A Review of Liquid Chromatography-Mass Spectrometry Strategies for the Analyses of Metabolomics Induced by Microplastics
Abstract
:1. Introduction
2. Targeted Metabolomics
2.1. Data Acquisition Methods for Targeted Metabolomics
2.2. Targeted Metabolomics Studies of Aquatic Species
2.3. Targeted Metabolomics Studies of Terrestrial Species
3. Untargeted Metabolomics
3.1. Data Acquisition Methods of Untargeted Metabolomics
3.2. Untargeted Metabolomics Studies of Aquatic Species
3.3. Untargeted Metabolomics Studies of Terrestrial Species
3.4. Novel Data Acquisition of Untargeted Metabolomics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fotopoulou, K.N.; Karapanagioti, H.K. Degradation of various plastics in the environment. Hazard. Chem. Assoc. Plast. Mar. Environ. 2019, 78, 71–92. [Google Scholar]
- Tsuchimoto, I.; Kajikawa, Y. Recycling of Plastic Waste: A Systematic Review Using Bibliometric Analysis. Sustainability 2022, 14, 16340. [Google Scholar]
- Hale, R.C.; Seeley, M.E.; La Guardia, M.J.; Mai, L.; Zeng, E.Y. A global perspective on microplastics. J. Geophys. Res. Ocean. 2020, 125, e2018JC014719. [Google Scholar] [CrossRef]
- Yao, Y.; Glamoclija, M.; Murphy, A.; Gao, Y. Characterization of microplastics in indoor and ambient air in northern New Jersey. Environ. Res. 2022, 207, 112142. [Google Scholar] [PubMed]
- Tian, L.; Jinjin, C.; Ji, R.; Ma, Y.; Yu, X. Microplastics in agricultural soils: Sources, effects, and their fate. Curr. Opin. Environ. Sci. Health 2022, 25, 100311. [Google Scholar]
- Sun, Q.; Li, J.; Wang, C.; Chen, A.; You, Y.; Yang, S.; Liu, H.; Jiang, G.; Wu, Y.; Li, Y. Research progress on distribution, sources, identification, toxicity, and biodegradation of microplastics in the ocean, freshwater, and soil environment. Front. Environ. Sci. Eng. 2022, 16, 1. [Google Scholar]
- Li, Z.; Chao, M.; He, X.; Lan, X.; Tian, C.; Feng, C.; Shen, Z. Microplastic bioaccumulation in estuary-caught fishery resource. Environ. Pollut. 2022, 306, 119392. [Google Scholar] [CrossRef]
- Kwon, J.-H.; Kim, J.-W.; Pham, T.D.; Tarafdar, A.; Hong, S.; Chun, S.-H.; Lee, S.-H.; Kang, D.-Y.; Kim, J.-Y.; Kim, S.-B. Microplastics in food: A review on analytical methods and challenges. Int. J. Environ. Res. Public Health 2020, 17, 6710. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef]
- Qiao, R.; Deng, Y.; Zhang, S.; Wolosker, M.B.; Zhu, Q.; Ren, H.; Zhang, Y. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere 2019, 236, 124334. [Google Scholar]
- Meng, X.; Zhang, J.; Wang, W.; Gonzalez-Gil, G.; Vrouwenvelder, J.S.; Li, Z. Effects of nano-and microplastics on kidney: Physicochemical properties, bioaccumulation, oxidative stress and immunoreaction. Chemosphere 2022, 288, 132631. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Shi, W.; Tang, Y.; Zhou, W.; Sun, H.; Zhang, J.; Yan, M.; Hu, L.; Liu, G. Microplastics and bisphenol A hamper gonadal development of whiteleg shrimp (Litopenaeus vannamei) by interfering with metabolism and disrupting hormone regulation. Sci. Total Environ. 2022, 810, 152354. [Google Scholar]
- Kim, J.-H.; Yu, Y.-B.; Choi, J.-H. Toxic effects on bioaccumulation, hematological parameters, oxidative stress, immune responses and neurotoxicity in fish exposed to microplastics: A review. J. Hazard. Mater. 2021, 413, 125423. [Google Scholar] [PubMed]
- Liu, Z.; Zhuan, Q.; Zhang, L.; Meng, L.; Fu, X.; Hou, Y. Polystyrene microplastics induced female reproductive toxicity in mice. J. Hazard. Mater. 2022, 424, 127629. [Google Scholar] [PubMed]
- Lee, S.; Kang, K.-K.; Sung, S.-E.; Choi, J.-H.; Sung, M.; Seong, K.-Y.; Lee, S.; Yang, S.Y.; Seo, M.-S.; Kim, K. Toxicity study and quantitative evaluation of polyethylene microplastics in ICR mice. Polymers 2022, 14, 402. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017, 7, 46687. [Google Scholar] [PubMed]
- Shin, H.; Jeong, C.-B. Metabolism deficiency and oxidative stress induced by plastic particles in the rotifer Brachionus plicatilis: Common and distinct phenotypic and transcriptomic responses to nano-and microplastics. Mar. Pollut. Bull. 2022, 182, 113981. [Google Scholar] [CrossRef]
- Feussner, I.; Polle, A. What the transcriptome does not tell—Proteomics and metabolomics are closer to the plants’ patho-phenotype. Curr. Opin. Plant Biol. 2015, 26, 26–31. [Google Scholar] [CrossRef]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2007, 36, D480–D484. [Google Scholar] [CrossRef]
- Liu, L.; Wu, Q.; Miao, X.; Fan, T.; Meng, Z.; Chen, X.; Zhu, W. Study on toxicity effects of environmental pollutants based on metabolomics: A review. Chemosphere 2022, 286, 131815. [Google Scholar]
- Bhagat, J.; Zhang, L.; Nishimura, N.; Shimada, Y. Application of omics approaches for assessing microplastic and nanoplastic toxicity in fish and seafood species. Trends Anal. Chem. 2022, 154, 116674. [Google Scholar] [CrossRef]
- Cappello, T.; De Marco, G.; Conti, G.O.; Giannetto, A.; Ferrante, M.; Mauceri, A.; Maisano, M. Time-dependent metabolic disorders induced by short-term exposure to polystyrene microplastics in the Mediterranean mussel Mytilus galloprovincialis. Ecotoxicol. Environ. Saf. 2021, 209, 111780. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qin, Z.; Huang, Z.; Bao, Z.; Luo, T.; Jin, Y. Effects of polyethylene microplastics on the microbiome and metabolism in larval zebrafish. Environ. Pollut. 2021, 282, 117039. [Google Scholar] [CrossRef]
- An, Y.; Hong, S.; Kim, Y.; Kim, M.; Choi, B.; Won, E.-J.; Shin, K.-H. Trophic transfer of persistent toxic substances through a coastal food web in Ulsan Bay, South Korea: Application of compound-specific isotope analysis of nitrogen in amino acids. Environ. Pollut. 2020, 266, 115160. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.-Z.; Gonzalez, F.J. LC–MS-based metabolomics: An update. Arch. Toxicol. 2014, 88, 1491–1502. [Google Scholar] [CrossRef]
- Roberts, L.D.; Souza, A.L.; Gerszten, R.E.; Clish, C.B. Targeted metabolomics. Curr. Protoc. Mol. Biol. 2012, 98, 30.2.1–30.2.24. [Google Scholar] [CrossRef]
- Vinayavekhin, N.; Saghatelian, A. Untargeted metabolomics. Curr. Protoc. Mol. Biol. 2010, 90, 30.1.1–30.1.24. [Google Scholar]
- Kondrat, R.W.; McClusky, G.A.; Cooks, R.G. Multiple reaction monitoring in mass spectrometry/mass spectrometry for direct analysis of complex mixtures. Anal. Chem. 1978, 50, 2017–2021. [Google Scholar] [CrossRef]
- Griffiths, W.J.; Koal, T.; Wang, Y.; Kohl, M.; Enot, D.P.; Deigner, H.P. Targeted metabolomics for biomarker discovery. Angew. Chem. Int. Ed. 2010, 49, 5426–5445. [Google Scholar] [CrossRef]
- Jiang, P.; Yuan, G.-h.; Jiang, B.-r.; Zhang, J.-y.; Wang, Y.-q.; Lv, H.-j.; Zhang, Z.; Wu, J.-l.; Wu, Q.; Li, L. Effects of microplastics (MPs) and tributyltin (TBT) alone and in combination on bile acids and gut microbiota crosstalk in mice. Ecotoxicol. Environ. Saf. 2021, 220, 112345. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, Y.; Wang, Q.; Liu, N.; Li, M. Seasonal variations and feedback from microplastics and cadmium on soil organisms in agricultural fields. Environ. Int. 2022, 161, 107096. [Google Scholar] [CrossRef] [PubMed]
- Bobori, D.C.; Dimitriadi, A.; Feidantsis, K.; Samiotaki, A.; Fafouti, D.; Sampsonidis, I.; Kalogiannis, S.; Kastrinaki, G.; Lambropoulou, D.A.; Kyzas, G.Z. Differentiation in the expression of toxic effects of polyethylene-microplastics on two freshwater fish species: Size matters. Sci. Total Environ. 2022, 830, 154603. [Google Scholar] [CrossRef]
- Jeong, S.; Jang, S.; Kim, S.S.; Bae, M.A.; Shin, J.; Lee, K.-B.; Kim, K.-T. Size-dependent seizurogenic effect of polystyrene microplastics in zebrafish embryos. J. Hazard. Mater. 2022, 439, 129616. [Google Scholar] [CrossRef]
- Dimitriadi, A.; Papaefthimiou, C.; Genizegkini, E.; Sampsonidis, I.; Kalogiannis, S.; Feidantsis, K.; Bobori, D.C.; Kastrinaki, G.; Koumoundouros, G.; Lambropoulou, D.A. Adverse effects polystyrene microplastics exert on zebrafish heart–Molecular to individual level. J. Hazard. Mater. 2021, 416, 125969. [Google Scholar] [CrossRef]
- Li, S.; Wang, T.; Guo, J.; Dong, Y.; Wang, Z.; Gong, L.; Li, X. Polystyrene microplastics disturb the redox homeostasis, carbohydrate metabolism and phytohormone regulatory network in barley. J. Hazard. Mater. 2021, 415, 125614. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Marsal, S.; Julià, A. Analytical methods in untargeted metabolomics: State of the art in 2015. Front. Bioeng. Biotechnol. 2015, 3, 23. [Google Scholar] [PubMed]
- Ivanisevic, J.; Want, E.J. From samples to insights into metabolism: Uncovering biologically relevant information in LC-HRMS metabolomics data. Metabolites 2019, 9, 308. [Google Scholar] [CrossRef]
- Guo, J.; Huan, T. Comparison of full-scan, data-dependent, and data-independent acquisition modes in liquid chromatography–mass spectrometry based untargeted metabolomics. Anal. Chem. 2020, 92, 8072–8080. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chen, G.; Wang, J. Microplastics in the marine environment: Sources, fates, impacts and microbial degradation. Toxics 2021, 9, 41. [Google Scholar] [CrossRef]
- Medriano, C.A.; Bae, S. Acute exposure to microplastics induces metabolic disturbances and gut dysbiosis in adult zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2022, 245, 114125. [Google Scholar]
- Lu, X.; Zhang, J.-X.; Zhang, L.; Wu, D.; Tian, J.; Yu, L.-J.; He, L.; Zhong, S.; Du, H.; Deng, D.-F. Comprehensive understanding the impacts of dietary exposure to polyethylene microplastics on genetically improved farmed tilapia (Oreochromis niloticus): Tracking from growth, microbiota, metabolism to gene expressions. Sci. Total Environ. 2022, 841, 156571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wen, K.; Ding, D.; Liu, J.; Lei, Z.; Chen, X.; Ye, G.; Zhang, J.; Shen, H.; Yan, C. Size-dependent adverse effects of microplastics on intestinal microbiota and metabolic homeostasis in the marine medaka (Oryzias melastigma). Environ. Int. 2021, 151, 106452. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Sun, S.; Han, Y.; Tang, Y.; Zhou, W.; Du, X.; Liu, G. Microplastics impair olfactory-mediated behaviors of goldfish Carassius auratus. J. Hazard. Mater. 2021, 409, 125016. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-K.; Yang, B.-K.; Zhang, C.-N.; Xu, S.-X.; Sun, P. Effects of polystyrene microplastics acute exposure in the liver of swordtail fish (Xiphophorus helleri) revealed by LC-MS metabolomics. Sci. Total Environ. 2022, 850, 157772. [Google Scholar] [CrossRef] [PubMed]
- Sussarellu, R.; Suquet, M.; Thomas, Y.; Lambert, C.; Fabioux, C.; Pernet, M.E.J.; Le Goïc, N.; Quillien, V.; Mingant, C.; Epelboin, Y.; et al. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci. USA 2016, 113, 2430–2435. [Google Scholar] [CrossRef]
- Huang, W.; Wang, X.; Chen, D.; Xu, E.G.; Luo, X.; Zeng, J.; Huan, T.; Li, L.; Wang, Y. Toxicity mechanisms of polystyrene microplastics in marine mussels revealed by high-coverage quantitative metabolomics using chemical isotope labeling liquid chromatography mass spectrometry. J. Hazard. Mater. 2021, 417, 126003. [Google Scholar] [CrossRef]
- Teng, J.; Zhao, J.; Zhu, X.; Shan, E.; Zhang, C.; Zhang, W.; Wang, Q. Toxic effects of exposure to microplastics with environmentally relevant shapes and concentrations: Accumulation, energy metabolism and tissue damage in oyster Crassostrea gigas. Environ. Pollut. 2021, 269, 116169. [Google Scholar] [CrossRef]
- Duan, Y.; Xiong, D.; Wang, Y.; Zhang, Z.; Li, H.; Dong, H.; Zhang, J. Toxicological effects of microplastics in Litopenaeus vannamei as indicated by an integrated microbiome, proteomic and metabolomic approach. Sci. Total Environ. 2021, 761, 143311. [Google Scholar] [CrossRef]
- Wang, P.; Li, Q.-Q.; Hui, J.; Xiang, Q.-Q.; Yan, H.; Chen, L.-Q. Metabolomics reveals the mechanism of polyethylene microplastic toxicity to Daphnia magna. Chemosphere 2022, 307, 135887. [Google Scholar] [CrossRef]
- Blackburn, K.; Green, D. The potential effects of microplastics on human health: What is known and what is unknown. Ambio 2022, 51, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Han, X.; Guo, W.; Wu, Q.; Yang, X.; Wang, Y.; Tang, G.; Wang, S.; Wang, Z.; Liu, Y.; et al. Disturbed Gut-Liver axis indicating oral exposure to polystyrene microplastic potentially increases the risk of insulin resistance. Environ. Int. 2022, 164, 107273. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Wang, D.; Zhao, H.; Wang, Y.; Zhang, Y.; Liu, Y.; Li, B.; Xing, M. Polystyrene microplastics up-regulates liver glutamine and glutamate synthesis and promotes autophagy-dependent ferroptosis and apoptosis in the cerebellum through the liver-brain axis. Environ. Pollut. 2022, 307, 119449. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yin, Y.; Zhu, Z.-J. Advancing untargeted metabolomics using data-independent acquisition mass spectrometry technology. Anal. Bioanal. Chem. 2019, 411, 4349–4357. [Google Scholar] [CrossRef] [PubMed]
- Zha, H.; Cai, Y.; Yin, Y.; Wang, Z.; Li, K.; Zhu, Z.-J. SWATHtoMRM: Development of High-Coverage Targeted Metabolomics Method Using SWATH Technology for Biomarker Discovery. Anal. Chem. 2018, 90, 4062–4070. [Google Scholar] [CrossRef] [PubMed]
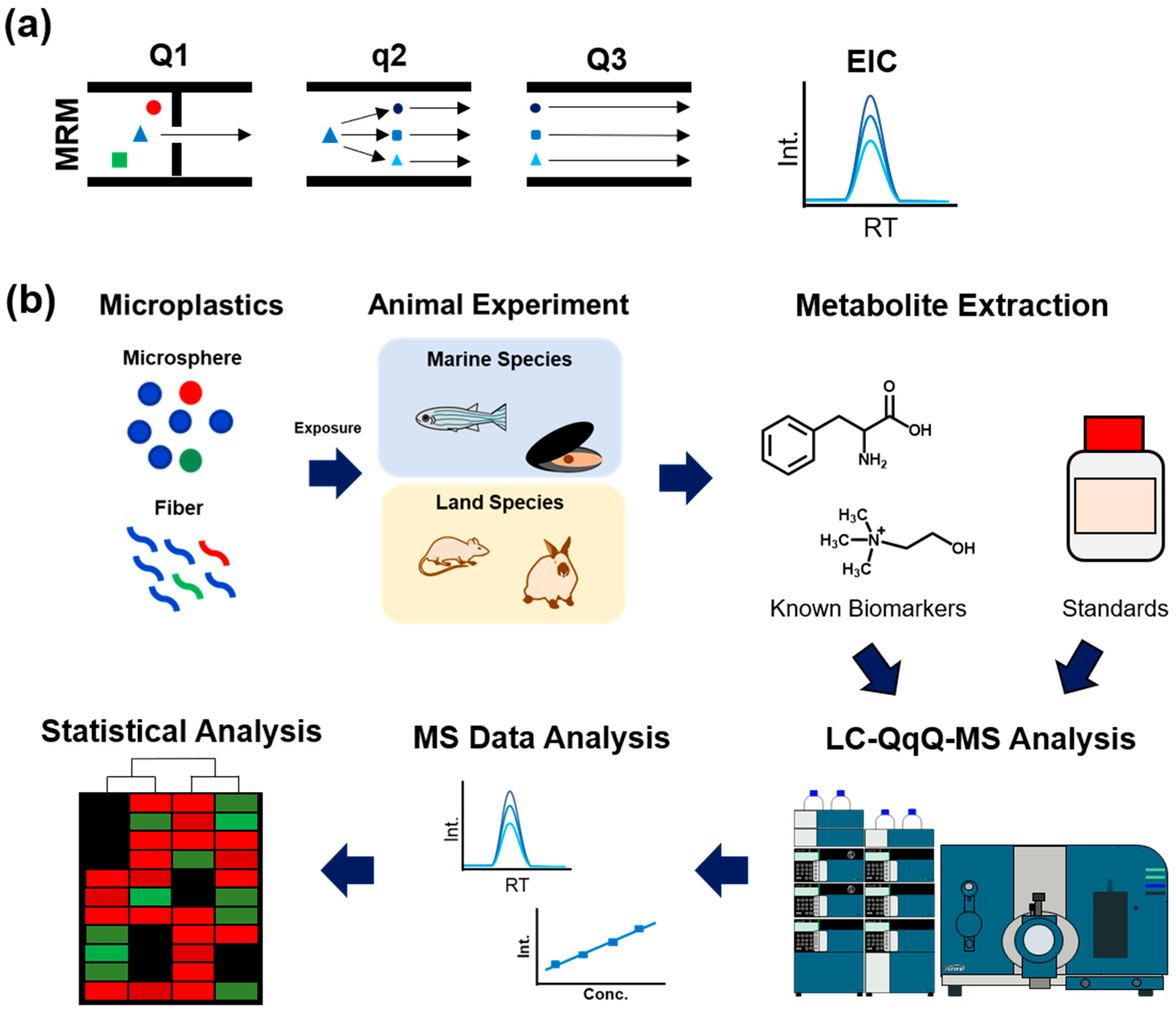
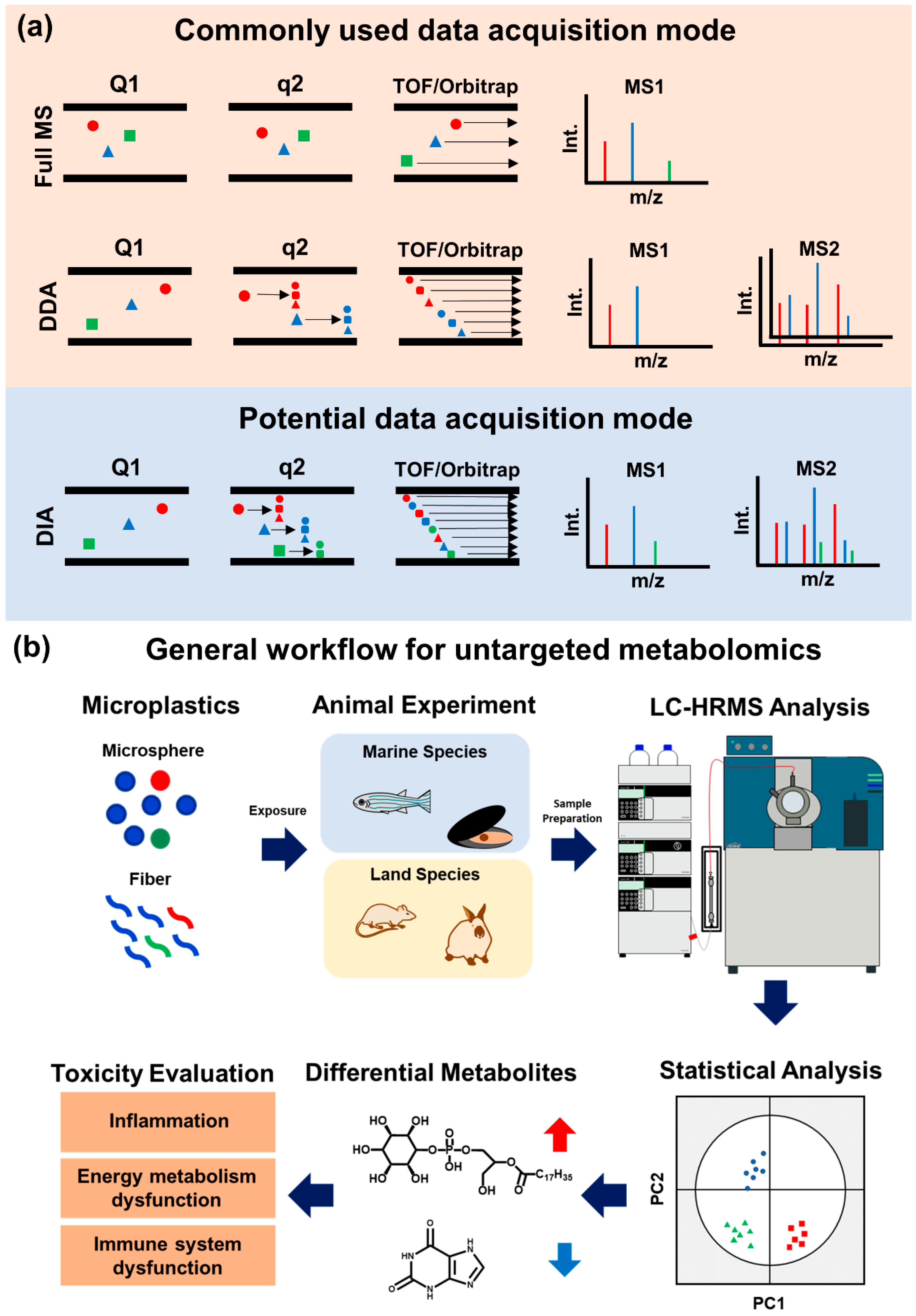

| MPs | Model; Tissue; Sample Numbers | Exposure Dose | Selected Biomarkers | Ref |
|---|---|---|---|---|
| PS (<5 µm) | Mice (Mus musculus); serum and fecal; n = 10 | 0.1 mg/day | Aspartate aminotransferase (AST) 7-Ketolithocholic acid (7-ketoLCA) Taurocholic acid (TCA) | [30] |
| PS (<0.5 mm) | Barley (Hordeum vulgare); leaves and roots; n = 3 | 2 mg/L | Indole-3-acetic acid (IAA) Indole-3-butyric acid (IBA) 3-Indolepropionic acid (IPA) | [31] |
| PE (10–45 and 106–125 µm) | Perch (Perca fluviatilis) and zebrafish (Danio rerio); gills and liver; n = 10 | 10 mg/g | Aromatic Amino acids Choline | [32] |
| PS (<0.5 mm) | ICR mice (Mus musculus); gut; n = 8 | 100 μg/L 1000 μg/L | Succinyl acetone, 11 Amino acids 25 Carnitines | [33] |
| PS (10 µm) | Zebrafish (Danio rerio); embryos; n = 30 | 0 particles/mL 500 particles/mL 5000 particles/mL 50,000 particles/mL | Choline Betaine Dopamine 3-Methoxytyramine γ-Aminobutyric acid | [34] |
| PS (3–12 µm) | Zebrafish (Danio rerio); heart; n = 7 | In vivo: 10 mg/g Ex vivo: 26 and 260 mg/L | Pyruvic acid Acetylcarnitine Carnitine Succinic acid α-Ketoglutaric Amino acids | [35] |
| PE (< 5 mm) | Earthworm (Amynthas corticis); intestines; n = 10 | Earth sample | L-phenylalanine Succinic acid | [36] |
| MPs | Modell; Tissue; Sample Numbers | Analysis Method (LC-MS) | Exposure Dose | Results | Ref |
|---|---|---|---|---|---|
| PE (1–4 μm) | Zebrafish (Danio rerio); Larval; n = 5 | LC-Q/Orbitrap-MS | 0 μg/L 10 μg/L 100 μg/L 1000 μg/L |
| [23] |
| PE and PES (100 µm) | Zebrafish (Danio rerio); whole fish; n = 9 | LC-Q/TOF-MS | 0 mg/L 1 mg/L PE 1 mg/L PES |
| [41] |
| PE (50 and 125 μm) | Nile tilapia (Oreochromis niloticus); liver; n = 9 | LC-Q/Orbitrap-MS | 1–3 weeks: 5% body weight 4–9 weeks: 3% body weight |
| [42] |
| PS (500 nm and 30 µm) | Goldfish (Carassius auratus); brain; n = 5 | - | 0 mg/L 500 nm PS: 0.26 mg/L 0.69 mg/L 30 µm PS: 0.26 mg/L 0.69 mg/L |
| [44] |
| PS (1 μm) | Swordtail fish (Xiphophorus helleri); liver; n = 6 | LC-Q/TOF-MS | 0 particles/L 106 particles/L 107 particles/L |
| [45] |
| PS (2 μm) | Marine mussels (Mytilus coruscus); hemolymph; n = 6 | LC-Q/TOF-MS(CIL methods) | 0 particles/L 10 particles/L 104 particles/L 106 particles/L |
| [47] |
| PE (10.7–93.9 μm) PET (9.0–56.0 μm) | Oysters (Crassostrea gigas); digestive gland; n = 8 | LC-Q/Orbitrap-MS | 0 μg/L 10 μg/L PE 1000 μg/L PE 10 μg/L PET 1000 μg/L PET |
| [48] |
| PE (6–18 μm) PTFE (1–8 μm) PP (1.77–18 μm) PS (100–200 μm) PVC (1–13 μm) | White shrimp (Litopenaeus vannamei); hemolymph; n = 6 | LC-Q/Orbitrap-MS | 1.0 mg/L |
| [49] |
| PE (20 and 30 μm) | Daphnia magna; whole; n = 6 | LC-Q/Orbitrap-MS | 0 mg/L 20 mg/L 60 mg/L |
| [50] |
| PS (1 μm) | ICR mice (Mus musculus); liver; n = 8 | LC-Q/Orbitrap-MS | 4.5 × 104 − 4.3 × 106 particles/day * |
| [52] |
| PS (2 μm) | Chicken (Gallus gallus domesticus); liver; n = 6 | LC-Q/TOF-MS | 0 mg/L 1 mg/L 10 mg/L 100 mg/L |
| [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.-L.; Liao, W.-R.; Lin, W.-C.; Chen, S.-F. A Review of Liquid Chromatography-Mass Spectrometry Strategies for the Analyses of Metabolomics Induced by Microplastics. Separations 2023, 10, 257. https://doi.org/10.3390/separations10040257
Wu K-L, Liao W-R, Lin W-C, Chen S-F. A Review of Liquid Chromatography-Mass Spectrometry Strategies for the Analyses of Metabolomics Induced by Microplastics. Separations. 2023; 10(4):257. https://doi.org/10.3390/separations10040257
Chicago/Turabian StyleWu, Kuan-Lu, Wan-Rou Liao, Wei-Chen Lin, and Sung-Fang Chen. 2023. "A Review of Liquid Chromatography-Mass Spectrometry Strategies for the Analyses of Metabolomics Induced by Microplastics" Separations 10, no. 4: 257. https://doi.org/10.3390/separations10040257
APA StyleWu, K. -L., Liao, W. -R., Lin, W. -C., & Chen, S. -F. (2023). A Review of Liquid Chromatography-Mass Spectrometry Strategies for the Analyses of Metabolomics Induced by Microplastics. Separations, 10(4), 257. https://doi.org/10.3390/separations10040257





