Phytochemical Characterization and Antifungal Efficacy of Camphor (Cinnamomum camphora L.) Extract against Phytopathogenic Fungi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Sampling and Extract Preparation
2.2. Fungal Isolates, Culture Media, and Growth Conditions
2.3. Morphological and Molecular Characterization
2.4. Antifungal Activity
2.5. Phytochemical Analysis of Camphor Extracts
2.6. HPLC Conditions for Phenolic and Flavonoid Compounds
2.7. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
2.8. Statistical Tests
3. Results
3.1. Isolation Trails and Identification
3.2. Effect of Plant Extracts on the Fungal Mat Weight
3.3. Phytochemical Screening of C. camphora Methanolic Extract
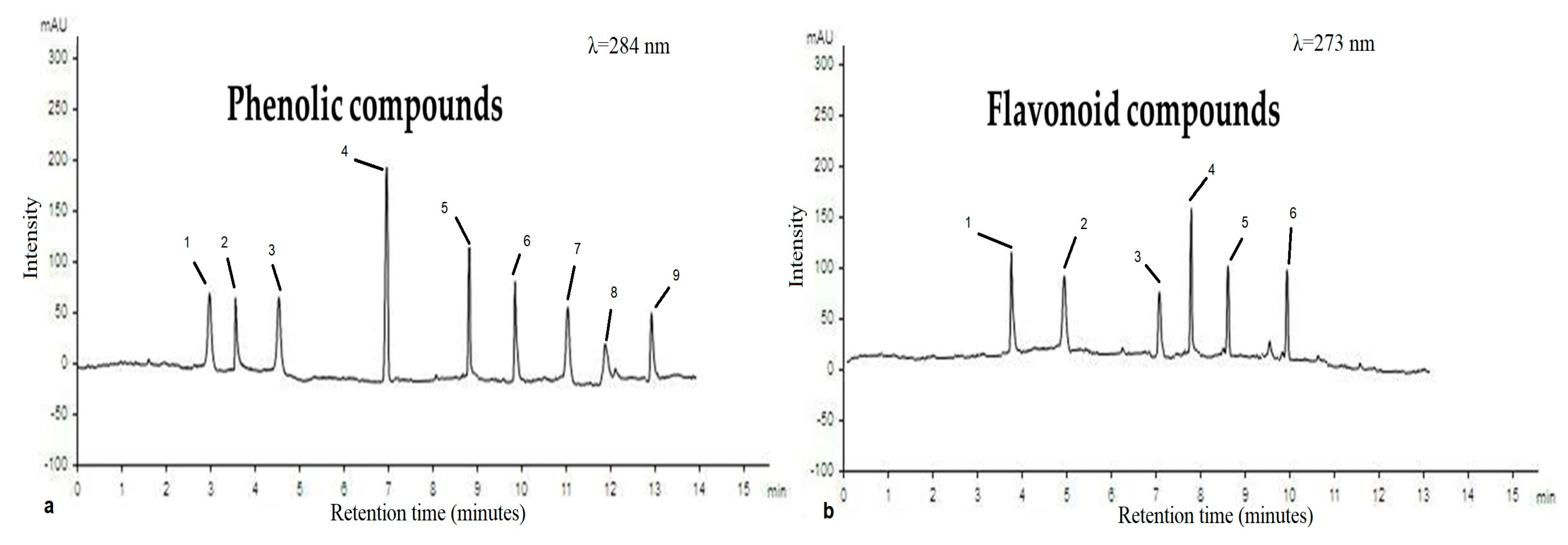
3.4. HPLC Analysis
3.5. GC-MS Analysis of the C. camphora Methanolic Extract
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdelkhalek, A.; Behiry, S.I.; Al-Askar, A.A. Bacillus velezensis PEA1 Inhibits Fusarium oxysporum Growth and Induces Systemic Resistance to Cucumber Mosaic Virus. Agronomy 2020, 10, 1312. [Google Scholar] [CrossRef]
- Baysal-Gurel, F.; Gardener, B.M.; Miller, S.A. Soil Borne Disease Management in Organic Vegetable Production. 2012. Available online: https://eorganic.org/node/7581 (accessed on 5 February 2023).
- Hibar, K.; Daami-Remadi, M.; Ayed, F.; El-Mahjoub, M. Fusarium crown and root rot of tomato and its chemical control. Int. J. Agric. Res. 2007, 2, 687–695. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, K.P.; Saleem, M.Y.; Asghar, M.; Haq, M.A. New report of Alternaria alternata causing leaf blight of tomato in Pakistan. Plant Pathol. 2004, 53, 816. [Google Scholar] [CrossRef]
- Chao, C.-C.T.; Parfitt, D.E.; Michailides, T.J. Alternaria late blight (Alternaria alternata) resistance in pistachio (Pistacia vera) and selection of resistant genotypes. J. Am. Soc. Hortic. Sci. 2001, 126, 481–485. [Google Scholar] [CrossRef] [Green Version]
- Abdelkhalek, A.; Hafez, E. Plant Viral Diseases in Egypt and Their Control. In Cottage Industry of Biocontrol Agents and Their Applications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 403–421. [Google Scholar]
- Heflish, A.A.; Abdelkhalek, A.; Al-Askar, A.A.; Behiry, S.I. Protective and Curative Effects of Trichoderma asperelloides Ta41 on Tomato Root Rot Caused by Rhizoctonia solani Rs33. Agronomy 2021, 11, 1162. [Google Scholar] [CrossRef]
- Pragadheesh, V.S.; Saroj, A.; Yadav, A.; Chanotiya, C.S.; Alam, M.; Samad, A. Chemical characterization and antifungal activity of Cinnamomum camphora essential oil. Ind. Crops Prod. 2013, 49, 628–633. [Google Scholar] [CrossRef]
- El-Gendi, H.; Al-Askar, A.A.; Király, L.; Samy, M.A.; Moawad, H.; Abdelkhalek, A. Foliar Applications of Bacillus subtilis HA1 Culture Filtrate Enhance Tomato Growth and Induce Systemic Resistance against Tobacco mosaic virus Infection. Horticulturae 2022, 8, 301. [Google Scholar] [CrossRef]
- Behiry, S.I.; Soliman, S.A.; Al-Askar, A.A.; Alotibi, F.O.; Basile, A.; Abdelkhalek, A.; Elsharkawy, M.M.; Salem, M.Z.M.; Hafez, E.E.; Heflish, A.A. Plantago lagopus extract as a green fungicide induces systemic resistance against Rhizoctonia root rot disease in tomato plants. Front. Plant Sci. 2022, 13, 2818. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Al-Askar, A.A.; Alsubaie, M.M.; Behiry, S.I. First Report of Protective Activity of Paronychia argentea Extract against Tobacco Mosaic Virus Infection. Plants 2021, 10, 2435. [Google Scholar] [CrossRef]
- Youssef, N.H.; Qari, S.H.; Behiry, S.I.; Dessoky, E.S.; El-Hallous, E.I.; Elshaer, M.M.; Kordy, A.; Maresca, V.; Abdelkhalek, A.; Heflish, A.A. Antimycotoxigenic Activity of Beetroot Extracts against Alternaria alternata Mycotoxins on Potato Crop. Appl. Sci. 2021, 11, 4239. [Google Scholar] [CrossRef]
- Ismail, I.A.; Qari, S.H.; Shawer, R.; Elshaer, M.M.; Dessoky, E.S.; Youssef, N.H.; Hamad, N.A.; Abdelkhalek, A.; Elsamra, I.A.; Behiry, S.I. The Application of Pomegranate, Sugar Apple, and Eggplant Peel Extracts Suppresses Aspergillus flavus Growth and Aflatoxin B1 Biosynthesis Pathway. Horticulturae 2021, 7, 558. [Google Scholar] [CrossRef]
- Al-Huqail, A.A.; Behiry, S.I.; Salem, M.Z.M.; Ali, H.M.; Siddiqui, M.H.; Salem, A.Z.M. Antifungal, antibacterial, and antioxidant activities of Acacia saligna (Labill.) HL Wendl. flower extract: HPLC analysis of phenolic and flavonoid compounds. Molecules 2019, 24, 700. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Zheng, T.; Ye, C.; Huannixi, W.; Yakefu, Z.; Meng, Y.; Peng, X.; Tian, Z.; Wang, J.; Ma, Y. Algicidal properties of extracts from Cinnamomum camphora fresh leaves and their main compounds. Ecotoxicol. Environ. Saf. 2018, 163, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Ghabraie, M.; Vu, K.D.; Tata, L.; Salmieri, S.; Lacroix, M. Antimicrobial effect of essential oils in combinations against five bacteria and their effect on sensorial quality of ground meat. LWT Food Sci. Technol. 2016, 66, 332–339. [Google Scholar] [CrossRef] [Green Version]
- Satyal, P.; Paudel, P.; Poudel, A.; Dosoky, N.S.; Pokharel, K.K.; Setzer, W.N. Bioactivities and compositional analyses of Cinnamomum essential oils from Nepal: C. camphora, C. tamala, and C. glaucescens. Nat. Prod. Commun. 2013, 8, 1934578X1300801232. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, R.; Pant, A.K.; Prakash, O. Chemical composition and biological activities of essential oils of Cinnamomum tamala, Cinnamomum zeylenicum and Cinnamomum camphora growing in Uttarakhand. In Chemistry of Phytopotentials: Health, Energy and Environmental Perspectives; Springer: Berlin/Heidelberg, Germany, 2012; pp. 87–92. [Google Scholar]
- Simmons, E.G. Alternaria: An Identification Manual: Fully Illustrated and with Catalogue Raisonné 1796–2007; CBS Fungal Biodiversity Centre: Utrecht, The Netherlands, 2007; ISBN 9789070351687. [Google Scholar]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; John Wiley & Sons: New York, NY, USA, 2007; ISBN 0813819199. [Google Scholar]
- Wang, Y.-B.; Yu, H.; Dai, Y.-D.; Wu, C.-K.; Zeng, W.-B.; Yuan, F.; Liang, Z.-Q. Polycephalomyces agaricus, a new hyperparasite of Ophiocordyceps sp. infecting melolonthid larvae in southwestern China. Mycol. Prog. 2015, 14, 70. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guid. Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Jindal, K.K.; Singh, R.N. Phenolic content in male and female Carica papaya: A possible physiological marker for sex identification of vegetative seedlings. Physiol. Plant. 1975, 33, 104–107. [Google Scholar] [CrossRef]
- Oser, B.L.; Hawk, P.B. Hawk’s Physiological Chemistry; McGraw-Hill: New York, NY, USA, 1965. [Google Scholar]
- Hiai, S.; Oura, H.; Hamanaka, H.; Odaka, Y. A color reaction of panaxadiol with vanillin and sulfuric acid. Planta Med. 1975, 28, 131–138. [Google Scholar] [CrossRef]
- Broadhurst, R.B.; Jones, W.T. Analysis of condensed tannins using acidified vanillin. J. Sci. Food Agric. 1978, 29, 788–794. [Google Scholar] [CrossRef]
- Lee, Y.P.; Takahashi, T. An improved colorimetric determination of amino acids with the use of ninhydrin. Anal. Biochem. 1966, 14, 71–77. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and mechanisms of antioxidant activity using the DPPH·free radical method. LWT Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Rao, L.J.; Sakariah, K.K. Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Chem. 2006, 98, 720–724. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Al-Askar, A.A.; Behiry, S.I. Bacillus licheniformis strain POT1 mediated polyphenol biosynthetic pathways genes activation and systemic resistance in potato plants against Alfalfa mosaic virus. Sci. Rep. 2020, 10, 16120. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; El-Gendi, H.; Al-Askar, A.A.; Maresca, V.; Moawad, H.; Elsharkawy, M.M.; Younes, H.A.; Behiry, S.I. Enhancing systemic resistance in faba bean (Vicia faba L.) to Bean yellow mosaic virus via soil application and foliar spray of nitrogen-fixing Rhizobium leguminosarum bv. viciae strain 33504-Alex1. Front. Plant Sci. 2022, 13, 933498. [Google Scholar] [CrossRef]
- Okla, M.K.; Alamri, S.A.; Salem, M.Z.M.; Ali, H.M.; Behiry, S.I.; Nasser, R.A.; Alaraidh, I.A.; Al-Ghtani, S.M.; Soufan, W. Yield, phytochemical constituents, and antibacterial activity of essential oils from the leaves/twigs, branches, branch wood, and branch bark of Sour Orange (Citrus aurantium L.). Processes 2019, 7, 363. [Google Scholar] [CrossRef] [Green Version]
- Abdelkhalek, A.; Al-Askar, A.A.; Elbeaino, T.; Moawad, H.; El-Gendi, H. Protective and Curative Activities of Paenibacillus polymyxa against Zucchini yellow mosaic virus Infestation in Squash Plants. Biology 2022, 11, 1150. [Google Scholar] [CrossRef]
- Youssef, N.H.; Qari, S.H.; Matar, S.; Hamad, N.A.; Dessoky, E.S.; Elshaer, M.M.; Sobhy, S.; Abdelkhalek, A.; Zakaria, H.M.; Heflish, A.A. Licorice, Doum, and Banana Peel Extracts Inhibit Aspergillus flavus Growth and Suppress Metabolic Pathway of Aflatoxin B1 Production. Agronomy 2021, 11, 1587. [Google Scholar] [CrossRef]
- Kim, D.-I.; Park, J.-D.; Kim, S.-G.; Kuk, H.; Jang, M.-S.; Kim, S.-S. Screening of some crude plant extracts for their acaricidal and insecticidal efficacies. J. Asia-Pac. Entomol. 2005, 8, 93–100. [Google Scholar] [CrossRef]
- Chuanmao, W.; Zhouhe, W.; Yesong, W. Study on antibacterial activity of ethanol extract of Cinamomum camphora. Amino Acids Biot. Resour. 2000, 22, 41–42. [Google Scholar]
- Chua, M.-T.; Tung, Y.-T.; Chang, S.-T. Antioxidant activities of ethanolic extracts from the twigs of Cinnamomum osmophloeum. Bioresour. Technol. 2008, 99, 1918–1925. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Mishra, A.K.; Tan, R.X.; Tang, C.; Yang, H.; Shen, Y.F. Repellent and insecticidal activities of essential oils from Artemisia princeps and Cinnamomum camphora and their effect on seed germination of wheat and broad bean. Bioresour. Technol. 2006, 97, 1969–1973. [Google Scholar] [CrossRef]
- Walch, S.G.; Kuballa, T.; Stühlinger, W.; Lachenmeier, D.W. Determination of the biologically active flavour substances thujone and camphor in foods and medicines containing sage (Salvia officinalis L.). Chem. Cent. J. 2011, 5, 44. [Google Scholar] [CrossRef] [Green Version]
- Pandey, A.K.; Bora, H.R.; Deka, S.C.; Rastogi, R.C.; Baruah, A.K.S. Composition of the essential oil of the bark of Cinnamomum camphora. J. Med. Aromat. Plant Sci. 1997, 19, 408–409. [Google Scholar]
- Poudel, D.K.; Rokaya, A.; Ojha, P.K.; Timsina, S.; Satyal, R.; Dosoky, N.S.; Satyal, P.; Setzer, W.N. The chemical profiling of essential oils from different tissues of Cinnamomum camphora L. and their antimicrobial activities. Molecules 2021, 26, 5132. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, D.-S.; Park, S.-H.; Park, H. Phytochemistry and applications of Cinnamomum camphora essential oils. Molecules 2022, 27, 2695. [Google Scholar] [CrossRef] [PubMed]
- Duda-Madej, A.; Stecko, J.; Sobieraj, J.; Szymańska, N.; Kozłowska, J. Naringenin and Its Derivatives—Health-Promoting Phytobiotic against Resistant Bacteria and Fungi in Humans. Antibiotics 2022, 11, 1628. [Google Scholar] [CrossRef]
- Teodoro, G.R.; Ellepola, K.; Seneviratne, C.J.; Koga-Ito, C.Y. Potential use of phenolic acids as anti-Candida agents: A review. Front. Microbiol. 2015, 6, 1420. [Google Scholar] [CrossRef] [Green Version]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Alves, C.T.; Ferreira, I.C.F.R.; Barros, L.; Silva, S.; Azeredo, J.; Henriques, M. Antifungal activity of phenolic compounds identified in flowers from North Eastern Portugal against Candida species. Future Microbiol. 2014, 9, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Ekeberg, D.; Flæte, P.-O.; Eikenes, M.; Fongen, M.; Naess-Andresen, C.F. Qualitative and quantitative determination of extractives in heartwood of Scots pine (Pinus sylvestris L.) by gas chromatography. J. Chromatogr. A 2006, 1109, 267–272. [Google Scholar] [CrossRef]
- Yakefu, Z.; Huannixi, W.; Ye, C.; Zheng, T.; Chen, S.; Peng, X.; Tian, Z.; Wang, J.; Yang, Y.; Ma, Z. Inhibitory effects of extracts from Cinnamomum camphora fallen leaves on algae. Water Sci. Technol. 2018, 77, 2545–2554. [Google Scholar] [CrossRef] [PubMed]
- Tomazoni, E.Z.; Pauletti, G.F.; da Silva Ribeiro, R.T.; Moura, S.; Schwambach, J. In vitro and in vivo activity of essential oils extracted from Eucalyptus staigeriana, Eucalyptus globulus and Cinnamomum camphora against Alternaria solani Sorauer causing early blight in tomato. Sci. Hortic. 2017, 223, 72–77. [Google Scholar] [CrossRef]
- Manilal, A.; Idhayadhulla, A. Potential in vitro antimicrobial efficacy of Holigarna arnottiana (Hook F). Asian Pac. J. Trop. Biomed. 2014, 4, 25–29. [Google Scholar] [CrossRef]
- Kalpashree, M.M.; Raveesha, K.A. Efficacy of aqueous plant extracts against Alternaria solani, the causative agent of early blight of tomato. Int. J. Herb. Med 2016, 4, 184–188. [Google Scholar]
- Pinto, E.; Gonçalves, M.J.; Oliveira, P.; Coelho, J.; Cavaleiro, C.; Salgueiro, L. Activity of Thymus caespititius essential oil and α-terpineol against yeasts and filamentous fungi. Ind. Crops Prod. 2014, 62, 107–112. [Google Scholar] [CrossRef]
- Mishra, A.K.; Dwivedi, S.K.; Kishore, N.; Dubey, N.K. Fungistatic properties of essential oil of Cinnamomum camphora. Int. J. Pharmacogn. 1991, 29, 259–262. [Google Scholar] [CrossRef]
- Govindappa, M.; Prathap, S.; Vinay, V.; Channabasava, R. Chemical composition of methanol extract of endophytic fungi, Alternaria sp. of Tebebuia argentea and their antimicrobial and antioxidant activity. Int. J. Biol. Pharm. Res. 2014, 5, 861–869. [Google Scholar]
- Krishnan, K.; Mani, A.; Jasmine, S. Cytotoxic Activity of Bioactive Compound 1, 2-Benzene Dicarboxylic Acid, Mono 2-Ethylhexyl Ester Extracted from a Marine Derived Streptomyces sp. VITSJK8. Int. J. Mol. Cell. Med. 2014, 3, 246–254. [Google Scholar] [PubMed]
- Mukund, S.; Jegan, G.; Palanisamy, M.; Muthukumaran, M.; Sivasubramanian, V. GC-MS and FT-IR analysis of some bioactive constituents from Oscillatoria terebriformis. Indian J. Pharm. Sci. Res. 2014, 4, 102–107. [Google Scholar]
- Ali, A.; Javaid, A.; Shoaib, A. GC-MS analysis and antifungal activity of methanolic root extract of Chenopodium album against Sclerotium rolfsii. Planta Daninha 2017, 35, e017164713. [Google Scholar] [CrossRef] [Green Version]
- Sousa, R.M.F.; de Morais, S.A.L.; Vieira, R.B.K.; Napolitano, D.R.; Guzman, V.B.; Moraes, T.S.; Cunha, L.C.S.; Martins, C.H.G.; Chang, R.; de Aquino, F.J.T. Chemical composition, cytotoxic, and antibacterial activity of the essential oil from Eugenia calycina Cambess. leaves against oral bacteria. Ind. Crops Prod. 2015, 65, 71–78. [Google Scholar] [CrossRef]
- Tan, N.; Satana, D.; Sen, B.; Tan, E.; Altan, H.B.; Demirci, B.; Uzun, M. Antimycobacterial and Antifungal Activities of Selected Four Salvia Species; ACG Publicaitons: Kocaeli, Türkiye, 2016. [Google Scholar]
- Hameed, I.H.; Hussein, H.J.; Kareem, M.A.; Hamad, N.S. Identification of five newly described bioactive chemical compounds in methanolic extract of Mentha viridis by using gas chromatography-mass spectrometry (GC-MS). J. Pharmacogn. Phyther. 2015, 7, 107–125. [Google Scholar]
- Al-Mawla, A.H.; Abu-Serag, N.A. Determination of bioactive compounds in leaves of Urospermum picroides (L.) Desf. Plant Arch. 2019, 19, 1299–1308. [Google Scholar]
- Arora, S.; Kumar, G. Gas Chromatography-Mass Spectrometry (GC-MS) determination of bioactive constituents from the methanolic and ethyl acetate extract of Cenchrus setigerus Vahl (Poaceae). Pharma Innov. J. 2017, 6, 635–640. [Google Scholar]
- Rosato, A.; Vitali, C.; De Laurentis, N.; Armenise, D.; Milillo, M.A. Antibacterial effect of some essential oils administered alone or in combination with Norfloxacin. Phytomedicine 2007, 14, 727–732. [Google Scholar] [CrossRef]
- Sonboli, A.; Babakhani, B.; Mehrabian, A.R. Antimicrobial activity of six constituents of essential oil from Salvia. Zeitschrift für Naturforschung C 2006, 61, 160–164. [Google Scholar] [CrossRef]
- Safaei-Ghomi, J.; Ahd, A.A. Antimicrobial and antifungal properties of the essential oil and methanol extracts of Eucalyptus largiflorens and Eucalyptus intertexta. Pharmacogn. Mag. 2010, 6, 172–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorman, H.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.-T.; Chua, M.-T.; Wang, S.-Y.; Chang, S.-T. Anti-inflammation activities of essential oil and its constituents from indigenous cinnamon (Cinnamomum osmophloeum) twigs. Bioresour. Technol. 2008, 99, 3908–3913. [Google Scholar] [CrossRef]
- Hammami, S.; Jmii, H.; El Mokni, R.; Khmiri, A.; Faidi, K.; Dhaouadi, H.; El Aouni, M.H.; Aouni, M.; Joshi, R.K. Essential oil composition, antioxidant, cytotoxic and antiviral activities of Teucrium pseudochamaepitys growing spontaneously in Tunisia. Molecules 2015, 20, 20426–20433. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.-Q.; Kenney, P.M.; Lam, L.K.T. Sesquiterpenes from clove (Eugenia caryophyllata) as potential anticarcinogenic agents. J. Nat. Prod. 1992, 55, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.P.; Singh, R.K.; Malik, P. Analgesic and anti-inflammatory activities of Annona squamosa Linn bark. J. Sci. Innov. Res 2014, 3, 60–64. [Google Scholar] [CrossRef]
| Concentration (µg/mL) | Weight of Fungal Mat (g) | |||||
|---|---|---|---|---|---|---|
| F. solani | Inhibition % | A. alternata | Inhibition % | F. oxysporum | Inhibition % | |
| Control (10% DMSO) | 3.38 ± 0.2 a | 0.00 | 4.12 ± 0.12 a | 0.00 | 1.37 ± 0.1 a | 0.00 |
| 1000 | 3.27 ± 0.1 ab | 3.25 | 3.65 ± 0.14 b | 11.41 | 1.20 ± 0.1 a | 12.41 |
| 2000 | 3.22 ± 0.2 ab | 4.73 | 2.95 ± 0.3 c | 28.40 | 0.81 ± 0.1 b | 40.88 |
| 3000 | 3.05 ± 0.2 b | 9.76 | 2.33 ± 0.1 d | 43.45 | 0.66 ± 0.1 bc | 51.82 |
| 4000 | 2.56 ± 0.1 c | 24.26 | 2.09 ± 0.2 d | 49.27 | 0.55 ± 0.1 c | 59.85 |
| LSD 0.05 | 0.24 ** | 0.33 ** | 0.18 ** | |||
| Classification | Tested Parameters | Concentration (mg/g DM) |
|---|---|---|
| Antioxidants | Flavonoids | 48.0 ± 2.3 |
| Phenols | 22.7 ± 1.1 | |
| Ascorbic acid | 46.6 ± 1.9 | |
| Secondary metabolites | Saponin | 12.0 ± 0.9 |
| Tannins | 16.5 ± 0.6 | |
| Osmo-regulatory molecules | Proline | 2.6 ± 0.4 |
| Glycine betaine (GB) | 16.0 ± 1.2 | |
| Total amino acids | 37.4 ± 3.4 | |
| Antioxidant activity | Total antioxidant activity (PMA) | 22.0 ± 2.1 |
| DPPH scavenging activity % | 13.7 ± 1.6 |
| Phenolic Compounds | Flavonoid Compounds | ||||
|---|---|---|---|---|---|
| Compound | * RT | µg/mL | Compound | RT | µg/mL |
| Syringic acid | 2.8 | 1.08 | Naringin | 3.8 | 3.78 |
| p-coumaric acid | 3.5 | 0.98 | Quercetin | 5.0 | 2.46 |
| Caffeic acid | 4.8 | 1.11 | Hesperidin | 7.0 | 1.25 |
| Ferulic acid | 7.0 | 7.22 | Catechin | 7.8 | 6.21 |
| Gallic acid | 8.9 | 6.98 | 7-OH flavone | 8.9 | 4.36 |
| Benzoic acid | 10.0 | 1.55 | Apigenin | 10.0 | 3.98 |
| Ellagic acid | 11.0 | 2.11 | |||
| Iso-Ferulic acid | 12.0 | 0.87 | |||
| Catechol | 13.0 | 1.65 | |||
| Retention Time | m/z | Area | Height | Name | Molecular Formula | Class | Chemical Structure |
|---|---|---|---|---|---|---|---|
| 10.475 | 43.00 | 18,660 | 8351 | 2-Oxabicyclo[2.2.2]octan-6-ol, 1,3,3-trimethyl- (1,8-Cineole) | C10H18O2 | monoterpene |  |
| 10.547 | 43.00 | 40,620 | 22,112 | Bicyclo(3.1.1)heptane-2,3-diol, 2,6,6-trimethyl- (Pinanediol) | C10H18O2 | terpene | 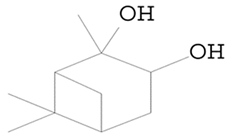 |
| 10.595 | 111.00 | 113,130 | 34,818 | 2-Butenamide, 2-ethyl-3-methyl-N-phenyl (Crotamiton) | C13H17NO | anilides | 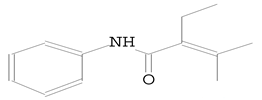 |
| 11.253 | 43.00 | 16,397 | 7665 | 5-Hexenal, 4-(acetyloxy)-4-methyl-, | C9H14O3 | acetate ester | 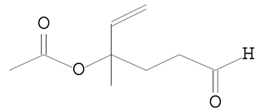 |
| 11.460 | 98.00 | 30,566 | 14,037 | 2-Cyclohexen-1-one, 4-hydroxy-3-methyl-6-(1-methylethyl)-, (Barosma camphor) | C10H16O2 | cyclic monoterpene ketone | 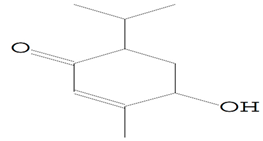 |
| 11.712 | 41.00 | 22,150 | 12,307 | 7-Oxabicyclo[4.1.0]heptan-2-one, 3-methyl-6-(1-methylethyl) | C10H16O2 | ketone |  |
| 12.211 | 43.00 | 40,594 | 22,972 | 7-Methyl-Z-tetradecen-1-ol acetate | C17H32O2 | fatty acid esters | 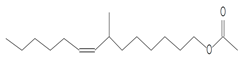 |
| 12.971 | 43.00 | 87,247 | 55,041 | 1H-Cycloprop[e]azulen-7-ol, decahydro-1,1,7-trimethyl-4-methylene- (Spathulenol) | C15H24O | tricyclic sesquiterpene | 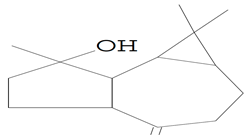 |
| 14.115 | 43.00 | 45,451 | 32,086 | 9,19-Cyclolanostan-3-ol, acetate, (3.beta)- | C32H54O2 | triterpene | 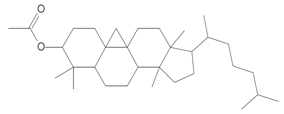 |
| 14.150 | 43.00 | 14,824 | 9135 | Caryophyllene oxide | C15H24O | cyclic sesquiterpene |  |
| 14.258 | 43.00 | 15,666 | 10,273 | Ledol | C15H26O | sesquiterpene | 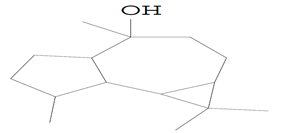 |
| 14.317 | 43.00 | 20,595 | 12,396 | trans-Z-alpha.-Bisabolene epoxide | C32H54O2 | sesquiterpene | 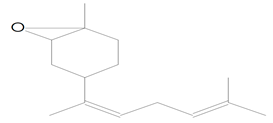 |
| 14.859 | 41.00 | 9363 | 7179 | Andrographolide | C20H30O5 | diterpenoids |  |
| 23.267 | 149.00 | 437,984 | 83,389 | 1,2-Benzenedicarboxylic acid, mono(2-ethylhexyl) ester | C16H22O4 | phthalate esters | 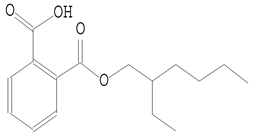 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobhy, S.; Al-Askar, A.A.; Bakhiet, E.K.; Elsharkawy, M.M.; Arishi, A.A.; Behiry, S.I.; Abdelkhalek, A. Phytochemical Characterization and Antifungal Efficacy of Camphor (Cinnamomum camphora L.) Extract against Phytopathogenic Fungi. Separations 2023, 10, 189. https://doi.org/10.3390/separations10030189
Sobhy S, Al-Askar AA, Bakhiet EK, Elsharkawy MM, Arishi AA, Behiry SI, Abdelkhalek A. Phytochemical Characterization and Antifungal Efficacy of Camphor (Cinnamomum camphora L.) Extract against Phytopathogenic Fungi. Separations. 2023; 10(3):189. https://doi.org/10.3390/separations10030189
Chicago/Turabian StyleSobhy, Sherien, Abdulaziz A. Al-Askar, Elsayed K. Bakhiet, Mohsen M. Elsharkawy, Amr A. Arishi, Said I. Behiry, and Ahmed Abdelkhalek. 2023. "Phytochemical Characterization and Antifungal Efficacy of Camphor (Cinnamomum camphora L.) Extract against Phytopathogenic Fungi" Separations 10, no. 3: 189. https://doi.org/10.3390/separations10030189
APA StyleSobhy, S., Al-Askar, A. A., Bakhiet, E. K., Elsharkawy, M. M., Arishi, A. A., Behiry, S. I., & Abdelkhalek, A. (2023). Phytochemical Characterization and Antifungal Efficacy of Camphor (Cinnamomum camphora L.) Extract against Phytopathogenic Fungi. Separations, 10(3), 189. https://doi.org/10.3390/separations10030189









