A New Method for the Determination of Amisulpride in a Small Volume (200 μL) of Human Saliva Using LC-DAD Supported by SPE
Abstract
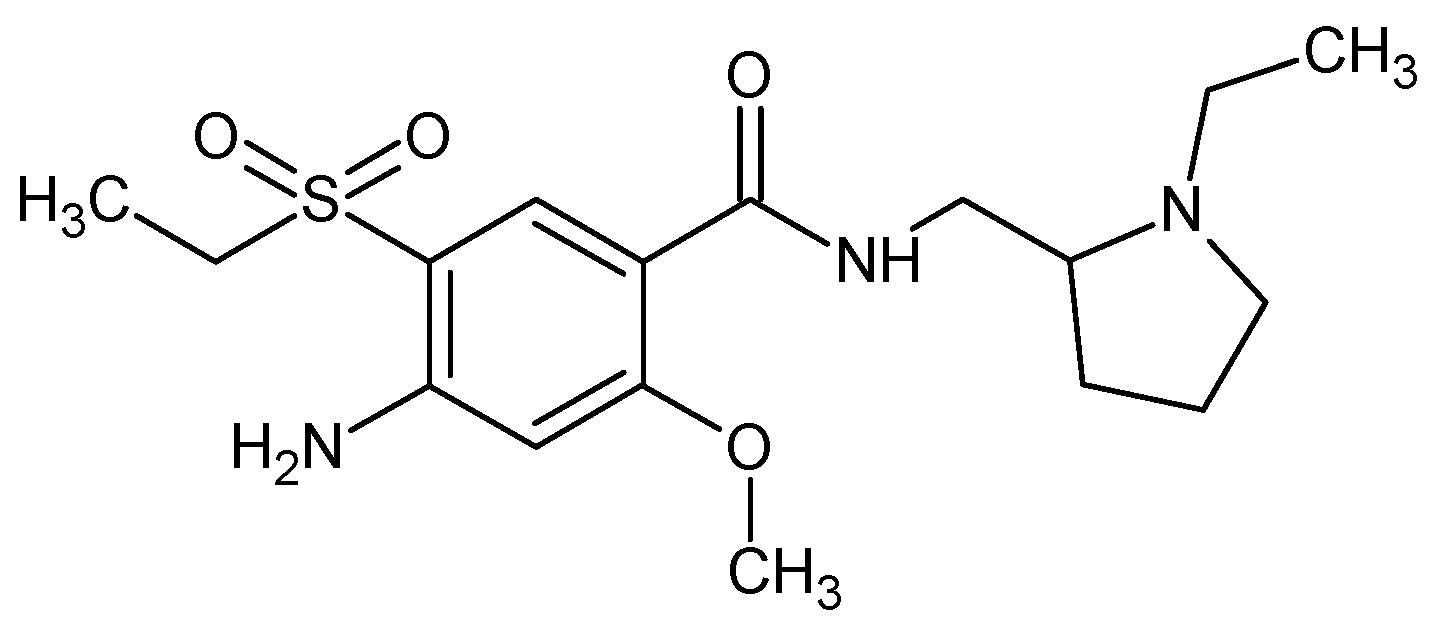
1. Introduction
2. Materials and Methods
2.1. Chemicals and Solvents
2.2. LC-DAD Conditions
2.3. Preparation of Quality Control (QC) and Standard Samples
2.4. Collection of Saliva Samples
2.5. Extraction of Amisulpride from Saliva Samples
2.6. Validation
2.6.1. Linearity
2.6.2. Intra and Inter-Day Precision
2.6.3. Limit of Detection (LOD) and Lower Limit of Quantification (LLOQ)
2.6.4. Selectivity
2.6.5. Extraction and Absolute Recovery
2.6.6. Stability
2.7. Clinical Application
3. Results
3.1. Chromatographic Analysis
3.2. Solid Phase Extraction
3.3. Method Validation
3.3.1. Extraction and Absolute Recovery
3.3.2. Stability
3.4. Clinical Application
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meyer, J.M. Pharmacotherapy of Psychosis and Mania. In Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 14th ed.; Laurence, L.B., Björn, C.K., Eds.; McGraw Hill: New York, NY, USA, 2023; Available online: https://accesspharmacy-1mhmedical-1com-16n9axldd00ce.han.gumed.edu.pl/content.aspx?bookid=3191§ionid=269307491 (accessed on 21 March 2023).
- Medicines Complete. Available online: https://www-1medicinescomplete-1com-1988e0mqa0056.han.gumed.edu.pl/#/content/martindale/1759-d (accessed on 21 March 2023).
- Kranke, P.; Bergese, S.D.; Minkowitz, H.S.; Melson, T.I.; Leiman, D.G.; Candiotti, K.A.; Liu, N.; Eberhart, L.; Habib, A.S.; Wallenborn, J.; et al. Amisulpride prevents postoperative nausea and vomiting in patients at high risk: A randomized, double-blind, placebo-controlled trial. Anesthesiology 2018, 128, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Haber, S.L.; Graybill, A.; Minasian, A. Amisulpride: A new drug for management of postoperative nausea and vomiting. Ann. Pharmacother. 2021, 55, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.S.; Partridge, S.J.; Handley, S.A.; Flanagan, R.J. Stability of some atypical antipsychotics in human plasma, haemolysed whole blood, oral fluid, human serum and calf serum. Forensic Sci. Int. 2013, 229, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.S.; van Schalkwyk, G.I.; Seedat, S.; Curran, S.R.; Flanagan, R.J. Plasma, oral fluid, and whole-blood distribution of antipsychotics and metabolites in clinical samples. Ther. Drug Monit. 2013, 35, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Proença, P.; Monteiro, C.; Mustra, C.; Claro, A.; Franco, J.; Corte-Real, F. Identification and quantification of antipsychotics in blood samples by LC-MS-MS: Case reports and data from three years of routine analysis. J. Anal. Toxicol. 2020, 44, 915–922. [Google Scholar] [CrossRef]
- Péhourcq, F.; Ouariki, S.; Bégaud, B. Rapid high-performance liquid chromatographic measurement of amisulpride in human plasma: Application to manage acute intoxication. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 789, 101–105. [Google Scholar] [CrossRef]
- Sachse, J.; Härtter, S.; Weigmann, H.; Hiemke, C. Automated determination of amisulpride by liquid chromatography with column switching and spectrophotometric detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 784, 405–410. [Google Scholar] [CrossRef]
- Bergemann, N.; Kopitz, J.; Kress, K.R.; Frick, A. Plasma amisulpride levels in schizophrenia or schizoaffective disorder. Eur. Neuropsychopharmacol. 2004, 14, 245–250. [Google Scholar] [CrossRef]
- Gschwend, M.H.; Arnold, P.; Ring, J.; Martin, W. Selective and sensitive determination of amisulpride in human plasma by liquid chromatography-tandem mass spectrometry with positive electrospray ionisation and multiple reaction monitoring. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2006, 831, 132–139. [Google Scholar] [CrossRef]
- Nirogi, R.; Bhyrapuneni, G.; Kandikere, V.; Mudigonda, K.; Ajjala, D.; Suraneni, R.; Mukkanti, K. Liquid chromatography tandem mass spectrometry method for the quantification of amisulpride with LLOQ of 100 pg/mL using 100 microL of plasma. Biomed. Chromatogr. 2008, 22, 1424–1433. [Google Scholar] [CrossRef]
- Couchman, L.; Morgan, P.E.; Flanagan, R.J. Basic drug analysis by strong cation-exchange liquid chromatography-tandem mass spectrometry: Simultaneous analysis of amisulpride, and of metamfetamine and amfetamine in serum/plasma. Biomed. Chromatogr. 2011, 25, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Mogili, R.; Kanala, K.; Challa, B.R.; Chandu, B.R.; Bannoth, C.K. Development and validation of amisulpride in human plasma by HPLC coupled with tandem mass spectrometry and its application to a pharmacokinetic study. Sci. Pharm. 2011, 79, 583–599. [Google Scholar] [CrossRef] [PubMed]
- Kudris, I.V.; Skakun, N.N.; Orlova, I.N.; Libina, V.V.; Kulikov, A.U. Analysis of amisulpride in human plasma by SPE and LC with fluorescence detection. Chromatographia 2011, 73, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.S.; Partridge, S.J.; Handley, S.A.; Couchman, L.; Morgan, P.E.; Flanagan, R.J. LC-MS/MS of some atypical antipsychotics in human plasma, serum, oral fluid and haemolysed whole blood. Forensic Sci. Int. 2013, 229, 145–150. [Google Scholar] [CrossRef]
- OʼHalloran, S.J.; Wong, A.; Joyce, D.A. A liquid chromatography-tandem mass spectrometry method for quantifying amisulpride in human plasma and breast milk, applied to measuring drug transfer to a fully breast-fed neonate. Ther. Drug Monit. 2016, 38, 493–498. [Google Scholar] [CrossRef]
- Wang, S.T.; Li, Y. Development of a UPLC-MS/MS method for routine therapeutic drug monitoring of aripiprazole, amisulpride, olanzapine, paliperidone and ziprasidone with a discussion of their therapeutic reference ranges for Chinese patients. Biomed. Chromatogr. 2017, 31, e3928. [Google Scholar] [CrossRef]
- Fisher, D.S.; Beyer, C.; van Schalkwyk, G.; Seedat, S.; Flanagan, R.J. Measurement of clozapine, norclozapine, and amisulpride in plasma and in oral fluid obtained using 2 different sampling systems. Ther. Drug Monit. 2017, 39, 109–117. [Google Scholar] [CrossRef]
- Frahnert, C.; Rao, M.L.; Grasmäder, K. Analysis of eighteen antidepressants, four atypical antipsychotics and active metabolites in serum by liquid chromatography: A simple tool for therapeutic drug monitoring. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 794, 35–47. [Google Scholar] [CrossRef]
- Patteet, L.; Maudens, K.E.; Morrens, M.; Sabbe, B.; Dom, G.; Neels, H. Determination of common antipsychotics in quantisal-collected oral fluid by UHPLC-MS/MS: Method validation and applicability for therapeutic drug monitoring. Ther. Drug Monit. 2016, 38, 87–97. [Google Scholar] [CrossRef]
- European Medicines Agency. Available online: https://www.ema.europa.eu/en (accessed on 21 March 2023).
- Sun, F.; Yu, F.; Gao, Z.; Ren, Z.; Jin, W. Study on the relationship among dose, concentration and clinical response in Chinese schizophrenic patients treated with Amisulpride. Asian J. Psychiatry 2021, 62, 102694. [Google Scholar] [CrossRef]
- Urban, A.E.; Cubała, W.J. Therapeutic drug monitoring of atypical antipsychotics. Psychiatry Pol. 2017, 51, 1059–1077. [Google Scholar] [CrossRef] [PubMed]
- Sparshatt, A.; Taylor, D.; Patel, M.X.; Kapur, S. Amisulpride—Dose, plasma concentration, occupancy and response: Implications for therapeutic drug monitoring. Acta Psychiatry Scand. 2009, 120, 416–428. [Google Scholar] [CrossRef] [PubMed]



| Procedure | Dilution Solvent | Washing Solvent | Elution Solvent |
|---|---|---|---|
| 1. | methanol:water (1:1) | water | methanol |
| 2. | acetonitrile | ||
| 3. |
| methanol | |
| 4. | acetonitrile | ||
| 5. | 2% formic acid | 5% ammonium in methanol | |
| 6. | 5% ammonium in acetonitrile | ||
| 7. |
| 5% ammonium in methanol | |
| 8. | 5% ammonium in acetonitrile | ||
| 9. | 0.1 M K2HPO4 | methanol:water (10:90, v/v) | methanol |
| Calibration Curve y = ax + b (n = 4) | |
|---|---|
| Range (ng/mL) | 5–5000 |
| Determination coefficient (R2) | 0.9978 ± 0.0021 |
| Slope a ± Δa | 0.0005 ± 0.00005 |
| Intercept b ± Δb | −0.0147 ± 0.00700 |
| LOD (ng/mL) | 3.0 |
| LLOQ (ng/mL) | 5.0 |
| QC (ng/mL) | Intra-Day (CV%) | Inter-Day (CV%) | Extraction Recovery (%) | Absolute Recovery (%) | Stability (Difference %) | |
|---|---|---|---|---|---|---|
| 8 °C | −21 °C | |||||
| 25 | 4.79 | 8.84 | 101.22 | 61.03 | −2.50 | −0.50 |
| 400 | 4.41 | 4.72 | 91.81 | 83.10 | −2.81 | −1.24 |
| 2500 | 5.05 | 7.44 | 92.82 | 80.69 | −2.63 | −1.65 |
| 4000 | 1.79 | 4.95 | 96.02 | 79.91 | −3.89 | −2.49 |
| Patient | Age | Dose (mg/Day) | Amisulpride (ng/mL) |
|---|---|---|---|
| 1 | 58 | 400 | 2852.9 |
| 2 | 62 | 400 | 4713.1 |
| 3 | 56 | 400 | 4707.6 |
| 4 | 40 | 200 | 216.3 |
| 5 | 38 | 200 | 543.3 |
| 6 | 43 | 200 | 104.8 |
| Biological Matrices | Volume of the Sample (µL) | Isolation | Separation and Detection | LOQ (ng/mL) | Linearity (ng/mL) | R2 | Concentration (ng/mL) | References |
|---|---|---|---|---|---|---|---|---|
| Plasma | 1000 | LLE | HPLC-UV | 20 | 100–1000 | 0.985 | 107–14,824 | [8] |
| 1000 | SPE | HPLC-FL | 2 | 2–800 | >0.99 | 424.4 ± 292.8 | [10] | |
| 50 | LLE | LC-MS/MS | 0.5 | 0.5–500.52 | =0.9999 | 522.58 | [11] | |
| 100 | LLE | LC-MS/MS | 0.1 | 0.1–100 | >0.99 | - | [12] | |
| 100 | LLE | HPLC-MS/MS | 2 | 2–2500 | >0.99 | 947.9 1093.4 | [14] | |
| 1500 | SPE | LC-FL | 10 | 10–1000 | =0.9979 | 501 ± 216 515 ± 208 | [15] | |
| 20 | deproteinisation | UPLC-MS/MS | 20 | 20–2000 | >0.9977 | 21.9–773.7 | [18] | |
| Plasma/breast milk | plasma 25 milk 15 | deproteinisation | LC-MS/MS | breast milk 0.6 plasma 0.5 | breast milk 0.6 to 75 plasma 0.5–360 | >0.999 | maternal plasma 95 breast milk 1131 baby’s plasma 10 | [17] |
| Serum, plasma | 200 | LLE | LC-MS/MS | 0.5 | 1–400 | >0.98 | - | [13] |
| 100 | column-switching | HPLC-UV | 10 | 10–600 | >0.99 | - | [9] | |
| Serum | 1000 | SPE | HPLC-UV | 10 | 10–1000 | >0.9995 | - | [20] |
| Blood | 500 | SPE | HPLC-MS/MS | 5 | 5–500 | >0.99 | - | [7] |
| Whole blood, oral fluid, serum | 200 | LLE | LC-MS/MS | 10 | 10–500 | >0.99 | - | [5] |
| Plasma, oral fluid, whole blood | 500 | LLE | HPLC-MS/MS | - | - | - | plasma 193,241; oral fluid 190, 208; whole blood 243, 381 | [6] |
| Plasma, serum, oral fluid | 200 | LLE | LC-MS/MS | 6 | 10–500 | >0.99 | plasma/serum 413–2348 | [16] |
| Plasma, oral fluid | 500 | LLE | LC-MS/MS | 5 | 5–800 | >0.99 | plasma 40–1338 oral fluid 16–1827 | [19] |
| Oral fluid | 500 | LLE | UPLC-MS/MS | 3.20 | 3.20–1920 | 0.995 | 1.04–68.66 | [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziurkowska, E.; Kosinska, S.; Plenis, A.; Wesolowski, M. A New Method for the Determination of Amisulpride in a Small Volume (200 μL) of Human Saliva Using LC-DAD Supported by SPE. Separations 2023, 10, 277. https://doi.org/10.3390/separations10050277
Dziurkowska E, Kosinska S, Plenis A, Wesolowski M. A New Method for the Determination of Amisulpride in a Small Volume (200 μL) of Human Saliva Using LC-DAD Supported by SPE. Separations. 2023; 10(5):277. https://doi.org/10.3390/separations10050277
Chicago/Turabian StyleDziurkowska, Ewelina, Sandra Kosinska, Alina Plenis, and Marek Wesolowski. 2023. "A New Method for the Determination of Amisulpride in a Small Volume (200 μL) of Human Saliva Using LC-DAD Supported by SPE" Separations 10, no. 5: 277. https://doi.org/10.3390/separations10050277
APA StyleDziurkowska, E., Kosinska, S., Plenis, A., & Wesolowski, M. (2023). A New Method for the Determination of Amisulpride in a Small Volume (200 μL) of Human Saliva Using LC-DAD Supported by SPE. Separations, 10(5), 277. https://doi.org/10.3390/separations10050277







