Separation of Metal and Cathode Materials from Waste Lithium Iron Phosphate Battery by Electrostatic Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
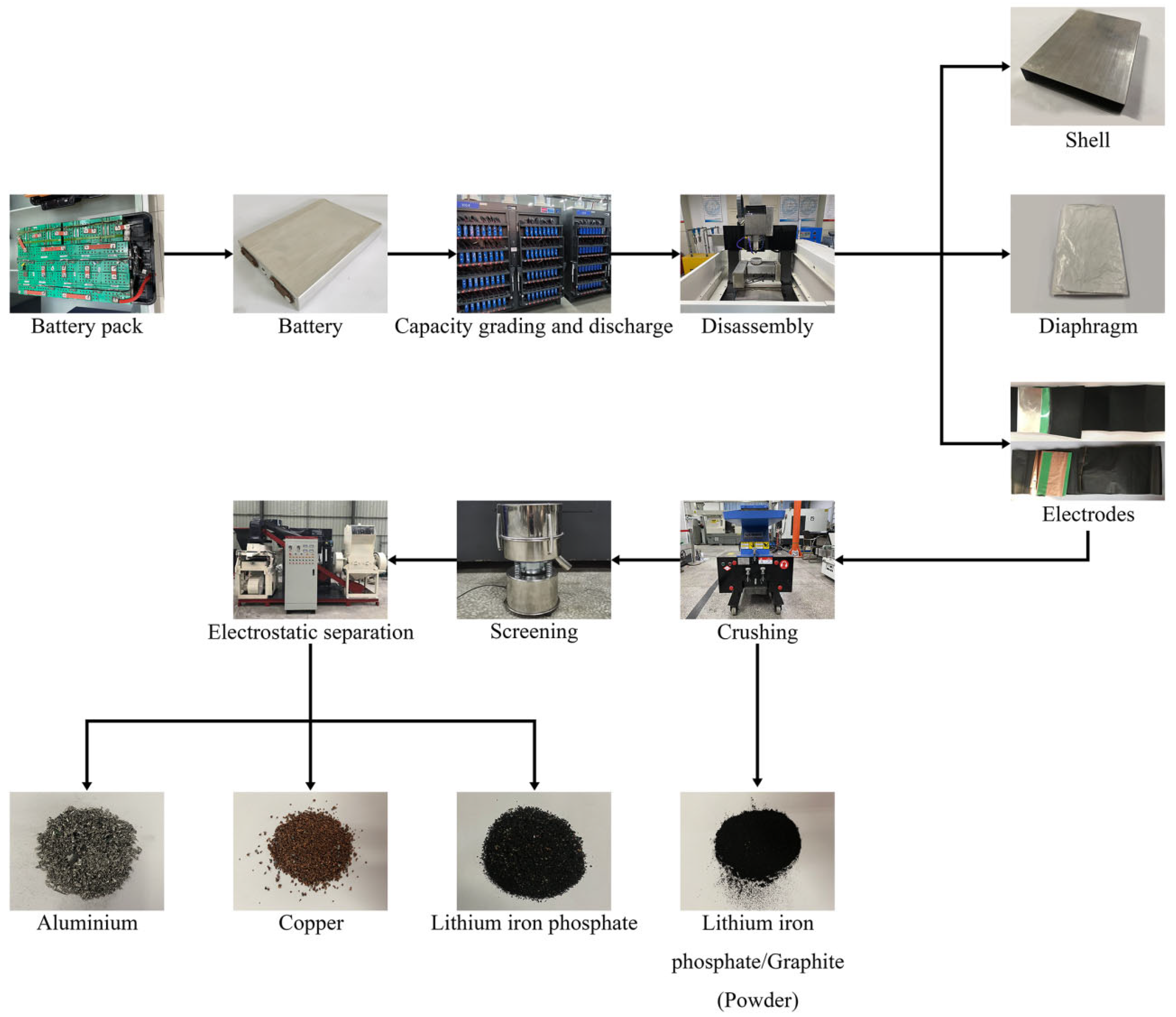
2.2. Discharge and Disassembly
2.3. Pretreatment and Crushing
2.4. Establishment of High-Voltage Electrostatic Separation Model
2.5. High Voltage Electrostatic Separation Test
3. Results and Discussion
3.1. Pretreatment and Characterization
3.2. Crushing and Screening
3.3. Simulation and Analysis of High-Voltage Electrostatic Separation Process of Crushed Products
3.4. Fitting Results of Particle Motion Trajectory
3.5. Analysis of Electrostatic Separation Test Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, J.; Neiber, R.R.; Park, J.; Soomro, R.A.; Greene, G.W.; Mazari, S.A.; Seo, H.Y.; Lee, J.H.; Shon, M.; Chang, D.W.; et al. Recent progress in sustainable recycling of LiFePO4-type lithium-ion batteries: Strategies for highly selective lithium recovery. Chem. Eng. J. 2022, 431, 133993. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, B.; Shen, M.; Wu, Y.; Qu, S.; Hu, Y.; Feng, Y. Environmental impact assessment of second life and recycling for LiFePO4 power batteries in China. J. Environ. Manag. 2022, 314, 115083. [Google Scholar] [CrossRef]
- Makwarimba, C.P.; Tang, M.; Peng, Y.; Lu, S.; Zheng, L.; Zhao, Z.; Zhen, A.G. Assessment of recycling methods and processes for lithium-ion batteries. Iscience 2022, 25, 104321. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Mu, D.; Lu, Z.; Li, R.; Liu, Z.; Wang, Y.; Tian, S.; Dai, C. A comprehensive review on the recycling of spent lithium-ion batteries: Urgent status and technology advances. J. Clean. Prod. 2022, 340, 130535. [Google Scholar] [CrossRef]
- Liu, W.; Zhong, X.; Han, J.; Qin, W.; Liu, T.; Zhao, C.; Chang, Z. Kinetic study and pyrolysis behaviors of spent LiFePO4 batteries. ACS Sustain. Chem. Eng. 2018, 7, 1289–1299. [Google Scholar] [CrossRef]
- Wang, M.; Liu, K.; Dutta, S.; Alessi, D.S.; Rinklebe, J.; Ok, Y.S.; Tsang, D.C. Recycling of lithium iron phosphate batteries: Status, technologies, challenges, and prospects. Renew. Sustain. Energy Rev. 2022, 163, 112515. [Google Scholar] [CrossRef]
- Chen, M.; Ma, X.; Chen, B.; Arsenault, R.; Karlson, P.; Simon, N.; Wang, Y. Recycling end-of-life electric vehicle lithium-ion batteries. Joule 2019, 3, 2622–2646. [Google Scholar] [CrossRef]
- Mao, J.; Li, J.; Xu, Z. Coupling reactions and collapsing model in the roasting process of recycling metals from LiCoO2 batteries. J. Clean. Prod. 2018, 205, 923–929. [Google Scholar] [CrossRef]
- Liang, Z.; Cai, C.; Peng, G.; Hu, J.; Hou, H.; Liu, B.; Liang, S.; Xiao, K.; Yuan, S.; Yang, J. Hydrometallurgical recovery of spent lithium ion batteries: Environmental strategies and sustainability evaluation. ACS Sustain. Chem. Eng. 2021, 9, 5750–5767. [Google Scholar] [CrossRef]
- Jung, J.C.Y.; Sui, P.C.; Zhang, J. A review of recycling spent lithium-ion battery cathode materials using hydrometallurgical treatments. J. Energy Storage 2021, 35, 102217. [Google Scholar] [CrossRef]
- Wang, F.; Sun, R.; Xu, J.; Chen, Z.; Kang, M. Recovery of cobalt from spent lithium ion batteries using sulphuric acid leaching followed by solid–liquid separation and solvent extraction. RSC Adv. 2016, 6, 85303–85311. [Google Scholar] [CrossRef]
- Dang, H.; Li, N.; Chang, Z.; Wang, B.; Zhan, Y.; Wu, X.; Liu, W.; Ali, S.; Li, H.; Guo, J.; et al. Lithium leaching via calcium chloride roasting from simulated pyrometallurgical slag of spent lithium ion battery. Sep. Purif. Technol. 2020, 233, 116025. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, L.; Xu, Z. Analysis of cobalt flows in mainland China: Exploring the potential opportunities for improving resource efficiency and supply security. J. Clean. Prod. 2020, 275, 122841. [Google Scholar] [CrossRef]
- Liu, G.; Wang, N.; Qi, F.; Lu, X.; Liang, Y.; Sun, Z. Novel Ni–Ge–P anodes for lithium-ion batteries with enhanced reversibility and reduced redox potential. Inorg. Chem. Front. 2023, 10, 699–711. [Google Scholar] [CrossRef]
- Roy, J.J.; Cao, B.; Madhavi, S. A review on the recycling of spent lithium-ion batteries (LIBs) by the bioleaching approach. Chemosphere 2021, 282, 130944. [Google Scholar] [CrossRef]
- Thompson, D.L.; Hartley, J.M.; Lambert, S.M.; Shiref, M.; Harper, G.D.; Kendrick, E.; Anderson, P.; Ryder, K.S.; Gaines, L.; Abbott, A.P. The importance of design in lithium ion battery recycling–a critical review. Green Chem. 2020, 22, 7585–7603. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef]
- King, S.; Boxall, N.J. Lithium battery recycling in Australia: Defining the status and identifying opportunities for the development of a new industry. J. Clean. Prod. 2019, 215, 1279–1287. [Google Scholar] [CrossRef]
- Bi, H.; Zhu, H.; Zu, L.; Bai, Y.; Gao, S.; Gao, Y. A new model of trajectory in eddy current separation for recovering spent lithium iron phosphate batteries. Waste Manag. 2019, 100, 1–9. [Google Scholar] [CrossRef]
- Bedeković, G.; Trbović, R. Electrostatic separation of aluminium from residue of electric cables recycling process. Waste Manag. 2020, 108, 21–27. [Google Scholar] [CrossRef]
- Rybarczyk, D.; Jędryczka, C.; Regulski, R.; Sędziak, D.; Netter, K.; Czarnecka-Komorowska, D.; Barczewski, M.; Barański, M. Assessment of the electrostatic separation effectiveness of plastic waste using a vision system. Sensors 2020, 20, 7201. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Yu, J.; Zheng, X. Experimental study on fine particle separation in a wet electrostatic classifier. Chem. Eng. Process. -Process Intensif. 2020, 156, 108108. [Google Scholar] [CrossRef]
- Martins, T.R.; Mrozinski, N.S.; Bertuol, D.A.; Tanabe, E.H. Recovery of copper and aluminium from coaxial cable wastes using comparative mechanical processes analysis. Environ. Technol. 2021, 42, 3205–3217. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Subasinghe, N.; Jeon, H.S. Separation of covering plastics from particulate copper in cable wastes by induction electrostatic separation. Mater. Trans. 2015, 56, 1140–1143. [Google Scholar] [CrossRef]
- Calin, L.; Catinean, A.; Bilici, M.; Samuila, A. A corona-electrostatic technology for zinc and brass recovery from the coarse fraction of the recycling process of spent alkaline and zinc–carbon batteries. J. Clean. Prod. 2021, 278, 123477. [Google Scholar] [CrossRef]
- Xu, P.; Dai, Q.; Gao, H.; Liu, H.; Zhang, M.; Li, M.; Chen, Y.; An, K.; Meng, Y.S.; Liu, P.; et al. Efficient direct recycling of lithium-ion battery cathodes by targeted healing. Joule 2020, 4, 2609–2626. [Google Scholar] [CrossRef]
- Li, J.; Ma, Z.F. Past and present of LiFePO4: From fundamental research to industrial applications. Chem 2019, 5, 3–6. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Z.; Hou, X.; Liu, L.; He, M.; He, X.; Huang, J.; Wen, Z. Fluorine doped carbon coating of LiFePO4 as a cathode material for lithium-ion batteries. Chem. Eng. J. 2020, 379, 122371. [Google Scholar] [CrossRef]
- Saxena, P.; Shukla, P. Comprehensive review on fundamental properties and applications of poly (vinylidene fluoride)(PVDF). Adv. Compos. Hybrid Mater. 2021, 4, 8–26. [Google Scholar] [CrossRef]
- Lu, H.; Li, J.; Guo, J.; Xu, Z. Movement behavior in electrostatic separation: Recycling of metal materials from waste printed circuit board. J. Mater. Process. Technol. 2008, 197, 101–108. [Google Scholar] [CrossRef]
- Chu, H.N.; Kim, S.U.; Rahimian, S.K.; Siegel, J.B.; Monroe, C.W. Parameterization of prismatic lithium–iron–phosphate cells through a streamlined thermal/electrochemical model. J. Power Sources 2020, 453, 227787. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, H.; Fan, T.; Liu, M.; Zhao, Y. DEM investigation of the conveyor belt sorting system for coated fuel particles with a large feeding rate. Powder Technol. 2022, 399, 117160. [Google Scholar] [CrossRef]







| Voltage of Static Electrode/kV | Difference Value of Leap Distance/m | Maximum Sorting Rate/% | |||
|---|---|---|---|---|---|
| Speed of Rotor (55 r/min) | Speed of Rotor (60 r/min) | Speed of Rotor (65 r/min) | Speed of Rotor (70 r/min) | ||
| 15 | 0.041 | 0.046 | 0.036 | 0.032 | 77.9 |
| 20 | 0.072 | 0.07 | 0.061 | 0.056 | 77.5 |
| 25 | 0.101 | 0.103 | 0.088 | 0.081 | 82.4 |
| 30 | 0.150 | 0.148 | 0.117 | 0.102 | 84.9 |
| Voltage of Static Electrode/kV | Difference Value of Leap Distance/m | Maximum Sorting Rate/% | |||
|---|---|---|---|---|---|
| Speed of Rotor (55 r/min) | Speed of Rotor (60 r/min) | Speed of Rotor (65 r/min) | Speed of Rotor (70 r/min) | ||
| 15 | 0.090 | 0.088 | 0.079 | 0.070 | 81.2 |
| 20 | 0.145 | 0.133 | 0.131 | 0.113 | 88.4 |
| 25 | 0.231 | 0.230 | 0.235 | 0.226 | 92.0 |
| 30 | 0.272 | 0.280 | 0.276 | 0.262 | 93.2 |
| Voltage of Static Electrode/kV | Difference Value of Leap Distance/m | Maximum Sorting Rate/% | |||
|---|---|---|---|---|---|
| Speed of Rotor (55 r/min) | Speed of Rotor (60 r/min) | Speed of Rotor (65 r/min) | Speed of Rotor (70 r/min) | ||
| 15 | 0.020 | 0.020 | 0.018 | 0.019 | 73.9 |
| 20 | 0.034 | 0.035 | 0.032 | 0.031 | 73.5 |
| 25 | 0.058 | 0.052 | 0.049 | 0.043 | 77.4 |
| 30 | 0.078 | 0.072 | 0.063 | 0.055 | 81.9 |
| Voltage of Static Electrode/kV | Difference Value of Leap Distance/m | Maximum Sorting Rate/% | |||
|---|---|---|---|---|---|
| Speed of Rotor (55 r/min) | Speed of Rotor (60 r/min) | Speed of Rotor (65 r/min) | Speed of Rotor (70 r/min) | ||
| 15 | 0.012 | 0.013 | 0.013 | 0.010 | 67.0 |
| 20 | 0.022 | 0.022 | 0.022 | 0.020 | 69.1 |
| 25 | 0.026 | 0.028 | 0.033 | 0.032 | 69.1 |
| 30 | 0.051 | 0.053 | 0.044 | 0.042 | 72.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Bai, Y.; Zu, L.; Bi, H.; Wen, J. Separation of Metal and Cathode Materials from Waste Lithium Iron Phosphate Battery by Electrostatic Process. Separations 2023, 10, 220. https://doi.org/10.3390/separations10030220
Zhu H, Bai Y, Zu L, Bi H, Wen J. Separation of Metal and Cathode Materials from Waste Lithium Iron Phosphate Battery by Electrostatic Process. Separations. 2023; 10(3):220. https://doi.org/10.3390/separations10030220
Chicago/Turabian StyleZhu, Huabing, Yuxuan Bai, Lei Zu, Haijun Bi, and Jian Wen. 2023. "Separation of Metal and Cathode Materials from Waste Lithium Iron Phosphate Battery by Electrostatic Process" Separations 10, no. 3: 220. https://doi.org/10.3390/separations10030220
APA StyleZhu, H., Bai, Y., Zu, L., Bi, H., & Wen, J. (2023). Separation of Metal and Cathode Materials from Waste Lithium Iron Phosphate Battery by Electrostatic Process. Separations, 10(3), 220. https://doi.org/10.3390/separations10030220






