Innovative Methods of Centrifugal Separation
Abstract
:1. Introduction
- (i)
- Separation of gaseous molecules of different weight by the Ultra Centrifuge with application to large scale uranium enrichment to serve for peaceful use as fuel for nuclear power plants;
- (ii)
- Separation of micron-sized particulate matter from fluids by mechanical separators based on centrifugation with special attention to the device of the Rotational Particle Separator RPS for dedusting and demisting gases in industry and domestic environment;
- (iii)
- Separation of mixtures of gases by Condensed Rotational Separation CRS, i.e., fast cooling by expansion leading to preferential condensation of one of the gaseous components in the form of micron-sized droplets which are separated by the RPS. Applications range from large scale upgrading of sour and acid natural gas to purification and liquefaction of the greenhouse gas CO2 emitted from carbon-based conversion processes;
- (iv)
- Separation of components from gases by applying absorbing liquid films in the rotating channels of the RPS. Applications range from H2S and CO2 removal from gases to separation and elimination of contagious aerosols such as aerosols containing harmful bacteria and viruses present in air.
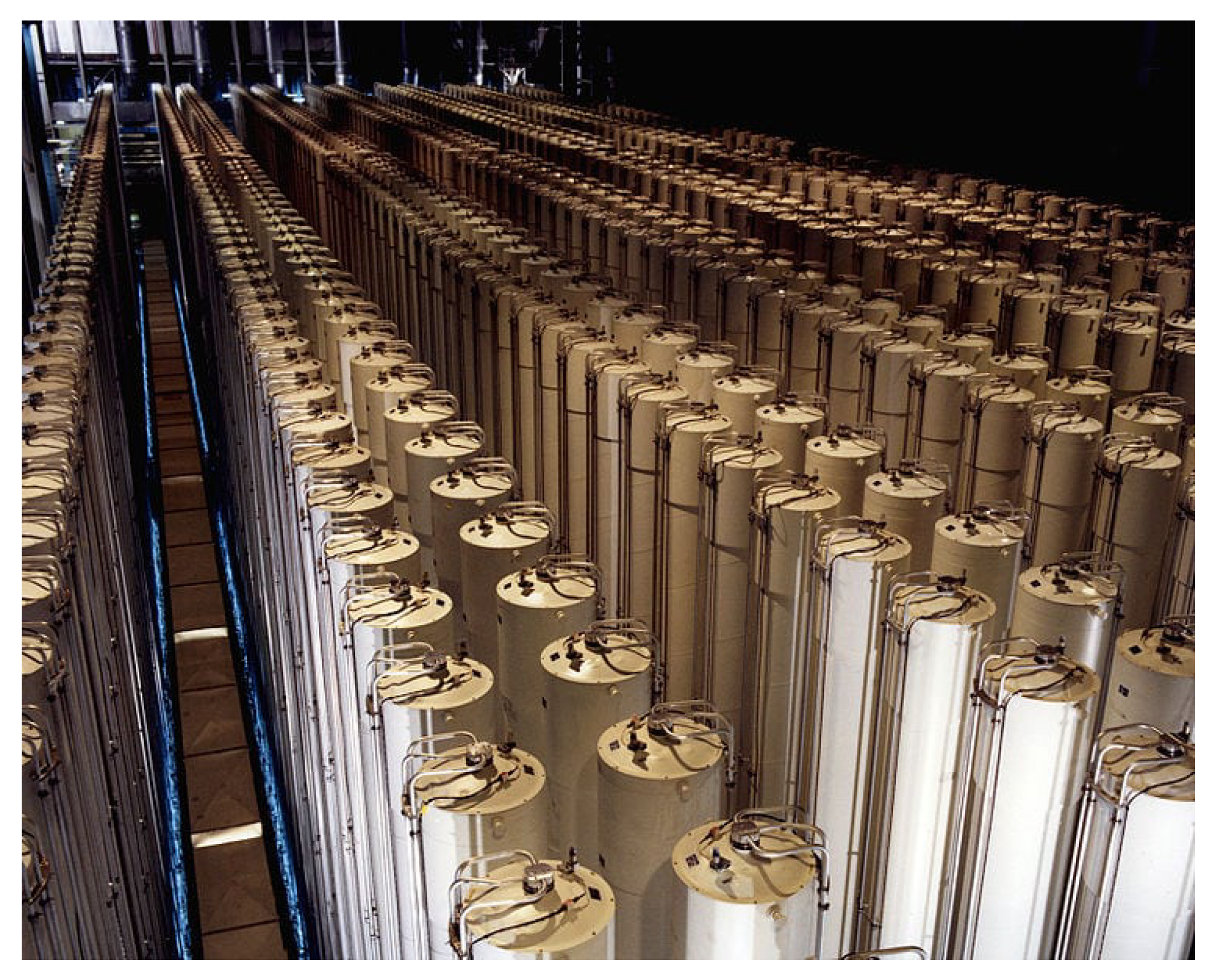
2. The Ultra Centrifuge for Separation of Gas Mixtures
2.1. Working of the Centrifuge
2.2. Separative Power
- (i)
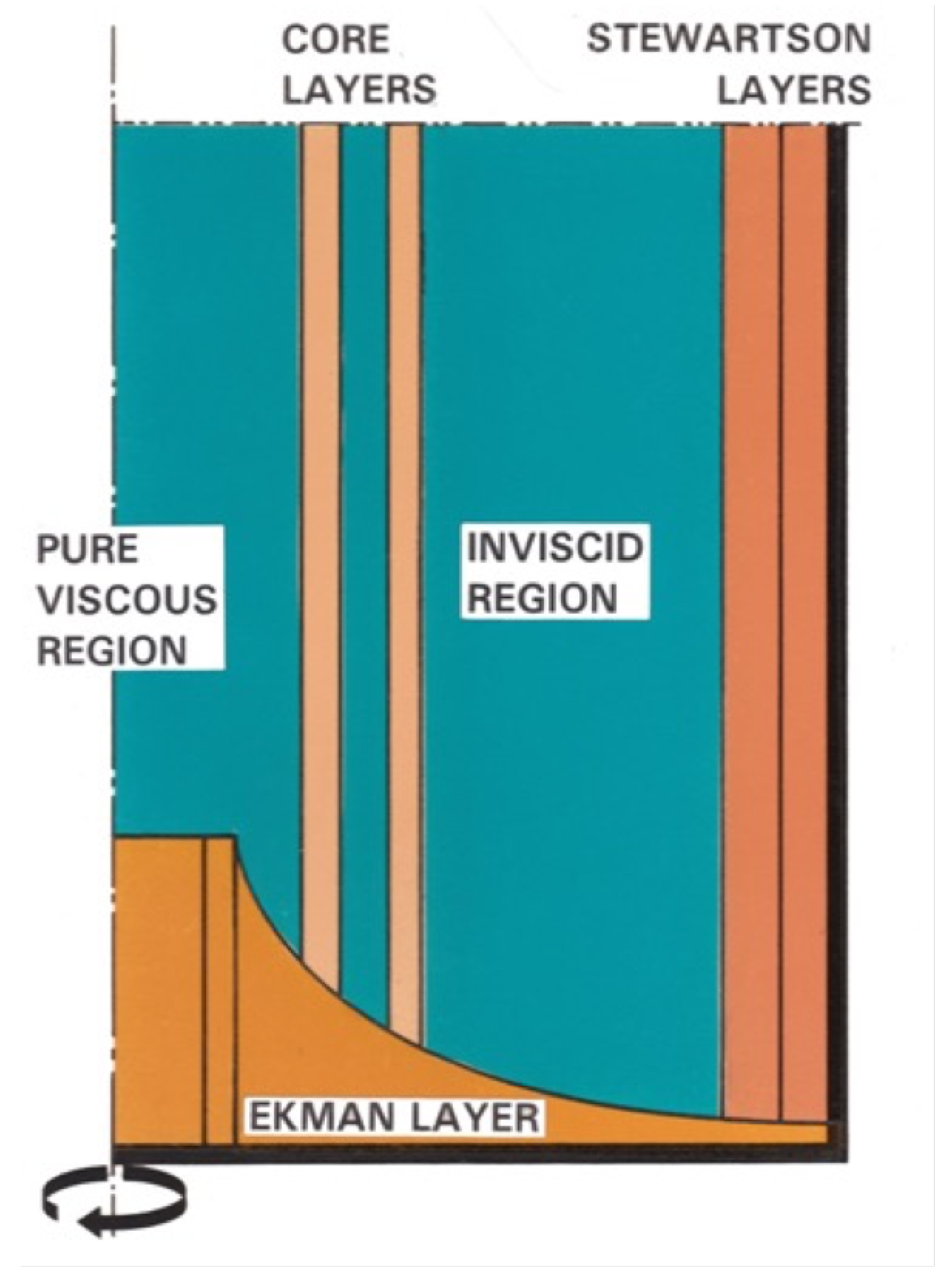
- The internal flow field represents a small perturbation on isothermal solid body rotation:Isothermal solid body rotation:Small perturbation:where v is gas velocity, r is radial position inside the rotor, p is gas pressure, M is average molecular weight, is small dimensionless parameter representing the perturbation on isothermal rigid body rotation, is perturbed velocity, is perturbed temperature, perturbed density, and perturbed pressure.
- (ii)
- The Navier–Stokes equations can be linearized by retaining terms linear in with the result that:Conservation of mass:Conservation of momentum:Conservation of energy:where is dynamic viscosity, is heat conductivity, and ∇ is nabla operator. In total there are five equations for five variables: , , and where is perturbed tangential velocity, u is radial velocity, and w is axial velocity.
- (iii)
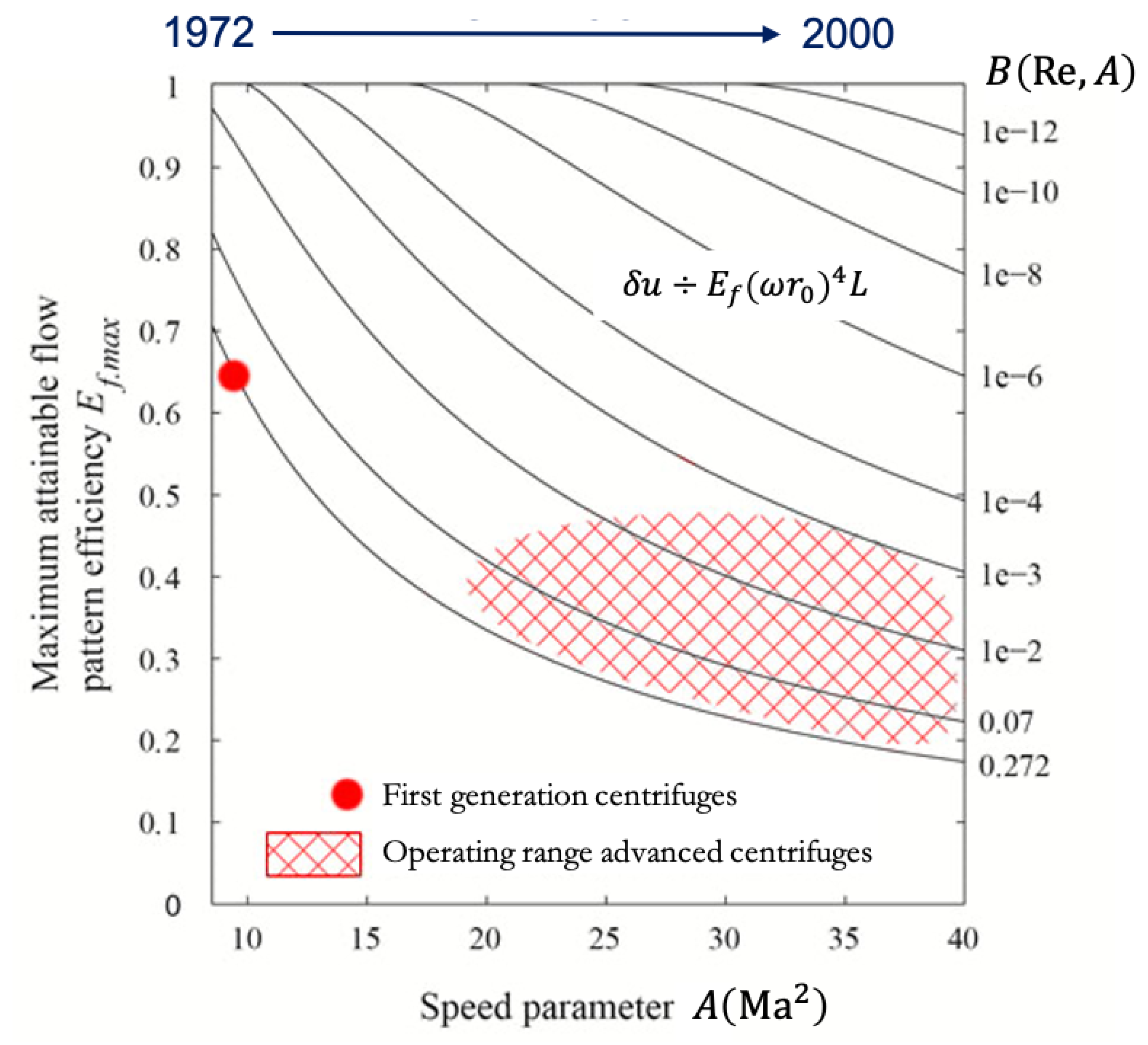
2.3. Limitations of Centrifugal Separation of Gas Mixtures
3. Centrifugal Particle Separation
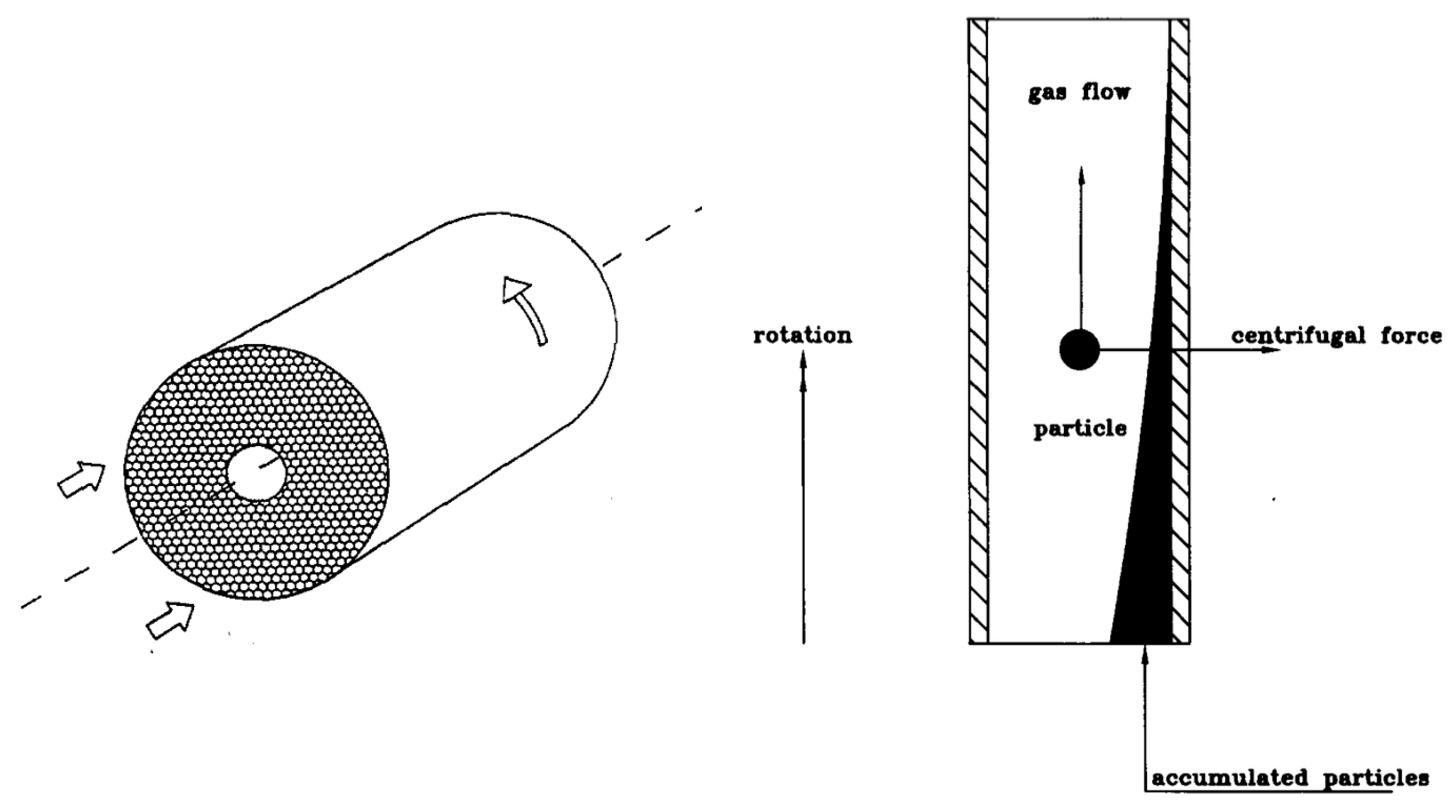
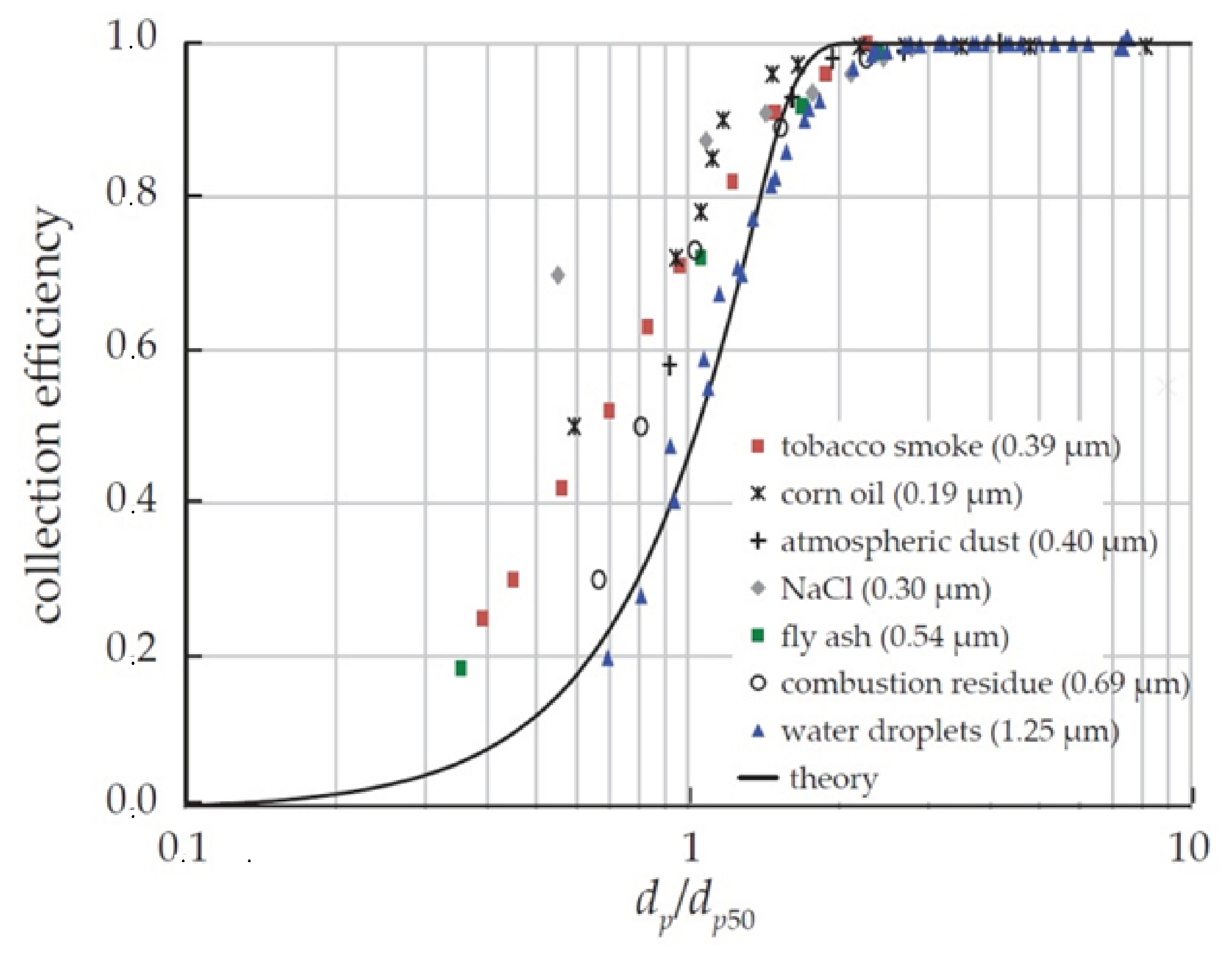
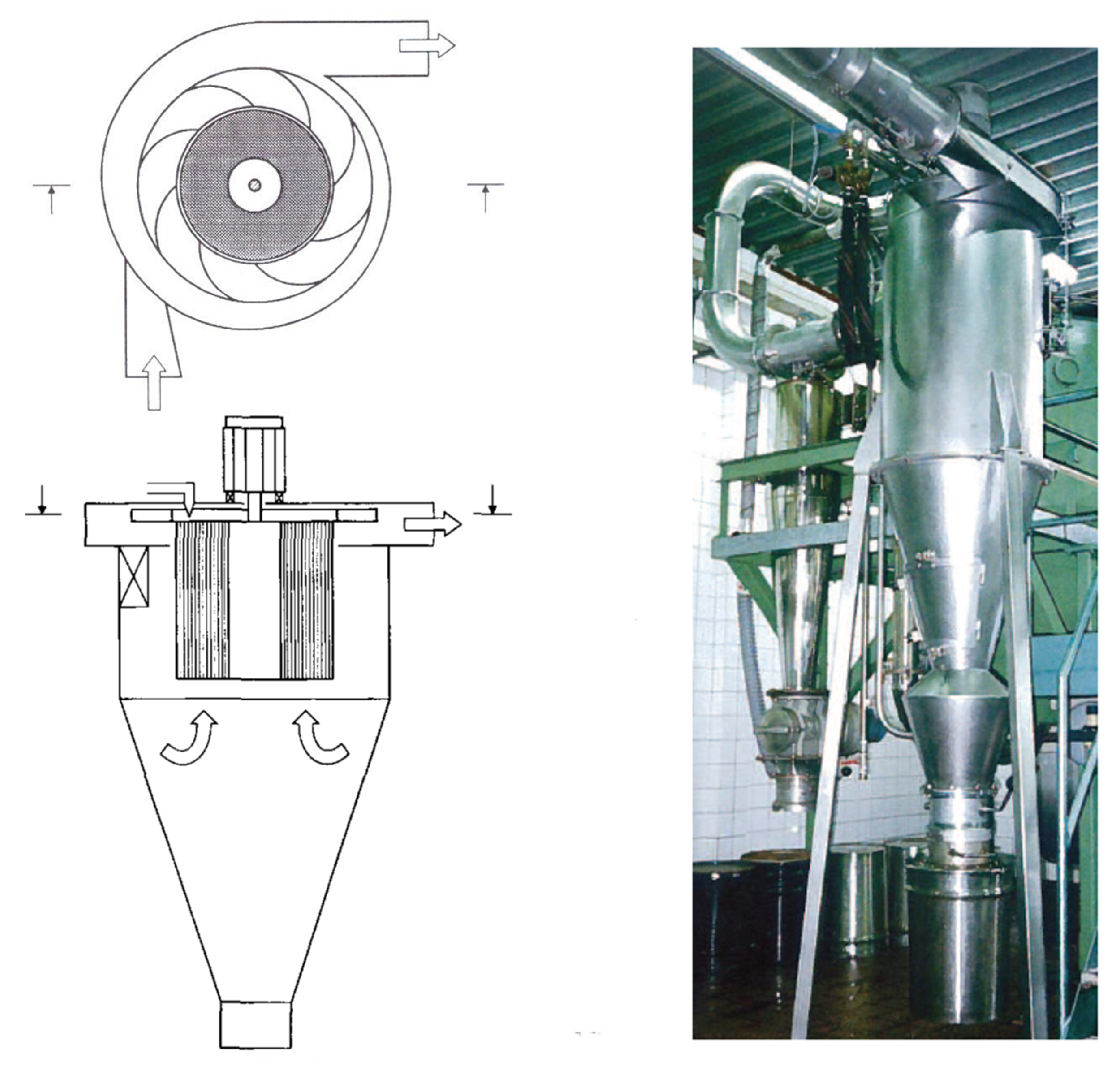
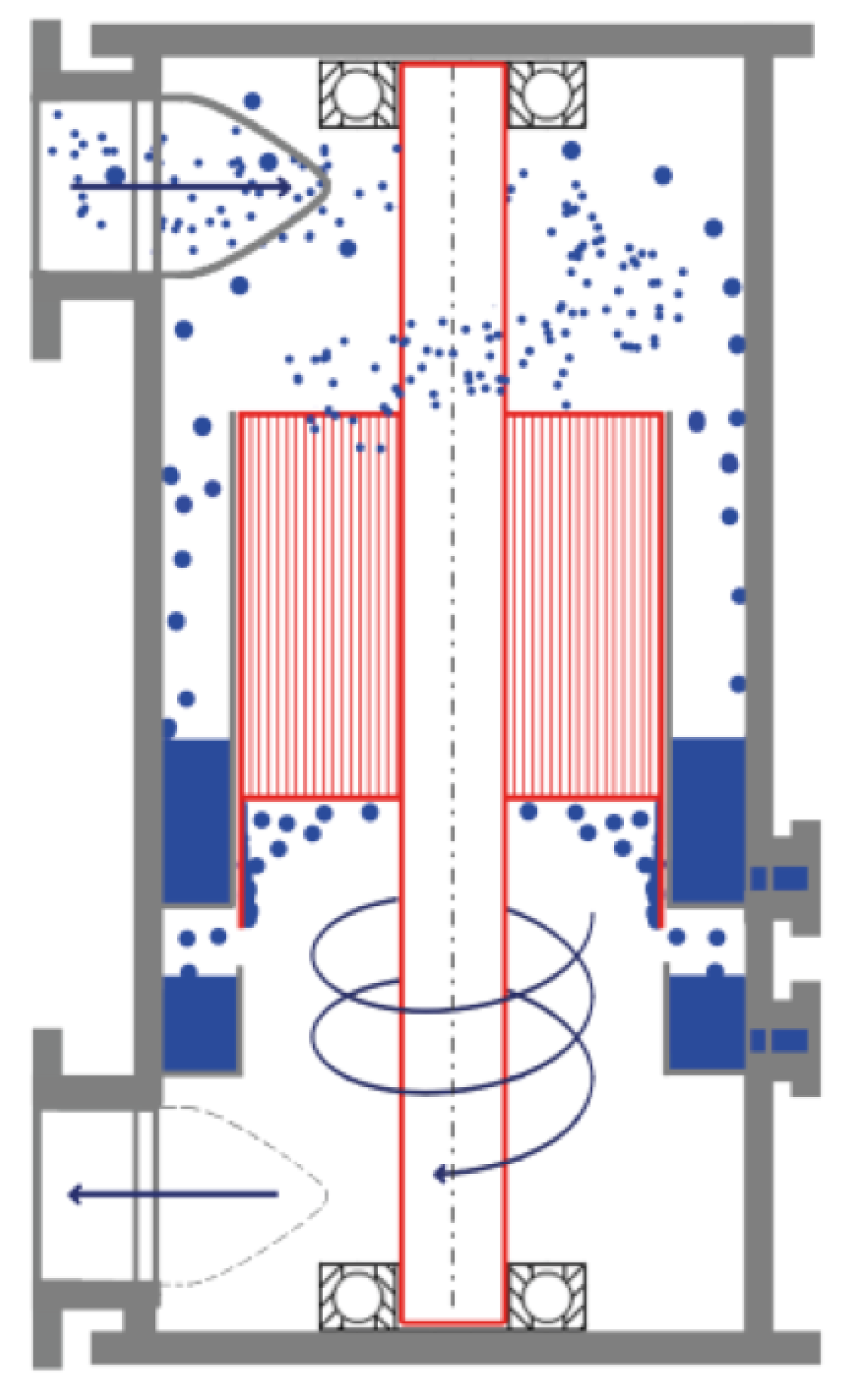
3.1. The Rotational Particle Separator
3.2. RPS Designs
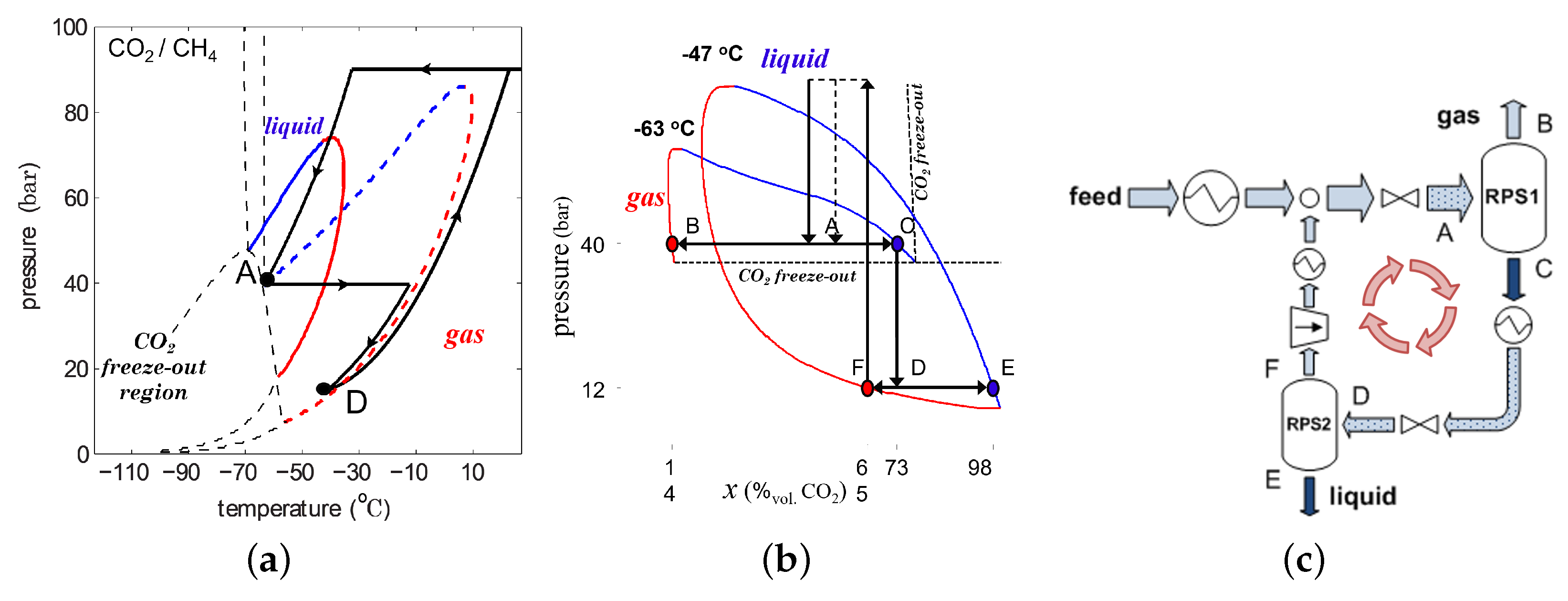
4. Condensed Rotational Separation
4.1. Liquefaction of Gases by CRS
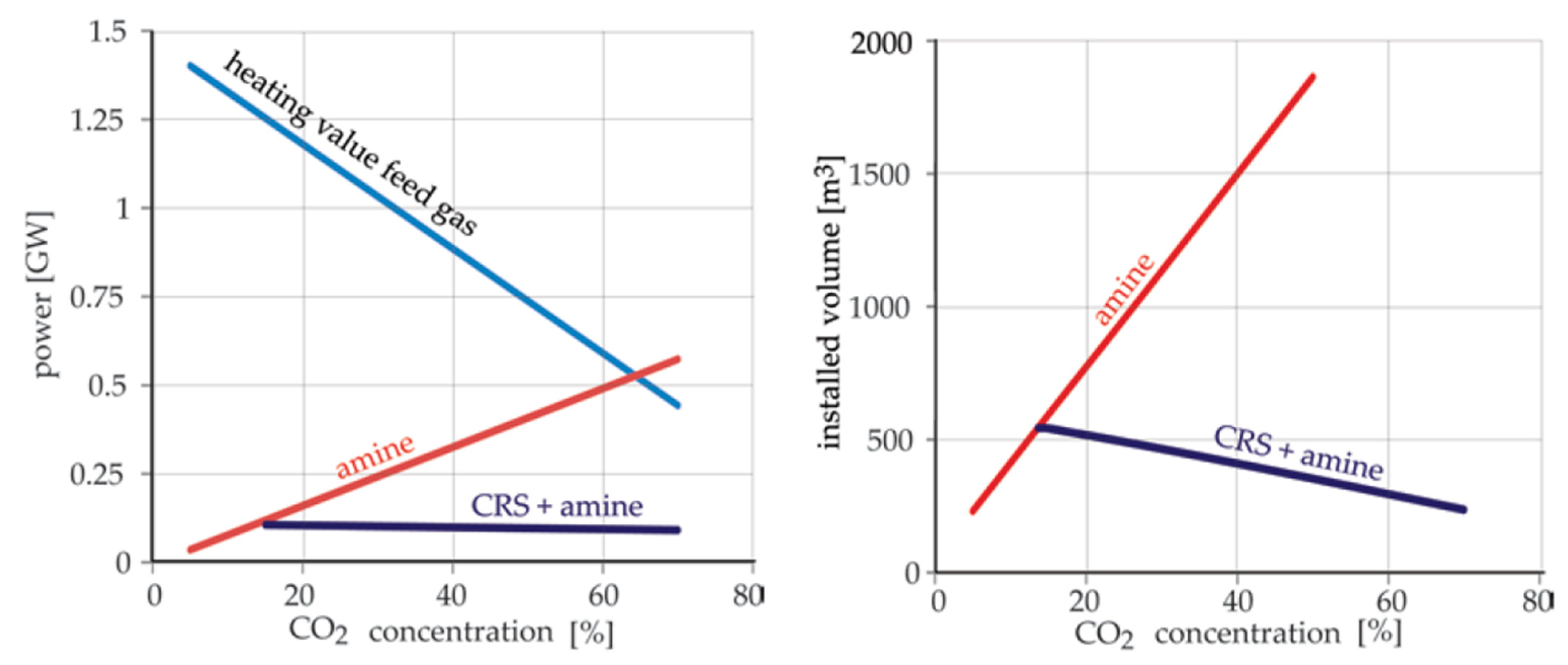
4.2. Upgrading a Sour Gas
5. The Rotational Absorber Device
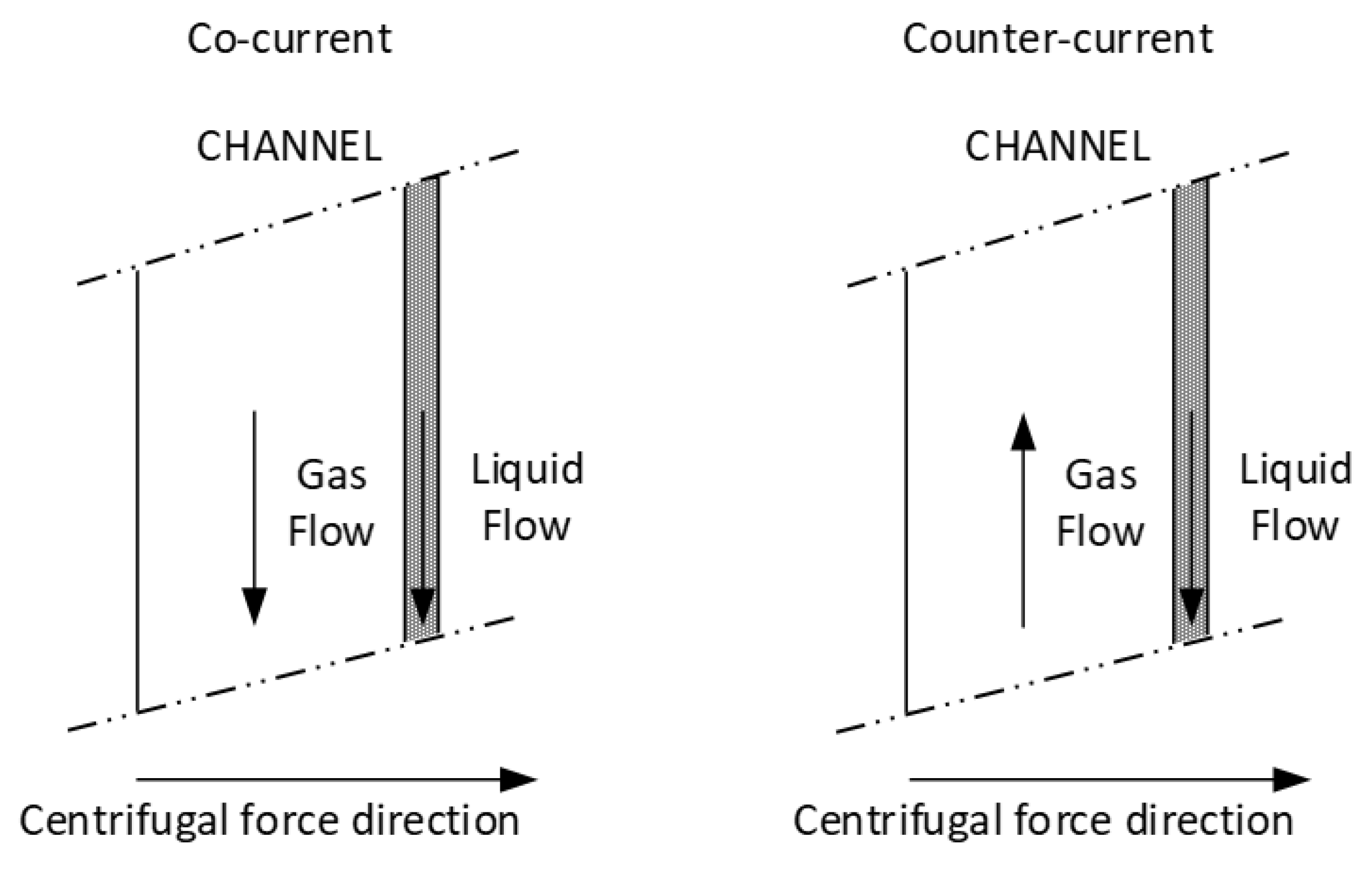
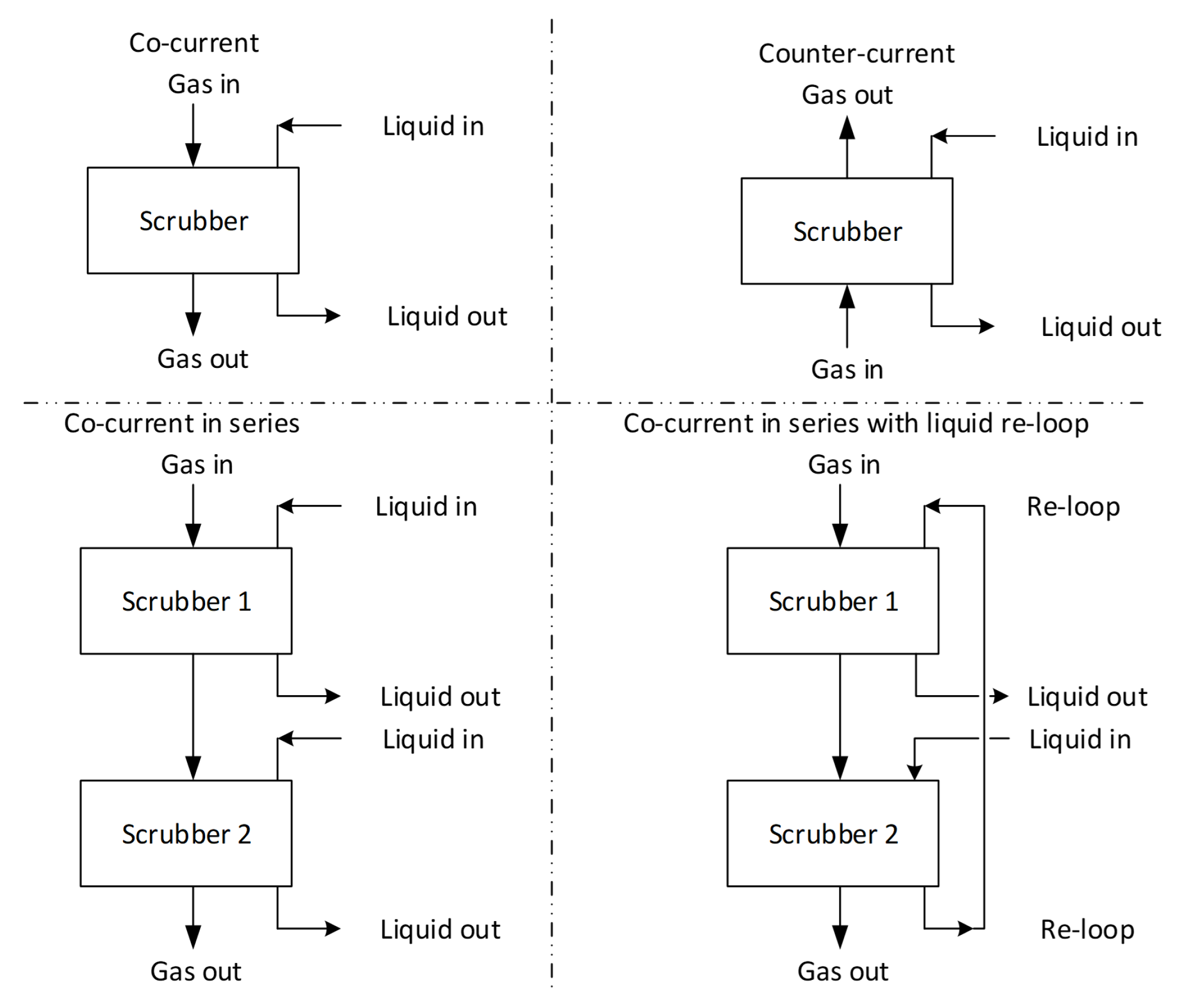
5.1. Design Layouts
5.2. Definition of
5.3. Definition of
5.4. Mass Transfer Coefficients
5.5. The Role of Rotation
- (i)
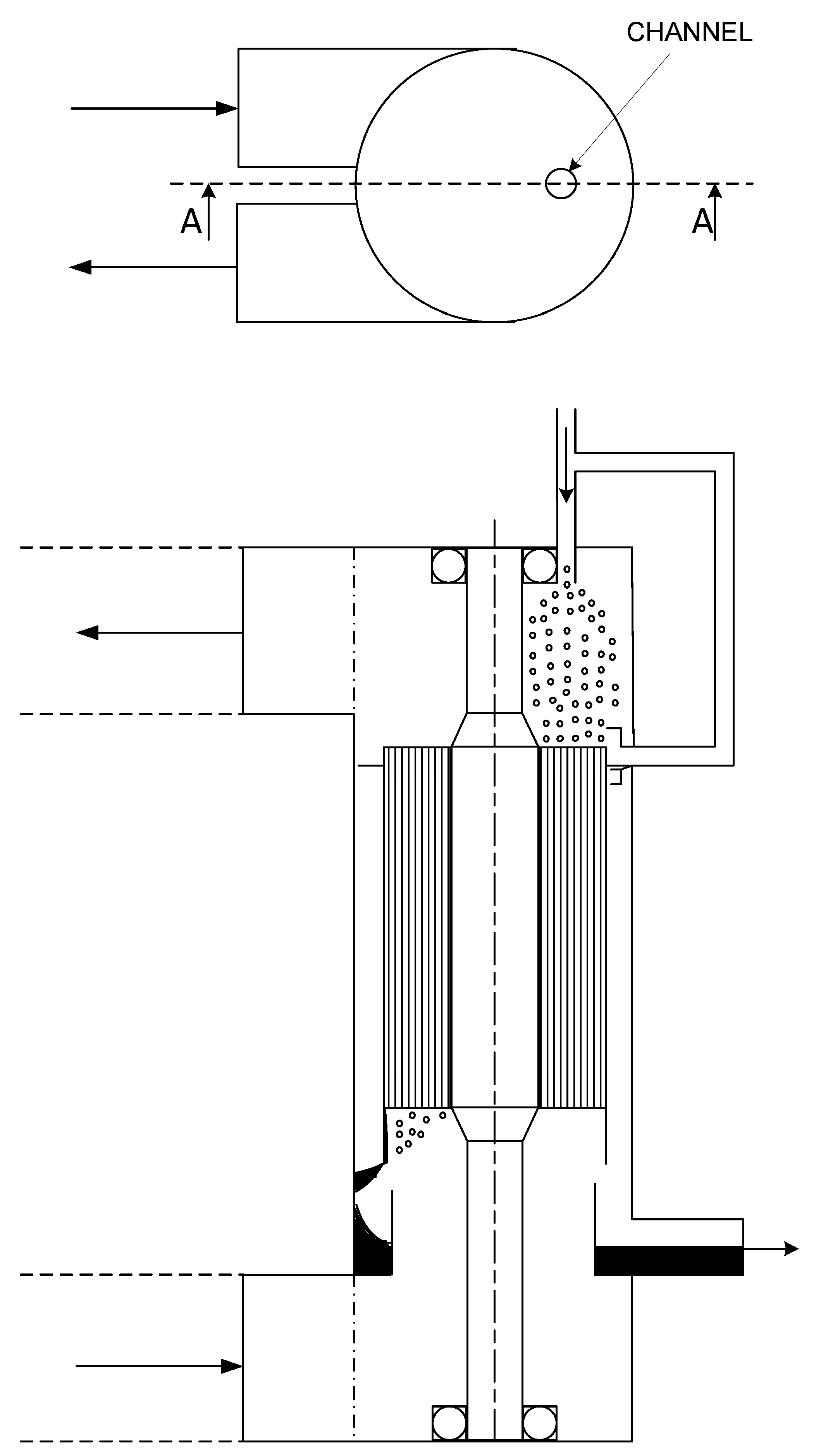
- The RAD element consists of a multitude of small channels. Because of rotation each channel receives about an equal amount of liquid from a stationary liquid source positioned above the rotating element. Some variation of injection in the circumference of the element will lead to some variation in time of the feeding of liquid from the stationary source in each channel. The degree of variation depends on rotational speed and inhomogeneity of injection in circumferential direction. The variation will lead to a wave through the liquid film which can be described by that of gravity waves in shallow water [30,31] by replacing g by . It enhances mass transfer in the liquid film in the manner described by Villermaux [32]. Its stimulating effect becomes important in case of high liquid loads where film thicknesses are large and mass transfer in the liquid is a limiting factor in the overall transfer process.
- (ii)
- The centrifugal force pushes the liquid to the outer wall of each channel. It results in the separation of gas flow and liquid film in the channel and a well-defined surface of mass transfer between both phases.
- (iii)
- Because of rotation, liquid which leaves the channels at the bottom is propelled in the form of relatively large droplets to a liquid collection chamber in the housing of the RAD: Figure 16.
- (iv)
- The RAD can also be used as a method for separating particles in addition to absorption by the liquid added to the rotating element. If the particles are very small and of micron/submicron size higher rotation speeds are required than those necessary for the functioning of the absorption processes of (i)–(iii). The particles will be transported by the liquid towards the liquid exit chamber. Applying a disinfecting liquid enables elimination of harmful bacteria and viruses [18].
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Cohen, K.P. The Theory of Isotope Separation as Applied to the Large Scale Production of U-235; McGraw-Hill: NewYork, NY, USA, 1951. [Google Scholar]
- Delbeke, J.F.A.; van Esch, B.P.M.; Eklund, G.; Janssens-Maenhout, G.; Janssens, W. Verifying the spin of centrifuges. Energy 2010, 35, 3123–3130. [Google Scholar] [CrossRef]
- Los, J. De Scheiding van Zware Isotopen in een Centrifugaal Veld. Ph.D. Thesis, Leiden University, Leiden, The Netherlands, 1963. Available online: https://www.lorentz.leidenuniv.nl/history/proefschriften/sources/Los_1963.pdf (accessed on 1 January 2020).
- Los, J. The Separative Power of Short Countercurrent Columns. Z. Naturforschung 1964, 19A, 106. [Google Scholar] [CrossRef]
- Brouwers, J.J.H. On the Motion of a Compressible Gas in a Rotating Cylinder. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 1976. Available online: https://pure.tue.nl/ws/portalfiles/portal/3503099/703721842548872.pdf (accessed on 1 January 2020).
- Brouwers, J.J.H. On compressible flow in a Rotating Cylinder. J. Eng. Math. 1978, 12, 265–285. [Google Scholar] [CrossRef] [Green Version]
- Brouwers, J.J.H. On compressible flow in a gas centrifuge and its effect on the maximum separative power. Nucl. Technol. 1978, 39, 311–322. [Google Scholar] [CrossRef]
- Dickinson, G.J.; Jones, I.P. Numerical solutions for the compressible flow in a rapidly rotating cylinder. J. Fluid Mech. 1981, 107, 89–107. [Google Scholar] [CrossRef]
- Van Ommen, K. Numerical Modelling of a Heavy Gas in Fast Rotation. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 2010. [Google Scholar]
- Brouwers, J.J.H. Rotational Particle Separator. European Patent EP0286160B1, 12 October 1988. [Google Scholar]
- Brouwers, J.J.H. Rotational Particle Separator. U.S. Patent 4,994,097, 19 February 1991. [Google Scholar]
- Fuchs, N.A. The Mechanics of Aerosols; Pergamon: Oxford, UK, 1964. [Google Scholar]
- Hinds, W.C. Aerosol Technology; John Wiley and Sons: New York, NY, USA, 1982. [Google Scholar]
- Brouwers, J.J.H. Particle collection efficiency of the rotational particle separator. Powder Technol. 1997, 92, 89–99. [Google Scholar] [CrossRef] [Green Version]
- Hoijtink, R.; Brouwers, J.J.H. Device and Method for Separating a Flowing Medium Mixture into Fractions. European Patent EP2021097B1, 7 October 2009. [Google Scholar]
- Kroes, J.P. Droplet Collection in a Scaled-Up Rotating Separator. Ph.D. Thesis, Eindhoven University of Technology, Eindhoven, The Netherlands, 2012. Available online: https://pure.tue.nl/ws/files/3658357/729780.pdf (accessed on 1 January 2020).
- Brouwers, J.J.H. Rotational particle separator: A new method for separating fine particles and mists from gases. Chem. Eng. Technol. 1996, 19, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Brouwers, J.J.H. Separation and Disinfection of Contagious Aerosols from the Perspective of SARS-CoV-2. Separations 2021, 8, 190. [Google Scholar] [CrossRef]
- Van Kemenade, H.P.; Brouwers, J.J.H. Hydrocarbon recovery by condensed rotational separation. J. Petrol. Explor. Prod. Technol. 2012, 2, 40–56. [Google Scholar] [CrossRef] [Green Version]
- Van Kemenade, H.P.; Brouwers, J.J.H.; De Rijke, S.J.M. Comparing the Volume and Energy Consumption of Sour-Gas Cleaning by Condensed Rotational Separation and Amine Treatments. Energy Technol. 2013, 1, 392–394. [Google Scholar] [CrossRef] [Green Version]
- Hoijtink, R.; Brouwers, H.; Brunner, T. Method for Separating a Medium Mixture into Fractions. U.S. Patent 8,915,988B2, 2014. [Google Scholar]
- Van Wissen, R.J.E. Centrifugal Separation for Cleaning Well Gas Streams: From Concept to Prototype. Ph.D. Thesis, Eindhoven University of Technology, Eindhoven, The Netherlands, 2006. Available online: https://pure.tue.nl/ws/portalfiles/portal/2328177/200612128.pdf (accessed on 1 January 2020).
- Willems, G.P. Condensed Rotational Cleaning of Natural Gas. Ph.D. Thesis, Eindhoven University of Technology, Eindhoven, The Netherlands, 2009. Available online: https://pure.tue.nl/ws/portalfiles/portal/2808690/200911885.pdf (accessed on 1 January 2020).
- Bansal, G.D. Condensing CO2 Droplets. Ph.D. Thesis, Eindhoven University of Technology, Eindhoven, The Netherlands, 2012. Available online: https://pure.tue.nl/ws/files/3603235/721543.pdf (accessed on 1 January 2020).
- Kohl, A.; Nielsen, R. Gas Purification; Gulf Professional Publishing: Houston, TX, USA, 1997. [Google Scholar]
- Van Benthum, R.J. CO2 Capture by Condensed Rotational Separation. Ph.D. Thesis, Eindhoven University of Technology, Eindhoven, The Netherlands, 2014. Available online: https://pure.tue.nl/ws/files/3768257/762323.pdf (accessed on 1 January 2020).
- Brouwers, J.J.H. Rotational Absorber Device and Method for Scrubbing an Absorbate from a Gas. European Patent EP3624923B1, 14 April 2021. [Google Scholar]
- Mondt, E. Compact Separator of Dispersed Phases. Ph.D. Thesis, Eindhoven University of Technology, Eindhoven, The Netherlands, 2005. Available online: https://pure.tue.nl/ws/files/3339334/200513466.pdf (accessed on 1 January 2020).
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Transport Phenemena; John Wiley and Sons: New York, NY, USA, 1960. [Google Scholar]
- Basset, A.B. A Treatise on Hydrodynamics; Bell and Co: Cambridge, UK, 1888; Volume 2. [Google Scholar]
- Lamb, H. Hydrodynamics; Cambridge University Press: Cambridge, UK, 1932. [Google Scholar]
- Villermaux, E. Mixing Versus Stirring. Annu. Rev. Fluid Mech. 2019, 51, 245–273. [Google Scholar] [CrossRef] [Green Version]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brouwers, J.J.H. Innovative Methods of Centrifugal Separation. Separations 2023, 10, 181. https://doi.org/10.3390/separations10030181
Brouwers JJH. Innovative Methods of Centrifugal Separation. Separations. 2023; 10(3):181. https://doi.org/10.3390/separations10030181
Chicago/Turabian StyleBrouwers, J. J. H. 2023. "Innovative Methods of Centrifugal Separation" Separations 10, no. 3: 181. https://doi.org/10.3390/separations10030181
APA StyleBrouwers, J. J. H. (2023). Innovative Methods of Centrifugal Separation. Separations, 10(3), 181. https://doi.org/10.3390/separations10030181




