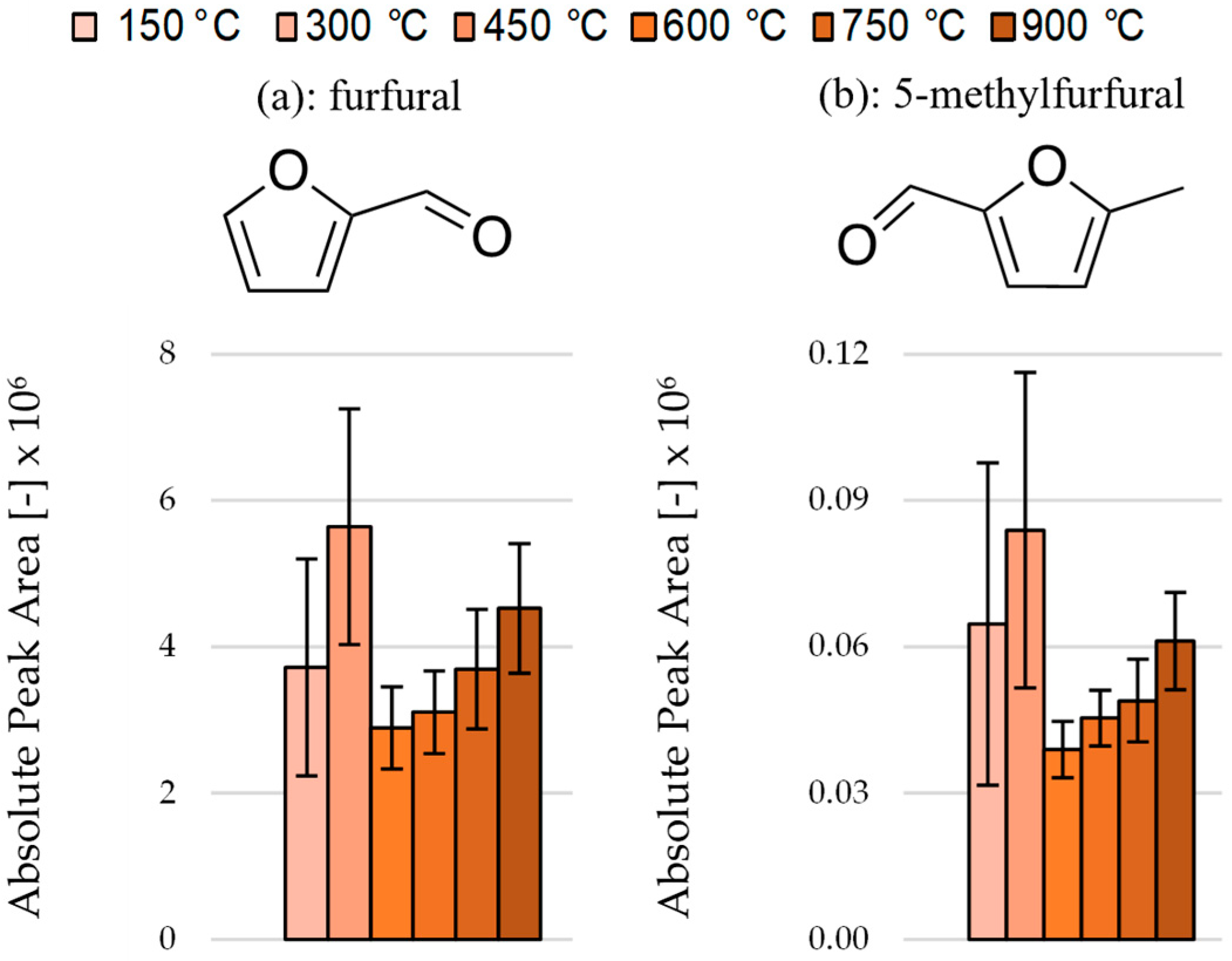
3.4. Effect of Pyrolysis Temperature on the Area and Ratios of Individual Carbonyls and Furans
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Glatz, J. Fleisch und Erzeugnisse aus Fleisch. In Handbuch für Lebensmittelchemiker; Frede, W., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 3, pp. 563–585. [Google Scholar]
- Toth, L.; Potthast, K. Chemical aspects of the smoking of meat and meat products. In Advances in Food Research; Elsevier: Amsterdam, The Netherlands, 1984; Volume 29, pp. 87–158. [Google Scholar]
- Foster, W.W.; Simpson, T.H. Studies of the smoking process for foods. I—The importance of vapours. J. Sci. Food Agric. 1961, 12, 363–374. [Google Scholar] [CrossRef]
- Foster, W.W.; Simpson, T.H.; Campbell, D. Studies of the smoking process for foods. II—The rǒle of smoke particles. J. Sci. Food Agric. 1961, 12, 635–644. [Google Scholar] [CrossRef]
- Sikorski, Z.; Sinkiewicz, I. Smoking: Traditional. In Encyclopedia of Meat Sciences, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Maga, J.A. Smoke in Food Processing; CRC Press: Boca Raton, FL, USA, 1988; Volume 1. [Google Scholar]
- Tóth, L.; Wittkowski, R. Das Räuchern–aus der Sicht der Chemie. Chem. Unserer Zeit 1985, 19, 48–58. [Google Scholar] [CrossRef]
- Lingbeck, J.M.; Cordero, P.; O’Bryan, C.A.; Johnson, M.G.; Ricke, S.C.; Crandall, P.G. Functionality of liquid smoke as an all-natural antimicrobial in food preservation. Meat Sci. 2014, 97, 197–206. [Google Scholar] [CrossRef]
- Toledo, R.T. Wood smoke components and functional properties. Smoked Seaf. Saf. 2007, 55-61, 55–61. [Google Scholar]
- Fiddler, W.; Parker, W.E.; Wasserman, A.E.; Doerr, R.C. Thermal decomposition of ferulic acid. J. Agric. Food Chem. 1967, 15, 757–761. [Google Scholar] [CrossRef]
- Gilbert, J.; Knowles, M.E. The chemistry of smoked foods: A review. Int. J. Food Sci. Technol. 1975, 10, 245–261. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Influence of Temperature. In Wood: Chemistry, Ultrastructure, Reactions; Fengel, D., Wegener, G., Eds.; De Gruyter, Inc.: Berlin Germany; Boston, MA, USA, 1983; pp. 319–344. [Google Scholar]
- Kawamoto, H. Lignin pyrolysis reactions. J. Wood Sci. 2017, 63, 117–132. [Google Scholar] [CrossRef]
- Fenner, R.A.; Lephardt, J.O. Examination of the thermal decomposition of kraft pine lignin by Fourier transform infrared evolved gas analysis. J. Agric. Food Chem. 1981, 29, 846–849. [Google Scholar] [CrossRef]
- Adler, E. Lignin chemistry—Past, present and future. Wood Sci. Technol. 1977, 11, 169–218. [Google Scholar] [CrossRef]
- Tóth, L. Chemie der Räucherung; Verlag Chemie: Weinheim, Germany, 1983; Volume 1. [Google Scholar]
- Weiss, J.; Gibis, M.; Zhang, Y. Project Proposal AiF 21343-N. 2019. Available online: http://www.fei-bonn.de/gefoerderte-projekte/projektdatenbank/aif-21343-n.projekt (accessed on 3 March 2023).
- Arthur, C.L.; Pawliszyn, J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Jeleń, H.H.; Majcher, M.; Dziadas, M. Microextraction techniques in the analysis of food flavor compounds: A review. Anal. Chim. Acta 2012, 738, 13–26. [Google Scholar] [CrossRef]
- Mahattanatawee, K.; Goodner, K.; Rouseff, R. Quantification of β-damascenone in orange juice using headspace standard addition SPME with selected ion GC-MS. Anal. Methods 2013, 5, 2630–2633. [Google Scholar] [CrossRef]
- Zhang, Y.; Fraatz, M.A.; Horlamus, F.; Quitmann, H.; Zorn, H. Identification of potent odorants in a novel nonalcoholic beverage produced by fermentation of wort with shiitake (Lentinula edodes). J. Agric. Food Chem. 2014, 62, 4195–4203. [Google Scholar] [CrossRef]
- Conde, F.J.; Afonso, A.M.; González, V.; Ayala, J.H. Optimization of an analytical methodology for the determination of alkyl-and methoxy-phenolic compounds by HS-SPME in biomass smoke. Anal. Bioanal. Chem. 2006, 385, 1162–1171. [Google Scholar] [CrossRef]
- Yu, A.-N.; Sun, B.-G.; Tian, D.-T.; Qu, W.-Y. Analysis of volatile compounds in traditional smoke-cured bacon (CSCB) with different fiber coatings using SPME. Food Chem. 2008, 110, 233–238. [Google Scholar] [CrossRef]
- Leible, M. The Effects of Variable Smoldering Temperatures and Flue Gas Compositions on Physical, Chemical, and Sensory Attributes of Frankfurters Who Are Similar in Their Casing Color. Master’s Thesis, University of Hohenheim, Stuttgart, Germany, October 2021. [Google Scholar]
- Rigling, M.; Heger, F.; Graule, M.; Liu, Z.; Zhang, C.; Ni, L.; Zhang, Y. A Robust Fermentation Process for Natural Chocolate-like Flavor Production with Mycetinis scorodonius. Molecules 2022, 27, 2503. [Google Scholar] [CrossRef] [PubMed]
- Ruedt, C.; Gibis, M.; Weiss, J. Effect of varying salt concentration on iridescence in precooked pork meat. Eur. Food Res. Technol. 2022, 248, 57–68. [Google Scholar] [CrossRef]
- Hunter, J.D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Harris, C.R.; Millman, K.J.; Van Der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J. Array programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef]
- McKinney, W. Data Structure for Statisticle Computing in Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010. [Google Scholar]
- Waskom, M.L. seaborn: Statistical data visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Porter, R.W.; Bratzler, L.J.; Pearson, A.M. Fractionation and study of compounds in wood smoke. J. Food Sci. 1965, 30, 615–619. [Google Scholar] [CrossRef]
- TGS. TGSC Information System. Available online: http://www.thegoodscentscompany.com (accessed on 17 February 2023).
- Fujimaki, M.; Kim, K.; Kurata, T. Analysis and comparison of flavor constituents in aqueous smoke condensates from various woods. Agric. Biol. Chem. 1974, 38, 45–52. [Google Scholar] [CrossRef]
- Kostyra, E.; Baryłko-Pikielna, N. Volatiles composition and flavour profile identity of smoke flavourings. Food Qual. Prefer. 2006, 17, 85–95. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure, Reactions; Walter de Gruyter: Berlin, Germany, 2011. [Google Scholar]
- Chen, L.; Liao, Y.; Guo, Z.; Cao, Y.; Ma, X. Products distribution and generation pathway of cellulose pyrolysis. J. Clean. Prod. 2019, 232, 1309–1320. [Google Scholar] [CrossRef]
- Daun, H.; Tilgner, D.J. The influence of generation parameters upon antioxidative properties of wood smoke. Acta Aliment. Pol. 1977, 3, 231–236. [Google Scholar]
- Cadwallader, K.R. Wood smoke flavor. In Handbook of Meat, Poultry and Seafood Quality; Blackwell Publishing: Ames, IA, USA, 2007; pp. 201–210. [Google Scholar]
- Kornreich, M.R.; Issenberg, P. Determination of phenolic wood smoke components as trimethylsilyl ethers. J. Agric. Food Chem. 1972, 20, 1109–1113. [Google Scholar] [CrossRef]
- Pöhlmann, M.; Hitzel, A.; Schwägele, F.; Speer, K.; Jira, W. Contents of polycyclic aromatic hydrocarbons (PAH) and phenolic substances in Frankfurter-type sausages depending on smoking conditions using glow smoke. Meat Sci. 2012, 90, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Doerr, R.C.; Fiddler, W. Partition ratios of some wood smoke phenols in two oil: Water systems. J. Agric. Food Chem. 1970, 18, 937–939. [Google Scholar] [CrossRef]
- Pöhlmann, M.; Hitzel, A.; Schwägele, F.; Speer, K.; Jira, W. Polycyclic aromatic hydrocarbons (PAH) and phenolic substances in smoked Frankfurter-type sausages depending on type of casing and fat content. Food Control 2013, 31, 136–144. [Google Scholar] [CrossRef]



| No. | RI | Compound | Odor Description 1 | Quantifier Ion m/z | Identification |
|---|---|---|---|---|---|
| carbonyls | |||||
| 1 | <1000 | diacetyl | butter-like, sweetish, creamy, pungent, caramel-like | 43 | RI, MS |
| 2 | 1283 | hydroxyacetone | pungent, sweetish, caramel-like, ethereal | 43 | RI, MS |
| 3 | 1352 | 2-methyl-2-cyclopenten-1-one | warm, spicy, sweetish | 67 | RI, MS |
| 4 | 1808 | cyclotene | sweetish, caramel-like, maple-like, sugar-like, coffee-like, woody | 112 | RI, MS |
| furans | |||||
| 5 | 1445 | furfural | sweetish, woody, almond-like, bread-like, baked | 97 | RI, MS |
| 6 | 1553 | 5-methyl-furfural | spicy, caramel-like, maple-like | 111 | RI, MS |
| lactones | |||||
| 7 | 1600 | butyrolactone | creamy, oily, fatty, caramel-like | 42 | RI, MS |
| 8 | 1742 | 2-(5H)-furanone | butter-like | 55 | RI, MS |
| phenols | |||||
| 9 | 1838 | guaiacol | spicy, clove-like, vanilla-like, phenolic, medicinal, leathery, woody, smoky | 109 | RI, MS |
| 10 | 1933 | creosol | spicy, clove-like, vanilla-like, phenolic, medicinal, leathery, woody, smoky, burnt | 138 | RI, MS |
| 11 | 1982 | phenol | musty, phenolic, plastic-like, medicinal, herbal, leathery | 94 | RI, MS |
| 12 | 1982 | o-cresol | musty, phenolic, plastic-like, medicinal, herbal, leathery | 108 | RI, MS |
| 13 | 2007 | 4-ethylguaiacol | spicy, smoky, bacon-like, phenolic, clove-like | 137 | RI, MS |
| 14 | 2058 | p-cresol | phenolic, narcissus-like, animalic, mimosa-like | 107 | RI, MS |
| 15 | 2143 | eugenol | sweetish, spicy, clove-like, woody | 164 | RI, MS |
| 16 | 2237 | syringol | smoky, phenolic, balsamic, bacon-like, powdery, woody | 154 | RI, MS |
| 17 | 2317 | trans-isoeugenol | sweetish, spicy, carnation-like, phenolic, floral | 164 | RI, MS |
| 18 | 2522 | vanillin | sweetish, vanilla-like, creamy, chocolate-like | 152 | RI, MS |
| Pyrolysis temperature [°C] | Relative Peak Area [%] | ||||
|---|---|---|---|---|---|
| guaiacol | syringol | phenol | creosol | 4-ethyl-guaiacol | |
| 150 | 17.17 ± 6.39 | 35.10 ± 12.10 ab | 3.79 ± 1.06 ab | 13.79 ± 4.20 | 10.39 ± 1.88 |
| 300 | 11.82 ± 3.92 | 36.38 ± 10.59 ab | 2.96 ± 0.66 ab | 11.66 ± 3.08 | 10.84 ± 2.18 |
| 450 | 7.43 ± 0.80 | 40.72 ± 1.38 b | 2.88 ± 0.23 a | 10.29 ± 0.63 | 10.88 ± 0.37 |
| 600 | 7.94 ± 0.64 | 38.24 ± 1.04 b | 3.16 ± 0.21 a | 10.94 ± 0.42 | 11.15 ± 0.24 |
| 750 | 8.15 ± 0.79 | 37.37 ± 0.97 b | 3.16 ± 0.21 a | 11.16 ± 0.41 | 11.51 ± 0.12 |
| 900 | 9.41 ± 0.78 | 31.95 ± 1.05 a | 4.73 ± 0.21 b | 12.11 ± 0.42 | 11.82 ± 0.21 |
| Pyrolysis temperature [°C] | Relative Peak Area [%] | ||||
| p-cresol | eugenol | o-cresol | vanillin | trans-isoeugenol | |
| 150 | 1.93 ± 0.35 a | 2.52 ± 0.09 a | 1.62 ± 0.49 ab | 2.18 ± 1.38 | 11.5 ± 0.99 a |
| 300 | 2.04 ± 0.34 a | 3.55 ± 0.40 acde | 1.34 ± 0.37 b | 1.46 ± 0.58 | 17.96 ± 0.78 b |
| 450 | 2.55 ± 0.12 a | 4.09 ± 0.04 c | 1.31 ± 0.11 b | 1.53 ± 0.10 | 18.33 ± 0.81 b |
| 600 | 2.84 ± 0.08 a | 4.29 ± 0.04 d | 1.48 ± 0.08 b | 1.58 ± 0.07 | 18.38 ± 0.52 b |
| 750 | 2.94 ± 0.05 a | 4.33 ± 0.10 cde | 1.60 ± 0.10 ab | 1.50 ± 0.08 | 18.30 ± 0.46 b |
| 900 | 3.94 ± 0.06 b | 4.67 ± 0.09 e | 2.28 ± 0.12 a | 1.38 ± 0.06 | 17.70 ± 0.60 b |
| Pyrolysis Temperature [°C] | Relative Peak Area [%] | |
|---|---|---|
| 2-(5H)-furanone | butyrolactone | |
| 150 | 87.73 ± 1.16 ab | 12.27 ± 1.16 ab |
| 300 | 87.95 ± 1.04 a | 12.05 ± 1.04 a |
| 450 | 91.89 ± 0.19 ab | 8.11 ± 0.19 ab |
| 600 | 91.92 ± 0.47 b | 8.08 ± 0.47 b |
| 750 | 91.74 ± 0.27 ab | 8.26 ± 0.27 ab |
| 900 | 91.49 ± 0.18 ab | 8.51 ± 0.18 ab |
| Pyrolysis Temperature [°C] | Relative Peak Area [%] | |||
|---|---|---|---|---|
| diacetyl | 2-methyl-2-cyclopenten-1-one | hydroxyacetone | cyclotene | |
| 150 | 0.52 ± 0.02 ac | 0.30 ± 0.13 | 62.93 ± 4.71 a | 36.25 ± 4.85 a |
| 300 | 0.65 ± 0.06 ac | 0.38 ± 0.11 | 63.25 ± 5.05 a | 35.72 ± 5.10 a |
| 450 | 0.93 ± 0.02 b | 0.41 ± 0.22 | 32.79 ± 1.27 b | 65.87 ± 1.22 b |
| 600 | 0.36 ± 0.12 c | 0.68 ± 0.07 | 37.67 ± 6.31 b | 61.29 ± 6.29 b |
| 750 | 0.64 ± 0.27 abc | 0.68 ± 0.41 | 37.69 ± 2.98 b | 60.99 ± 2.77 b |
| 900 | 0.91 ± 0.09 ab | 0.49 ± 0.47 | 32.60 ± 3.02 b | 66.00 ± 2.69 b |
| Pyrolysis Temperature [°C] | Relative Peak Area [%] | |
|---|---|---|
| furfural | 5-methyl-furfural | |
| 150 | 98.35 ± 0.22 | 1.65 ± 0.22 |
| 300 | 98.57 ± 0.17 | 1.43 ± 0.17 |
| 450 | 98.66 ± 0.07 | 1.34 ± 0.07 |
| 600 | 98.55 ± 0.12 | 1.45 ± 0.12 |
| 750 | 98.68 ± 0.13 | 1.32 ± 0.13 |
| 900 | 98.67 ± 0.04 | 1.33 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigling, M.; Höckmeier, L.; Leible, M.; Herrmann, K.; Gibis, M.; Weiss, J.; Zhang, Y. Characterization of the Aroma Profile of Food Smoke at Controllable Pyrolysis Temperatures. Separations 2023, 10, 176. https://doi.org/10.3390/separations10030176
Rigling M, Höckmeier L, Leible M, Herrmann K, Gibis M, Weiss J, Zhang Y. Characterization of the Aroma Profile of Food Smoke at Controllable Pyrolysis Temperatures. Separations. 2023; 10(3):176. https://doi.org/10.3390/separations10030176
Chicago/Turabian StyleRigling, Marina, Laura Höckmeier, Malte Leible, Kurt Herrmann, Monika Gibis, Jochen Weiss, and Yanyan Zhang. 2023. "Characterization of the Aroma Profile of Food Smoke at Controllable Pyrolysis Temperatures" Separations 10, no. 3: 176. https://doi.org/10.3390/separations10030176
APA StyleRigling, M., Höckmeier, L., Leible, M., Herrmann, K., Gibis, M., Weiss, J., & Zhang, Y. (2023). Characterization of the Aroma Profile of Food Smoke at Controllable Pyrolysis Temperatures. Separations, 10(3), 176. https://doi.org/10.3390/separations10030176









