Abstract
This study reports on the in vivo molluscicidal activity and Artemia salina lethality of Azorean Cryptomeria japonica leaf (CJL) essential oils (EOs) obtained by hydrodistillation (HD) and water-steam distillation (WSD) techniques, especially in light of the recent focus on the use of forestry and wood industry residues in a sustainable world economy. Molluscicidal activity was performed during several life stages of Radix peregra by the immersion method, under laboratory conditions. A first screening through a single-dose bioassay revealed that both EOs were highly active towards eggs, juveniles and adult snails (ca. 100% mortality). Concentration- and time-toxicity assays were carried out only on adult snails to determine the lethal parameters (LC50;90 and LT50;90). The LC50 values were 33 and 62 µg/mL for EO–WSD and EO–HD, respectively, after 48 h. The LT50 of EO–WSD required only 21 h for both 16 h and continous exposure periods, while that of EO–HD was slighty superior (21.8–25.6 h). Although not significant, EO–WSD was also slightly more toxic against A. salina than EO–HD (LC50 = 98 and 115 µg/mL after 24 h, respectively). In conclusion, Azorean CJL EOs, which are rich in α-pinene (mostly EO–WSD), have huge potential to be used as safe raw materials for the development of natural molluscicide products to control snails responsible for transmitting fascioliasis.
1. Introduction
Fascioliasis is a zoonosis propagated by parasitic trematodes from the genus Fasciola (Digenea: Fasciolidae), which affects a range of hosts, mainly livestock such as ruminants, horses, and pigs, and wild herbivores such as deer, rabbits, and hares [1]. This disease is not only recognized as a major veterinary issue, responsible for a decrease in animal productivity and economic loss, but also as a human disease with a high impact on public health [2]. The World Health Organization (WHO) includes fascioliasis in the list of the neglected tropical diseases and estimates that at least 2.4 million people are infected in more than 75 countries worldwide, with several million at risk of infection [3]. Infection can result in severe liver and lung diseases, and together these diseases are estimated to cause two million lives to be lost to disability and death worldwide every year. No continent is free from fascioliasis, and it is likely that where animal cases are reported, human cases also exist [3,4].
Freshwater snails belonging to the family Lymnaeidae play a crucial role in the development and transmission of fascioliasis. Among these snails, Radix (syn. Lymnaea) peregra (Müller), a European freshwater snail, is recognized as an intermediate host of trematodes, including Fasciola sp. Therefore, elimination of these hosts is important for preventing the transmission of fascioliasis [5].
Lymnaeid snails are capable of inhabiting a wide range of wet and aquatic environments, from lakes to ephemeral pools [4]. Treatments with synthetic molluscicides (e.g., niclosamide) are recommended measures stated by the WHO for the control of these intermediate hosts [3]. However, today many synthetic molluscicides are expensive and harmful to non-target species. Therefore, there is a need to develop alternative ecofriendly molluscicides that are less hazardous to aquatic systems and organisms.
Plants biosynthesize a wide diversity of secondary metabolites, such as essential oils (EOs), as a response to protect themselves against foreign agents such as pathogens. Plant EOs display a broad spectrum of biological activities against many pests, acting as contacts, fumigants, repellents, and/or feeding deterrents [6]. Moreover, various reports point out specific strategies that could help to optimize EOs and plant extracts application as part of integrated pest management programs (IPMPs) [7].
EOs are traditionally obtained by distillation processes, such as hydrodistillation (HD, water-steam distillation (WSD) and steam distillation (SD) methods. Another less-used process is the classic extraction with organic solvents, such as Soxhlet extraction, which is a non-eco-friendly method. Moreover, in order to improve conventional extraction methods, other innovative and green techniques have been developed, such as ultrasonic-assisted extraction (UAE), microwave-assisted extraction (MAE), and supercritical fluid extraction (SFE) [8,9,10].
Cryptomeria japonica (Thunb. ex L.f.) D. Don (Cupressaceae) is one of the main plantation conifer tree species in Far Eastern countries (Japan, Korea, Taiwan, India, and China), as well as in the Azores Archipelago (Portugal). Due to the high demand for C. japonica wood products, a huge number of residues (i.e., leaves, bark, twigs, and sawdust) is generated. Nonetheless, it should be noted that these large unused biomass residues are still a raw material to obtain value-added products, such as EOs and plant extracts that are rich sources of compounds with multi-bioactivities. In fact, C. japonica EOs/extracts exhibit several bioactivities, including antibacterial, antifungal, larvicidal, acaricidal, termiticidal, molluscicidal, and insect repellency activities [11,12] with potential application in IPMPs.
Furthermore, as already documented by our research group, Azorean C. japonica leaf (CJL) EO is rich in α-pinene monoterpene [13]. Extensive use of this component in cosmetics, and medicine, especially for its antioxidant/antibacterial, and anti-cancer properties, and additionally as a biocidal agent, has made it a versatile product [14].
In our continuous search for C. japonica residues valorization, we previously reported the yield and chemical profile of Azorean CJL EOs obtained by two different distillation methods (HD and WSD) [13], and herein we aimed to investigate, for the first time, the molluscicidal properties of these EOs against R. peregra under laboratory conditions, as well as the lethality against brine shrimp in order to estimate their toxic effects on the aquatic environment.
2. Materials and Methods
2.1. Chemicals
Ethanol (purity of 96%) and copper sulphate (CuSO4, purity > 99%) were both purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Plant Material
The fresh aerial parts of red heartwood-type C. japonica, provided by Marques, S.A. (the largest local producer of EOs), were collected in November 2016 (autumn season) in a Cryptomeria forest (ca. 30 to 40 years old, average height of 20 m and andosol-type soil) on the island of São Miguel (Azores Archipelago, Portugal). The aerial parts were randomly picked from healthy plants in two locations in the northeast region of São Miguel: Achadinha (altitude 733 m, latitude N 37°48′28.59″, longitude W 25°16′16.14″, code sample “Lote B”), and Algarvia (altitude 663 m, latitude N 37°49′00.45″, longitude W 25°13′39.22″, code sample “Lote 3 talhão 3”). The plant material was placed in plastic bags and brought to the laboratory at the University of the Azores, where its leaves were separated immediately prior to the distillation process. The moisture content of the fresh samples was 55.1%. A voucher specimen (UA-DCQFE 183) was deposited in the Department of Physics, Chemistry and Engineering, University of the Azores.
2.3. Extraction and Chemical Analyses of EOs
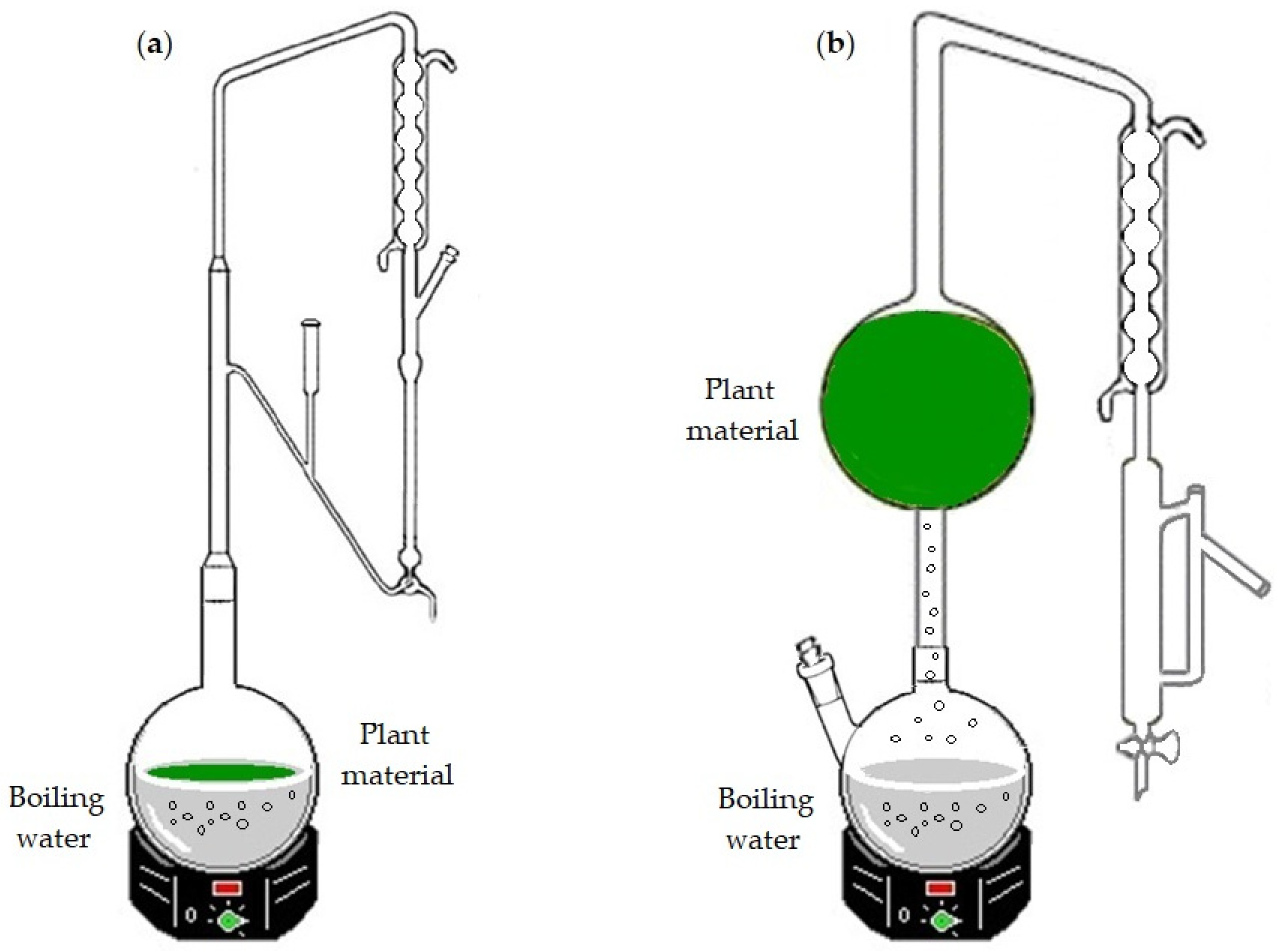
To obtain the EOs, fresh leaves of Azorean C. japonica sample were subjected to HD, using a Clevenger-type apparatus (Figure 1a), and to WSD (Figure 1b) processes, as detailed in Arruda et al. [13].
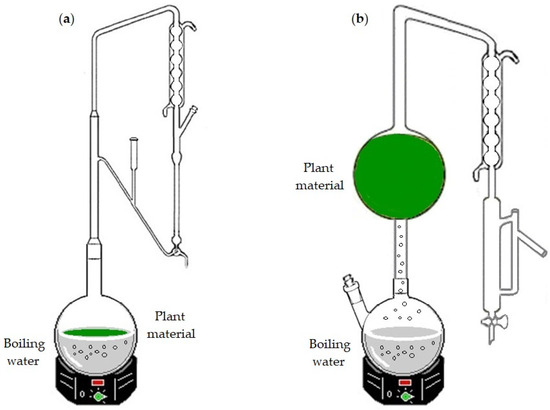
Figure 1.
Apparatus for (a) hydrodistillation (HD) and (b) water-steam distillation (WSD) processes. In the HD, the plant material is submerged in the boiling water, while in the WSD, it is packed above the steam source (boiling water).
Gas chromatography (GC) and GC–mass spectrometry (GC–MS) analysis of the EOs was performed using a DB-1 fused-silica column (30 m × 0.25 mm i.d. and 0.25 μm film thickness) from J & W Scientific Inc. (Rancho Cordova, CA, USA), as reported in Arruda et al. [13].
2.4. Preparation of EOs Emulsions
EOs stock solutions were prepared by mixing EOs with ethanol (300 mg/mL). Afterwards, 10 µL of EO stock solution was emulsified into spring water using an ultrasonic homogenizer (Sonopuls HD 2200, Bandelin, Berlin, Germany ) to obtain the working emulsions (30 mg EO per mL). Subsequently, according to the doses to be tested, different volumes were pipetted, and thoroughly mixed with spring water making up the final volumes required for the bioassays (10 mL for juveniles and eggs and 220 mL for adults). The emulsions were allowed to settle for 15 min before use.
2.5. Molluscicidal Activity
2.5.1. R. peregra Collection and Maintenance
R. peregra adult snails (1.00 ± 0.20 cm in length) were captured from water reservoirs (used for cattle breeding) in the localities of Lomba da Maia and Fenais da Ajuda (northeast of the island of São Miguel, Azores Archipelago, Portugal). Snails were kept in a plastic aquarium containing approximately 3 L of spring water obtained from the same collection site. The water was aerated using an air pump, and the snails were fed on a daily basis with lettuce leaves. Snails were acclimatized to laboratory conditions for a minimum of 72 h, with a natural photoperiod and a constant temperature of 23 ± 1 °C. The aquarium was cleaned three times a week, when excrement and dead snails were removed, and the water was replaced. Prior to the ovicidal assay, freshly laid eggs (0–48 h old) were examined under a stereoscopic microscope to verify their viability and confirm their stage of embryonic development (four to eight cells). Additionally, several egg masses were kept in a separate container with continuous aeration until hatching. Molluscicidal assays were conducted on juvenile snails aged between 48 and 72 h.
2.5.2. Ovicidal Activity
Egg masses of R. peregra that were less than 48 h old were subjected to a single-dose treatment of EOs at a concentration of 30 µg/mL in spring water to determine the ovicidal activity. Masses containing approximately 30 (±10.0) viable snail embryos were placed in 6-well plates containing freshly prepared emulsions (10 mL). Two negative control groups were also included and conducted simultaneously: one with spring water and the other with an ethanol aqueous solution that had an ethanol concentration equivalent to that of the tested EOs emulsions (0.1 µL/mL). Additionally, CuSO4 was used as a positive control at a concentration of 30 µg/mL. Each treatment was repeated at least three times. The egg masses were incubated at a controlled temperature of 23 ± 1 °C and exposed to a natural photoperiod. The eggs were monitored daily to observe their development, and the ovicidal activity of the treatments was evaluated by calculating the percentage of hatching (snails emerged from the capsule) 25 days later (by which time hatching in the control groups was ≥95%). Embryos were considered dead if their cells were dispersed or if they remained unhatched at the end of the experiment.
2.5.3. Single-Dose Screening Assay against Juveniles and Adults
Juvenile and adult snails were subjected to a single-dose evaluation of the molluscicidal activity of EOs, in accordance with the immersion method recommended by the WHO [15] and Teixeira et al. [16]. In each well of a 6-well plate, groups of ten juveniles were randomly assigned and 10 mL of freshly prepared emulsions (30 µg/mL) were cautiously added. Regarding the adults, groups of ten snails were randomly transferred into disposable cups containing 220 mL of freshly prepared EOs emulsions (at 100 µg/mL). To prevent adults from escaping, cups were covered with a Petri dish. Again, CuSO4 was included in both assays as a positive control at a concentration of 30 µg/mL. Two negative control groups were also included and conducted simultaneously: one with spring water and the other with ethanol aqueous solution (0.02 µL/mL for juveniles, and 0.1 µL/mL for adults). Each assay included three to four replicates of ten snails per EO treatment and controls. After 24 h of exposure, the mortality of the snails was assessed. The surviving snails were rinsed with spring water to remove emulsion residues and then transferred to new cups containing the same volume of spring water and fresh lettuce leaves for feeding. After a recovery period of 24 h, the mortality of juveniles and adults was re-evaluated using a stereo microscope. Juveniles were considered dead if they had no heartbeat, while adults were deemed to be dead if their tissues had deteriorated (visible foot discoloration and body fluid leakage) or if they failed to react to prodding (typical withdrawal movements). The experiments were conducted at a temperature of 23 ± 1 °C under natural photoperiod.
2.5.4. Dose-Response Assay on Adults (LCs)
The EOs were tested in the range of 20 to 80 µg/mL, following the aforementioned method. For the positive control (CuSO4), the concentrations used ranged from 0.1 to 0.75 µg/mL. To establish a relationship between dose and response, three to four replicates of ten snails per concentration were utilized, with at least four concentrations per EO. The mortality percentage was recorded at 24 h of exposure and after the 24-h recovery period to determine the lethal concentration of 50 and 90 (LC50 and LC90).
2.5.5. Time-Response Assay on Adults (LTs)
To evaluate the time-dependent lethality, the previously described methodology was followed. The purpose of this test was to determine whether the duration of exposure affects the effectiveness of treatments and to what extent. The concentration of 100 µg/mL was selected because it was closest to most of the estimated LC90 values determined after the 24-h recovery period. Similar to the above criteria, the positive control (CuSO4) was evaluated at 0.75 µg/mL. The lethality dependent on time was assessed by both continuous and short-term exposure periods of 8 and 16 h. After being exposed to the treatments for the designated time, adult snails were rinsed with spring water and left to recover in clean beakers filled with fresh water and lettuce leaves. Mortality was then monitored every 2 h. Snails that were continuously treated were also monitored every 2 h until the mortality percentage reached 90%. In each experiment, three replicates of ten individuals (n = 30) were used per treatment. In all three experiments, time was counted from the beginning of the exposure.
2.6. Ecotoxicology Assay on Brine Shrimp
The brine shrimp (Artemia salina Leach) lethality assay was performed to estimate the toxic effects of CJL EOs on the aquatic environment. Indeed, brine shrimp, a saltwater crustacean species, are frequently used as a bioindicator of ecotoxicological assays, such as for preliminary evaluation of bioactive metabolites [17,18]. A. salina mix (JBL GmbH & Co. KG, Neuhofen, Germany) was acquired in a local pet shop, introduced into a glass container filled with distilled water and incubated at 25 °C under constant aeration for 48 h to induce hatching. Active nauplii were collected, and the second instar shrimp larvae were used in the bioassays. EOs toxicity against brine shrimp was determined in 96-well microtiter plates, according to the methodology of Solis et al. [18], with slight modifications. A range of four or five optimized concentrations was obtained by adding stock solutions (1 mg/mL; EOs previously dissolved in ethanol and then in water) to wells containing filtered artificial seawater. A 100 µL suspension of nauplii containing 10–15 organisms was added to each well, and the covered microtiter plate was incubated at 25 °C. After 24 h, nauplii were examined under a stereomicroscope (×12.5) and the mortality was recorded (non-motile nauplii were considered dead). Acetone (100 µL) was added to kill any remaining living nauplii and determine the total numbers of nauplii. Two groups of negative controls were included: artificial seawater and ethanol (<0.1%). In all experiments, four or five replicates were used.
2.7. Statistical Analysis
Data obtained in the single-dose assays (hatching and mortality percentages) were transformed by the arcsine function prior to the one-way analysis of variance (one-way ANOVA). Means of treatments were separated at a 5% significance level by an LSD test, available on an IBM SPSS statistic package. Mortality percentages recorded in dose- and time-dependent experiments were corrected with the Abbott’s formula, whenever mortality in the negative control exceeded 5%. LC50 and LC90 after 48 h (24 h of exposure plus 24 h of recovery) were determined by probit analysis, using the IBM SPSS statistic package 28.0.1.0. The same linear model was applied to time-response data, to determine EOs LT50 and LT90 (times required to cause 50% and 90% of adults’ mortality) at the dose of 100 µg/mL. Treatments’ bioactivities were considered significantly different when the values from LC or LT failed to overlap the 95% of confidence limits (CL).
3. Results
3.1. Ecotoxicity Activity
Ecotoxicity assays are important tools that allow the establishment of safe environmental parameters regarding the use of biocidal products. In this context, a preliminary ecotoxicity evaluation of Azorean CJL EOs was made against the bioindicator A. salina and their LCs values (obtained after 24 h exposure) are reported in Table 1. Both EOs were toxic against A. salina, although there was not a significant difference between them, regarding their LC50 values (p > 0.05).

Table 1.
Estimated values of lethal concentration of 50 and 90 (LC50 and LC90) of essential oils (EOs) of Azorean Cryptomeria japonica leaf obtained by two distillation techniques against Artemia salina nauplii after 24 h of exposure.
3.2. Molluscicidal Activity
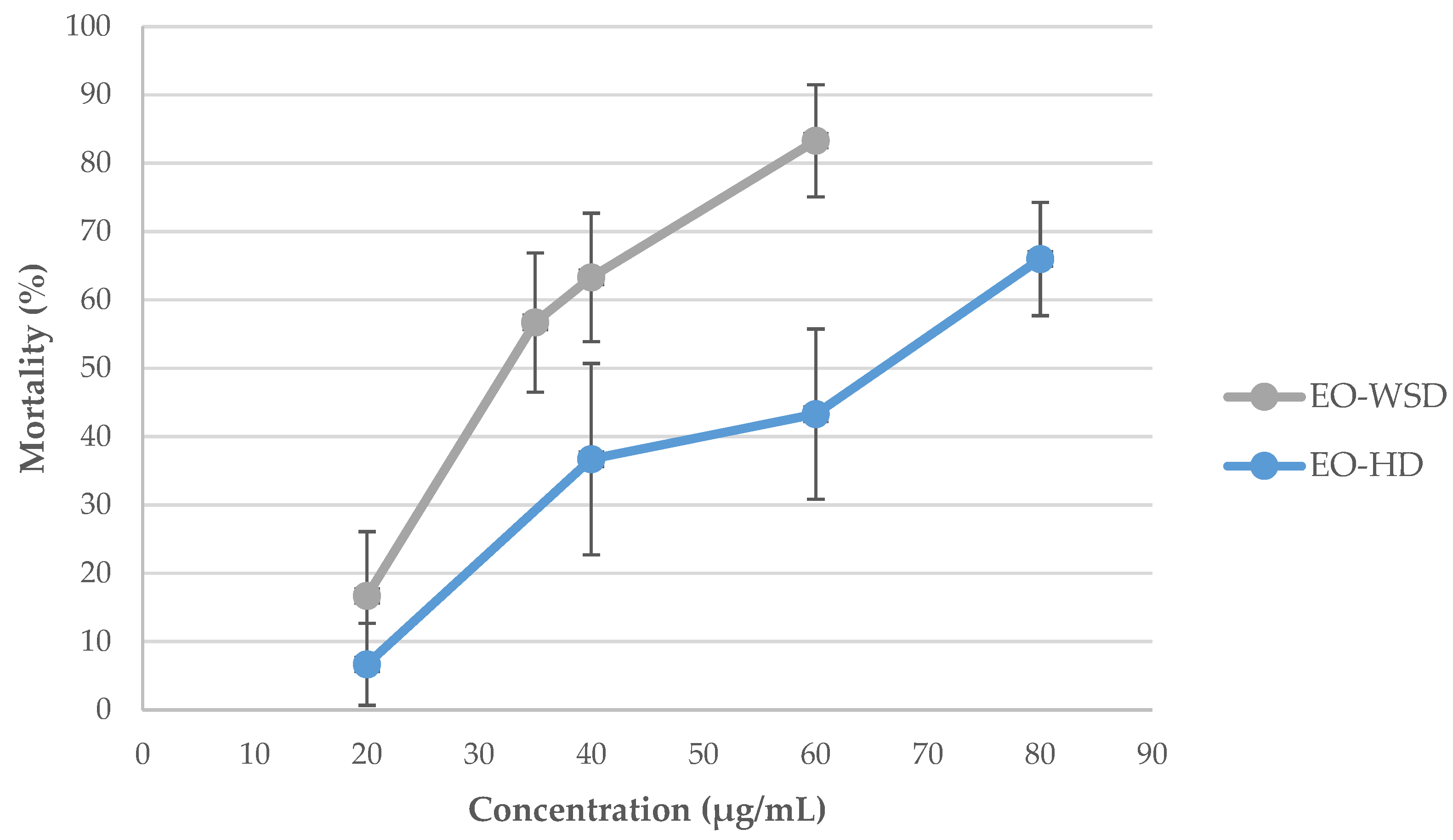
A single-dose screening was performed at the concentration of 100 µg/mL against adult snails. The mortality rate was recorded at 24 h of exposure followed by a 24-h recovery period. Again, both EOs were highly active with mortality rates higher than 97% (96.7 ± 4.7% for EO–HD vs. 100 ± 0.0% for EO–WSD, p > 0.05). In addition, both EOs were highly effective towards juveniles, presenting 100% mortality at 30 µg/mL. This information allowed for the establishment of a range of concentrations to determine the LC values against adult snails. The LC values for juveniles were not evaluated once they were more susceptible than older snails, as expected. As shown in Figure 2, the toxicity of these EOs (EO–HD and EO–WSD) was dose-dependent against R. peregra.
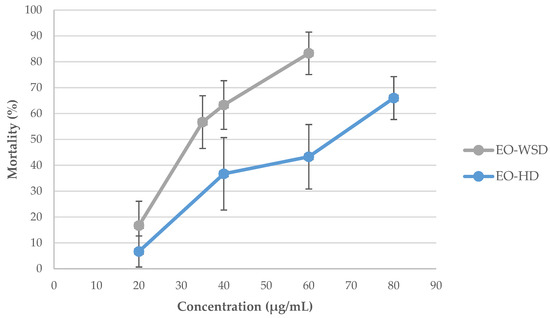
Figure 2.
Radix peregra adult mortality rate (%) at different concentrations of Azorean Cryptomeria japonica leaf essential oils (OEs) obtained by two distillation techniques, after 24 h of exposure followed by a 24-h recovery period. Legend: HD, hydrodistillation; WSD, water-steam distillation.
The LCs of Azorean CJL EOs against R. peregra adult snails are shown in Table 2. The χ2 values obtained in LC50 were not significant (p > 0.05) for any EOs in the study, indicating that data fit the assumptions of the Probit model. The EO–WSD was the most effective in molluscicidal activity with an LC50 of 33.3 µg/mL and a slope of 4.02 when compared to the EO–HD (LC50 of 61.8 µg/mL and a slope of 2.75). The same statistical pattern was observed regarding the LC90 values (Table 2). Nevertheless, both CJL EOs were highly toxic against adult snails (LC50 < 62 µg/mL) as can be observed in Figure 3a–c.

Table 2.
Estimated values of lethal concentration of 50 and 90 (LC50 and LC90) of the essential oils (EOs) of Azorean Cryptomeria japonica leaf obtained by two distillation techniques against adults of Radix peregra, after 24 h of exposure followed by a 24-h recovery period.
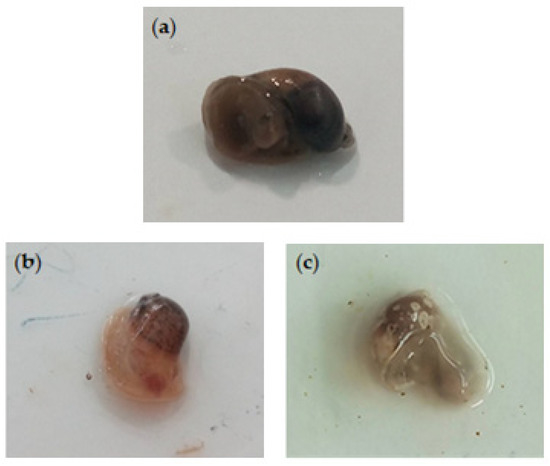
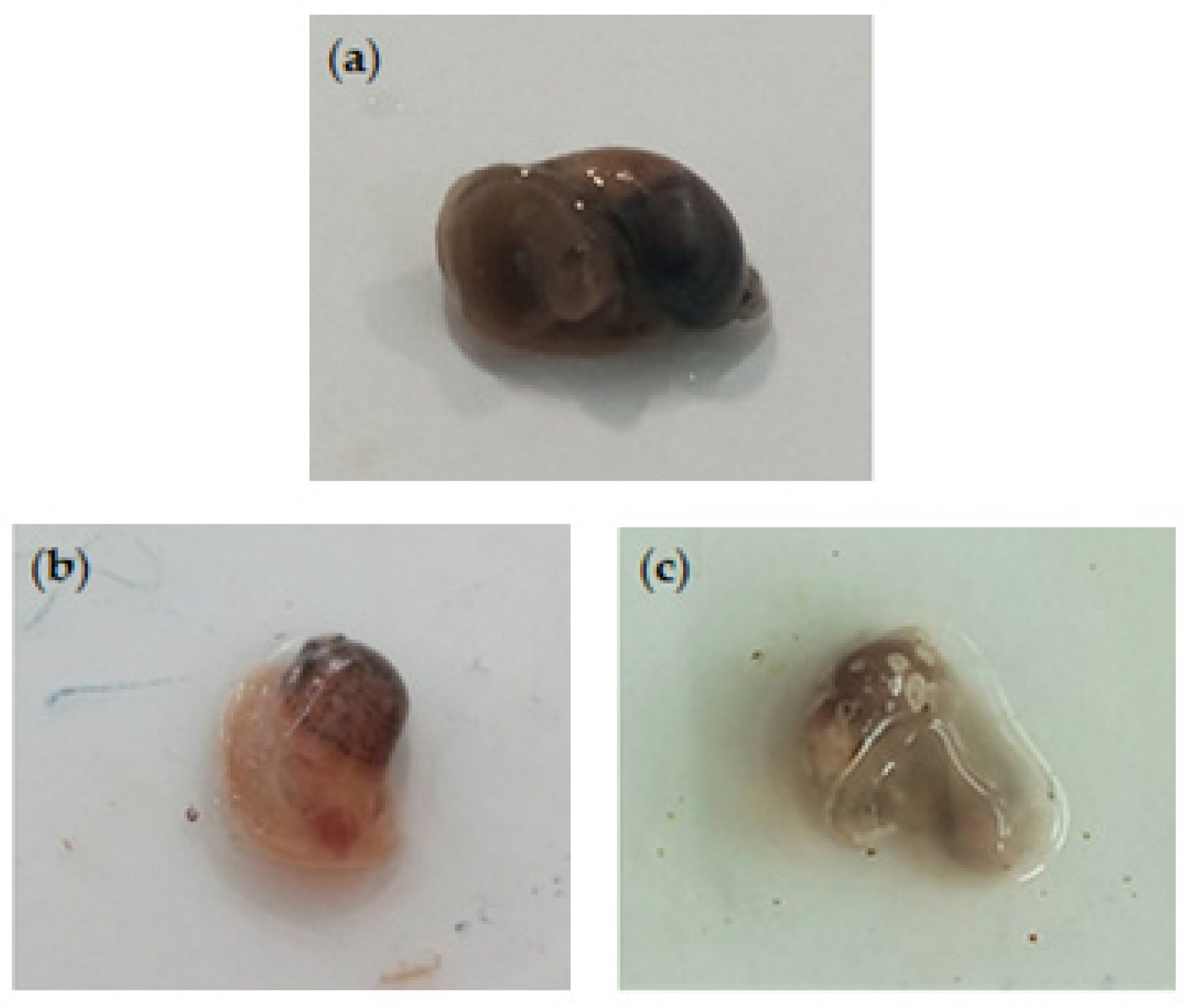
Figure 3.
Radix peregra adults in immersion test. Control snails (untreated) (a) vs. snails treated with Azorean Cryptomeria japonica leaf essential oils exhibited swelling of cephalopedal mass (b) and hemolymph leakage and tissue discoloration (c).
Table 3 presents the LTs of Azorean CJL EOs against R. peregra adult snails. The estimated LT50 for both EOs ranged from 20.7 to 31.8 h in all exposure periods analysed. It was not possible to determine the LT50 and LT90 for EO–HD at 8 h because the mortality rate was lower than 25% even after 47 h. Nevertheless, EO–WSD always showed the lowest LT50 and LT90 values at all exposure periods, being statistically different from EO–HD.

Table 3.
Estimated lethal time of 50 and 90 (LT50 and LT90) of the essential oils (EOs) of Azorean Cryptomeria japonica leaf obtained by two distillation techniques against adults of Radix peregra, assessed for different exposure periods at 100 µg/mL.
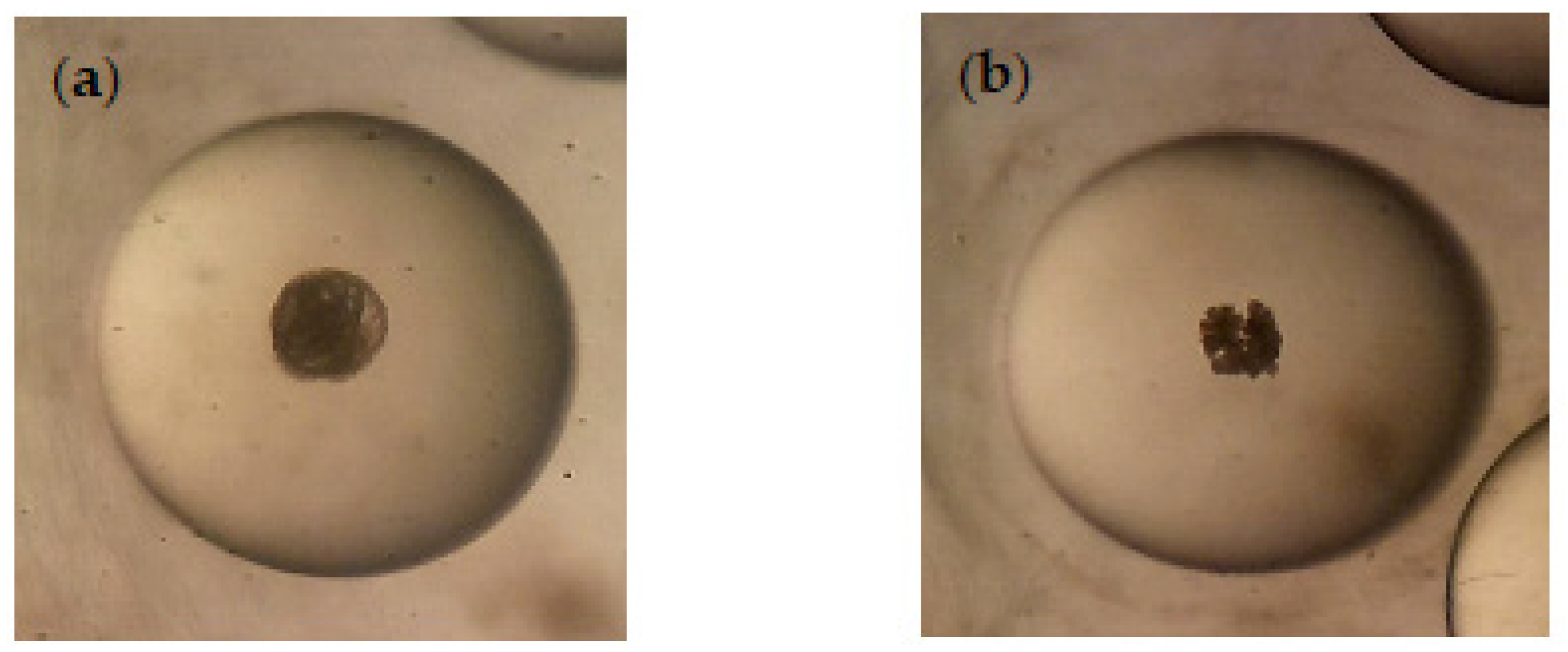
Regarding the ovicidal activity, both Azorean CJL EOs and CuSO4 were highly active in inhibiting the eggs hatching at 30 µg/mL, presenting 0% of hatching rate after 25 days, while control groups (untreated and ethanol) achieved 97 ± 2.2% of hatching rate (p < 0.05). A healthy embryo (untreated) and an embryo treated with the CJL EOs, which resulted in a disintegrated structure, are shown in Figure 4a and Figure 4b, respectively.
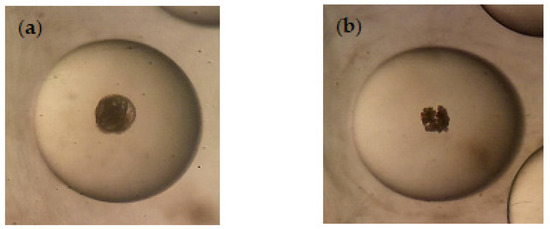
Figure 4.
Radix peregra embryo in immersion test. (a) Healthy embryo of R. peregra (untreated); (b) Disintegrated embryo of R. peregra treated with Azorean Cryptomeria japonica leaf essential oils.
3.3. Chemical Composition of EOs
Concerning the chemical characterization of EO–HD and EO–WSD from Azorean CJL, both EOs belong to α-pinene chemotype, as reported in Arruda et al. [13]. Nonetheless, some differences, mainly at the quantitative level, were found between the two EOs, as illustrated in Table 4.

Table 4.
Chemical composition (%) of Azorean Cryptomeria japonica leaf essential oils (EOs) obtained by hydrodistillation (HD) and by water-steam distillation (WSD) (adapted from Arruda et al. [13]).
4. Discussion
The molluscicidal activity of C. japonica EOs against R. peregra was demonstrated for the first time in the present study. In fact, we found that Azorean CJL EOs significantly affected egg hatching, juveniles and adults of R. peregra.
Regarding snail embryogenesis, Azorean CJL EOs display a highly biocidal activity (0% hatching rate) at 30 µg/mL, resulting in a disintegrated structure of the embryo (see Figure 4b). Sousa et al. [19] also demonstrated this effect for other EOs, namely from Petroselium crispum and Cuminum cyminum, but at 50 µg/mL. It is interesting to note that in this latter study, α-pinene (the major component of Azorean CJL EOs) and β-pinene also exhibited hatching values < 20%, which could help to explain why Azorean CJL EOs were very effective.
Regarding biocidal effects on young snails, Azorean CJL EOs revealed themselves again to be highly active (100% mortality rate after 24 h of exposure) at 30 µg/mL, showing no statistical difference between them. In fact, young snails were more susceptible than older ones that presented a 100% and 97% mortality rate after 48 h of exposure (at 100 µg/mL) for EO–WSD and EO–HD, respectively.
Both Azorean CJL EOs (EO–HD and EO–WSD) were very active against adult snails in a dose-dependent manner, as demonstrated by their low LC50 values. Nevertheless, the molluscicidal activity of EO–WSD was ca. twice that of the EO–HD against R. peregra adults. This difference in the lethality bioassay could be attributed to the quantitative variation in the components of the two EOs. Similar results are reported in Lahlou’s work [20] on Cedrus atlantica EO and its major component (α-pinene) against Bulinus truncates, also a freshwater snail. In comparison with Petroselium crispum EO efficacy (LC50 = 13.7–18.5 µg/mL), C. japonica EO–WSD exhibited lower molluscicidal activity against R. peregra (LC50 = 33.3 µg/mL), but similar activity with Cuminum cyminum EO (LC50 = 38.8 µg/mL) [19]. The LC values found for EO–WSD are in accordance with the molluscicide concentration value recommended by the WHO, which establishes that a plant extract with molluscicide properties will only have potential for use in the field if it promotes a mortality of 90% of snails at concentrations less than or equal to 100 µg/mL, within 24 h of exposure [15]. However, it is noteworthy that EO–WSD showed a lower yield than EO–HD [13], regardless of superior α-pinene content (ca. three times that of the EO–HD) and efficacy against snails. These results agree with the review by Pereira et al. [21], which stated that the molluscicidal effect of EOs extracted from the same plant species may vary according to their chemical composition.
The molluscicidal activity of both Azorean CJL EOs was also time-dependent; however, EO–WSD showed faster (requiring fewer hours) irreversible damage in adult snails than EO–HD, as can be observed from the lower LT50 values. Indeed, an 8 h period of exposure to EO–HD (at 100 µg/mL) was not enough to cause acute toxicity and high mortality percentages in adult snails. Time-dependent studies were previously performed on R. peregra with EOs from plant species of the Macaronesia flora found in the Azores [16] and from plants species from the Apiaceae family [19]. Contrariwise to those plants/herbs, C. japonica EOs seem to be more profitable, once they are abundant renewable resources from agroforestry biomass residues. Moreover, C. japonica unused biomass could be converted into EOs, in an economically and environmentally sustainable manner with zero competition for land areas.
In this work, adult snails under Azorean CJL EOs treatment exhibited swelling of the cephalopedal mass with haemolymph leakage, protrusion of the snail body, discoloration of the tissues and disintegration of the foot epithelium as already reported in the study by Sousa et al. [19]. However, the exact mechanism by which these EOs cause the death of snails is not known. In the study by Sousa et al. [19], α-pinene, β-pinene and γ-terpinene exhibited a ca. 50–90% mortality rate against adult snails at 50 µg/mL. Additionally, Ribeiro et al. [22] evaluated the toxic action of α-pinene and other monoterpenes on Biomphalaria glabrata, as well as the enzymatic activity of acetylcholinesterase (AChE), extracted from these snails. They found that this monoterpene is associated with a dose-dependent increase in snails’ mortality (LC50 = 7 µg/mL). In addition, α-pinene was a potent inhibitor of AChE from freshwater snails, with an IC50 value of 0.04 mg/mL [22] and it did not reveal toxicity to a non-target fish (Danio rerio) at the LC50 of the molluscicide (B. glabrata). Moreover, α-pinene showed itself to be a feeding deterrent of land snail pests [23]. According to Pereira et al. [21], the most toxic EOs for vector molluscs should include a high content of the monoterpene hydrocarbons fraction, such as α-phellandrene, p-cymene, α-pinene, β-pinene, limonene and β-myrcene in their composition. Therefore, Azorean CJL EOs, whose major fraction is monoterpene hydrocarbons, exhibited highly molluscicidal activity.
Further studies, which are ongoing, should isolate this target-monoterpene fraction from Azorean C. japonica EOs. Nonetheless, we cannot rule out the possibility that other minor compounds comprised in the remaining part of the C. japonica EOs could play a role in the dose and time effectiveness of the molluscicidal activity observed.
In summary, results suggest that C. japonica biomass residues from forest operations and wood industry could be converted into molluscicides that impair the life cycle of Fasciola sp., by reducing the number of intermediate hosts.
According to the results of toxicity to the bioindicator organism A. salina, the LC50 of both EOs was ca. two to three times higher than the LC50 determined for adults of R. peregra. In this sense, both Azorean CJL EOs can be used to control the spread of fascioliasis without significant risks to the environment at a molluscicidal dose. Furthermore, EOs, due to their volatile nature, are easily biodegradable and therefore non-persistent on the environment. Moreover, the LC50 values found in this study were significantly higher than those reported earlier by Cheng et al. [24] for CJL EO–HD from Taiwan (114.8 µg/mL vs. 31.8 µg/mL). However, CJL EO in this latter study belongs to another chemotype (elemol plus kaur-16-ene) [25].
5. Conclusions
The molluscicidal activity of the EOs from leaves of Azorean C. japonica (a conifer tree introduced in the Azores Archipelago, Portugal, in the mid-19th century) against the freshwater snail R. peregra, an intermediate host of the F. hepatica trematode, was first established in the present work. Among the studied CJL OEs (EO–HD and EO–WSD), the LC values of the EO–WSD sample are in accordance with WHO recommendations. In addition, both EOs have no significant impact on the aquatic environment at a molluscicidal dose, as demonstrated through bioassays with A. salina used in this study as a bioindicator. Therefore, these EOs are promising ecofriendly botanical molluscicides, because natural pesticides are preferred over synthetics due to their multi-component process affecting more than one system. Moreover, these EOs constitute inexpensive sources of natural biocides, once they can be obtained from abundant C. japonica biomass residues from forest operations and the timber industry. Thus, further studies should be carried out in semi-field conditions with ecotoxicological studies against non-target organisms.
Author Contributions
Conceptualization, F.A., A.L. and E.L.; methodology, F.A. and J.S.R.; software, F.A, A.L., L.O. and J.S.R.; writing, F.A., A.L., T.R., A.J. and E.L.; supervision, J.S.R. and E.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Portuguese national funds FCT under the project UIDP/05292/2020 and UIDB/05292/2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to Marques, S.A. from São Miguel, Azores, who provided the Cryptomeria japonica samples.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Beesley, N.J.; Caminade, C.; Charlier, J.; Flynn, R.J.; Hodgkinson, J.E.; Martinez-Moreno, A.; Martinez-Valladares, M.; Perez, J.; Rinaldi, L.; Williams, D.J.L. Fasciola and fasciolosis in ruminants in Europe: Identifying research needs. Transbound. Emerg. Dis. 2018, 65, 199–216. [Google Scholar] [CrossRef] [PubMed]
- Mas-Coma, S.; Bargues, M.; Valero, M. Human fascioliasis infection sources, their diversity, incidence factors, analytical methods and prevention measures. Parasitology 2018, 145, 1665–1699. [Google Scholar] [CrossRef]
- WHO. Neglected Tropical Diseases: Fascioliasis. Available online: https://www.who.int/news-room/questions-and-answers/item/q-a-on-fascioliasis (accessed on 19 January 2023).
- Correa, A.C.; Escobar, J.S.; Durand, P.; Renaud, F.; David, P.; Jarne, P.; Pointier, J.-P.; Hurtrez-Bousse`s, S. Bridging gaps in the molecular phylogeny of the Lymnaeidae (Gastropoda: Pulmonata), vectors of Fascioliasis. BMC Evol. Biol. 2010, 10, 381. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Yadav, R.P.; Singh, A. Molluscicides from some common medicinal plants of eastern Uttar Pradesh, India. J. Appl. Toxicol. 2010, 30, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sergeeva, V. Use of plant extracts and essential oils in modern plant protection. Acta. Hortic. 2016, 1125, 361–368. [Google Scholar] [CrossRef]
- de Oliveira, J.L. Nano-biopesticides: Present concepts and future perspectives in integrated pest management. In Advances in Nano-Fertilizers and Nano-Pesticides in Agriculture; Jogaiah, S., Singh, H.B., Fraceto, L.F., de Lima, R., Eds.; Woodhead Publishing: Cambridge, MA, USA, 2021; pp. 1–27. [Google Scholar] [CrossRef]
- Jin, Y.; Han, D.; Tian, M.; Row, K.H. Supercritical CO2 extraction of essential oils from Chamaecyparis obtusa. Nat. Prod. Commun. 2010, 5, 461–464. [Google Scholar] [CrossRef]
- Kusuma, H.S.; Altway, A.; Mahfud, M. Solvent-free microwave extraction of essential oil from dried patchouli (Pogostemon cablin Benth) leaves. J. Ind. Eng. Chem. 2018, 58, 343–348. [Google Scholar] [CrossRef]
- Febriana, I.D.; Gala, S.; Mahfud, M. Ultrasound assisted extraction of natural dye from jackfruit’s wood (Artocarpus heterophyllus): The effect of ethanol concentration as a solvent. AIP Conf. Proc. 2017, 1840, 070004. [Google Scholar] [CrossRef]
- Lima, A.; Arruda, F.; Medeiros, J.; Baptista, J.; Madruga, J.; Lima, E. Variations in essential oil chemical composition and biological activities of Cryptomeria japonica (Thunb. ex L.f.) D. Don from different geographical origins–A critical review. Appl. Sci. 2021, 11, 11097. [Google Scholar] [CrossRef]
- Lima, A.; Arruda, F.; Janeiro, A.; Medeiros, J.; Baptista, J.; Madruga, J.; Lima, E. Biological activities of organic extracts and specialized metabolites from different parts of Cryptomeria japonica (Cupressaceae)–A critical review. Phytochemistry 2023, 206, 113520. [Google Scholar] [CrossRef]
- Arruda, F.; Rosa, J.S.; Rodrigues, A.; Oliveira, L.; Lima, A.; Barroso, J.G.; Lima, E. Essential oil variability of Azorean Cryptomeria japonica leaves under different distillation methods, Part 1: Color, yield and chemical composition analysis. Appl. Sci. 2022, 12, 452. [Google Scholar] [CrossRef]
- Karimkhani, M.M.; Nasrollahzadeh, M.; Maham, M.; Jamshidi, A.; Kharazmi, M.S.; Dehnad, D.; Jafari, S.M. Extraction and purification of α-pinene; a comprehensive review. Crit. Rev. Food Sci. Nutr. 2022, 1–26. [Google Scholar] [CrossRef]
- WHO. Report of the Scientific Working Group on Plant Molluscicide and Guidelines for Evaluation of Plant Molluscicides; World Health Organization: Geneva, Switzerland, 1983; p. 9. [Google Scholar]
- Teixeira, T.; Rosa, J.S.; Rainha, N.; Baptista, J.; Rodrigues, A. Assessment of molluscicidal activity of essential oils from five Azorean plants against Radix peregra (Müller, 1774). Chemosphere 2012, 87, 1–6. [Google Scholar] [CrossRef]
- McLaughlin, J.L.; Rogers, L.L.; Anderson, J.E. The use of biological assays to evaluate botanicals. Drug Inf. J. 1998, 32, 513–524. [Google Scholar] [CrossRef]
- Solis, P.N.; Wright, C.W.; Anderson, M.M.; Gupta, M.P.; Phillipson, J.D. A microwell cytotoxicity assay using Artemia salina (brine shrimp). Planta Med. 1993, 59, 250–252. [Google Scholar] [CrossRef]
- Sousa, R.M.O.F.; Rosa, J.S.; Cunha, A.C.; Fernandes-Ferreira, M. Molluscicidal activity of four Apiaceae essential oils against the freshwater snail Radix peregra. J. Pest Sci. 2017, 90, 971–984. [Google Scholar] [CrossRef]
- Lahlou, M. Composition and molluscicidal properties of essential oils of five Moroccan Pinaceae. Pharm. Biol. 2003, 41, 207–210. [Google Scholar] [CrossRef]
- Pereira, L.P.L.A.; Ribeiro, E.C.G.; Brito, M.C.A.; Silveira, D.P.B.; Araruna, F.O.S.; Araruna, F.B.; Leite, J.A.C.; Dias, A.A.S.; Firmo, W.D.C.A.; Borges, M.O.D.R.; et al. Essential oils as molluscicidal agents against schistosomiasis transmitting snails—A review. Acta. Trop. 2020, 209, 105489. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, E.C.G.; Leite, J.A.C.; Luz, T.R.S.A.; Silveira, D.P.B.; Bezerra, S.A.; Frazão, G.C.C.G.; Pereira, L.P.L.A.; dos Santos, E.G.G.; Filho, P.R.C.F.R.; Soares, A.M.S.; et al. Molluscicidal activity of monoterpenes and their effects on inhibition of acetylcholinesterase activity on Biomphalaria glabrata, an intermediate host of Schistosoma mansoni. Acta. Trop. 2021, 223, 106089. [Google Scholar] [CrossRef]
- Abobakr, Y.; Al-Sarar, A.S.; Abdel-Kader, M.S. Fumigant toxicity and feeding deterrent activity of essential oils from Lavandula dentata, Juniperus procera, and Mentha longifolia against the land snail Monacha obstructa. Agriculture 2022, 12, 934. [Google Scholar] [CrossRef]
- Cheng, S.S.; Chang, H.T.; Chang, S.T.; Tsai, K.H.; Chen, W.J. Bioactivity of selected plant essential oils against the yellow fever mosquito Aedes aegypti larvae. Bioresour. Technol. 2003, 89, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.S.; Chua, M.T.; Chang, E.H.; Huang, C.G.; Chen, W.J.; Chang, S.T. Variations in insecticidal activity and chemical compositions of leaf essential oils from Cryptomeria japonica at different ages. Bioresour. Technol. 2009, 100, 465–470. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).